Abstract
Background
Liver resection is considered to offer the only hope of cure for patients with liver malignancy. However, there are concerns about its safety, particularly in view of the increasing efficacy of less invasive strategies. No systematic review of prognostic research in liver resections has yet been performed.
Methods
A systematic search identified articles published between 1999 and 2012 that performed a risk prediction analysis in patients undergoing liver resection. Studies were included if an outcome occurring within 90 days of surgery was identified, multivariable analysis performed and regression coefficients provided. The main endpoints were the outcomes and predictors chosen by the investigators, their definition, the performance and validity of the models, and the quality of the study as assessed using the QUIPS (quality in prognosis studies) tool.
Results
A total of 91 studies were included. Eleven were prospective, but only two of these were registered. Twenty-eight endpoints were identified. These focused on postoperative morbidity or mortality, but many were redundant or ill defined and other relevant patient-reported outcomes were lacking. Predictors were not standardized, were poorly defined and overlapped. Only nine studies assessed the performance of their models and seven made an internal or temporal validation, but none reported an external validation or impact analysis. The median QUIPS score was 34 out of 50, indicating a high risk for bias.
Conclusion
Prognostic research in liver resection is still at the developmental stage.
Introduction
Since the mid-1990s liver resection has been considered the optimal treatment and to offer the only hope of cure for patients with primary liver malignancy or colorectal liver metastasis.1 This supposition, however, has some limitations.
Firstly, liver resection is classified as a high-risk surgical procedure2 and there are still concerns about its safety.3 Low mortality rates published by some academic institutions match neither those reported by prospective studies4 nor population-based rates, which are 1.6-fold higher.5 Mortality rates have also remained stable or have decreased only marginally over the past 20 years, both at some high-volume academic units6 and at national levels.7 Morbidity rates have not declined and in the USA today, fewer patients are discharged directly to their homes than in the past,8 which suggests that recovery has not improved. In addition, liver surgeons do not accurately predict postoperative outcome.4
Secondly, innovative surgical strategies, such as portal vein embolization,9,10 two-stage hepatectomy11,12 and associated liver partition with portal vein ligation for staged hepatectomy,13 are being developed constantly to improve resection rates. However, not only is there still no consensus on the definition of a resectable tumour,14 but these new strategies are poorly evaluated and the complexity of liver surgery has increased.
Thirdly – and this is likely to be attributable to perceptions of the risk associated with the procedure and its potential benefit – liver resections are performed inconsistently, as indicated by highly variable national and regional rates of resection, which have increased only marginally in recent years.15,16
Fourthly, medical oncology and interventional radiology procedures increasingly represent effective alternatives to liver resection. They are also less invasive in patients in whom quality of life (QoL) (rather than simply quantity of life) is considered the optimal endpoint in determinations of the best therapeutic strategy.17
There are three possible reasons why both the associated risk and the acceptance of liver resection appear to have reached a plateau: the surgical procedure cannot be standardized beyond a certain point; the endpoints used to assess risk (with regard to benefit) are not adequate for the modern era, and the predictors of these endpoints have not been identified or taken into account.
To address these issues, the present authors performed a systematic review to amalgamate the available literature on risk prediction models developed in the setting of liver resection. The aims of this process were to critically analyse: (i) the outcomes chosen as endpoints, as well as their predictors, and (ii) the quality of the studies and their models, as well as their suitability for clinical use.
Materials and methods
Study design and identification
A systematic review was performed, in accordance with the PRISMA (preferred reporting items for systematic reviews and meta-analyses) Statement,18 to identify articles published during the past decade that addressed the postoperative risk associated with liver resection. An electronic search was formulated in collaboration with a medical librarian. The syntax, which included a previously validated strategy19 (Table S1, online), was used in PubMed and the search was restricted to English-language articles published between May 1999 and 31 March 2012. This review is part of a larger registered research program (clinicaltrials.gov: NCT01715402).
Eligibility criteria
To be eligible for inclusion, studies were required to meet the following criteria: (i) an outcome (endpoint) considered by the authors as a surrogate of the postoperative course must be identified; (ii) this outcome must be evaluated for up to 90 days after surgery (i.e. early outcome); (iii) multivariable analysis must be performed to identify independent predictors of this outcome; (iv) the regression coefficient, or the odds ratio (OR), of the predictors must be provided, and (v) the cohort must include at least 100 patients (to allow for meaningful statistical analysis).20
Articles and data extraction
All titles and abstracts of the articles retrieved were reviewed by two of the authors (CL and OF) independently. Duplicate articles and those that obviously failed to meet the eligibility criteria were removed. A full-text review of the remaining articles was performed to exclude duplicates and check eligibility. In the event of disagreement, a final decision was reached by consensus. To triangulate the search, the reference lists of all retained articles were checked for additional papers that might have been missed in the initial search.
Data extracted from retained articles included: the names of the first author, corresponding author and institution; year of publication; country of origin; design of study and statistical analysis; method of data collection (retrospective or prospective); study period; sample size; indication(s) for hepatectomy; outcomes measured and their definitions; predictors and their definitions, and ORs. Data required for quality assessment (see below) were also recorded. To identify registered studies (registration of prognostic research has been recommended21), the website http://www.clinicaltrials.gov was screened using the names of the first and corresponding authors.
Data analysis and definitions
Endpoints and predictors for each study were identified and their definitions compared across studies. A predictor was defined as: (i) a ‘candidate predictor’ if the variable had been considered in the multivariable analysis performed to identify independent risk factors of the given outcome, and (ii) an ‘independent predictor’ if the variable was found to be independent in multivariable analysis. Predictors were grouped in appropriate categories (preoperative, intraoperative and postoperative). Independent predictors in each study were listed and all studies were subsequently screened to identify the number of studies in which each predictor had been considered as a candidate predictor. Studies in which a risk prediction model had been developed were identified and the performance of the model, as well as the method used to validate the model, was recorded. As the studies were heterogeneous in terms of design and methodology, data pooling and meta-analysis were not performed.
Quality assessment
The quality of each study was assessed according to risk for bias using the QUIPS (quality in prognosis studies) tool.22 This is based on the identification of five domains of potential study bias (study participation, prognostic factor measurement, outcome measurement, confounding factor measurement, and analysis) with three to seven items per domain (Table S2, online). Each item is given a score according to whether its quality limits potential bias: a score of 2 indicates that it does; a score of 1 indicates that it does so ‘partly’, and a score of 0 indicates that it does not. Data were extracted independently by the authors and disagreements resolved by discussion.
Results
Selected studies
The electronic search identified 1407 potentially relevant articles. Figure 1 shows a flow diagram of the studies excluded and included. A total of 91 studies met the eligibility criteria (Appendix S1, online) and were included in the review.
Figure 1.
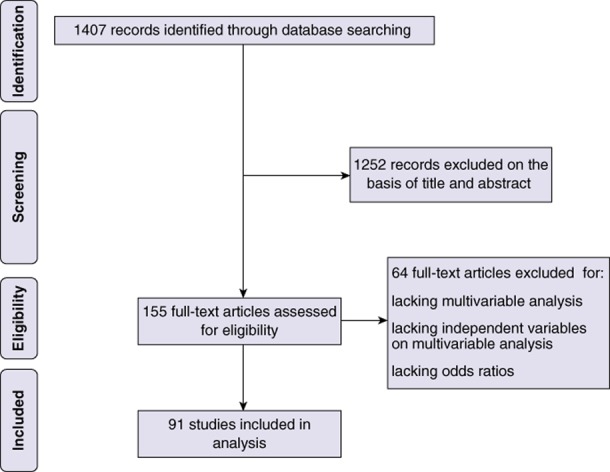
Flow diagram showing the selection of studies performing a risk prediction analysis in patients submitted to liver resection for systematic review
Characteristics of the selected studies
Fifty-five studies originated from Western and 36 from Eastern countries (Table 1). Seventy-nine studies were conducted using data extracted from clinical notes and 12 were based on regional or national administrative data. Of those based on clinical notes, 21 studies (27%) had collected the patient cohort over a period of less than 5 years, and 32 (41%) had collected the cohort over more than 10 years or did not specify the duration of inclusion. All of the studies that relied on administrative data originated in North America. The median number of patients in series for which data were sourced from clinical notes was 266, whereas the median number of patients in series that used administrative data was 2834. Eight of the 79 studies based on clinical notes were multicentre studies. Study design was described as prospective in 11 studies, but only two had been registered. Data collection in notes-based studies was described as prospective in 72 studies; however, none described (or had previously published) the full list of items recorded. Baseline characteristics of the included studies are summarized in Table S3 (online).
Table 1.
Summary of studies performing a risk prediction analysis in patients submitted to liver resection included for review
| All studies | Studies based on clinical data | Studies based on administrative data | |
|---|---|---|---|
| Studies, n | 91 | 79 | 12 |
| Origin, n | |||
| Western seriesa | 55 | 43 | 12 |
| Eastern series | 36 | 36 | 0 |
| Data sources, n | |||
| Clinical database | 79 | 79 | 0 |
| Administrative database | 12 | 0 | 12 |
| Study design, n | |||
| Prospective | 11 | 11 | 0 |
| Retrospective | 80 | 68 | 12 |
| Data collection, n | |||
| Prospective | 84 | 72 | 12 |
| Retrospective | 6 | 6 | 0 |
| Unclear | 1 | 1 | 0 |
| Number of patients, median (range) | 323 (99–50 537) | 266 (99–1803) | 2834 (569–50 537) |
| Inclusion period, n | |||
| <5 years | 27 | 21 | 6 |
| 5–10 years | 33 | 27 | 6 |
| >10 years | 28 | 28 | 0 |
| Unclear | 4 | 4 | 0 |
| Indications for surgery, n | |||
| Malignancy | 52 | 46 | 6 |
| Benign disease | 1 | 1 | 0 |
| Benign and malignant diseases | 31 | 25 | 6 |
| Benign, malignancy, living donor | 4 | 4 | 0 |
| Living donor | 2 | 2 | 0 |
| Unclear | 1 | 0 | 1 |
USA, Europe, Canada and New Zealand.
Endpoints
A total of 28 distinct postoperative endpoints were identified (Table 2). The most frequent related to overall morbidity, overall mortality or liver failure. Only five studies used hospital length of stay (LoS) as an endpoint, and none considered QoL, hospital readmission, or if and when adjuvant chemotherapy could be initiated in patients with colorectal liver metastases.
Table 2.
Endpoints in studies performing a risk prediction analysis in patients submitted to liver resection
| Endpointe | Studies assessing the endpoint of interest, n | Studies defining the endpoint of interest, n |
|---|---|---|
| Mortality | ||
| Overall mortality | 29 | 27a |
| Mortality from liver failure | 3 | 3 |
| Morbidity | ||
| Overall morbidity | 43 | 33b |
| Severe morbidity | 7 | 6 |
| Organ dysfunction | 1 | 1 |
| Major infectious morbidity | 1 | 1 |
| Intraoperative complications | ||
| Operation time | 1 | 1 |
| Intraoperative blood transfusion | 1 | 1 |
| Liver-related complications | ||
| Hepatobiliary complications | 1 | 1 |
| Haemorrhage | 1 | 1 |
| Biliary complications | 1 | 1 |
| Liver failure | 21 | 18c |
| Bile leak | 5 | 4d |
| Biliary complications | 1 | 1 |
| Ascites | 1 | 1 |
| Acute renal failure | 1 | 1 |
| Pulmonary complications | 1 | 1 |
| Pleural effusion | 1 | 1 |
| Pneumonia | 1 | 1 |
| Pulmonary embolism | 1 | 1 |
| Infection | 6 | 6 |
| Surgical site infection | 2 | 2 |
| Intra-abdominal abscess | 1 | 1 |
| Blood transfusion during hospital stay | 1 | 1 |
| Systemic complications | 1 | 1 |
| Postoperative complications | ||
| Ventilation for >48 h | 1 | 1 |
| Reoperation | 1 | 1 |
| Length of stay | 5 | 5 |
Six definitions of mortality were used, including 30-, 60- and 90-day, operative and in-hospital mortality and any death after surgery.
Multiple definitions of morbidity (when morbidity was defined) were used depending on severity (major as opposed to minor, specific, serious, resulting in organ failure, graded 3–5 on a scale of 1–5) origin (medical, surgical, both or unspecified) and timing (30-, 60- or 90-day, operative, in-hospital and after surgery).
Liver failure was defined by the association of many parameters including serum bilirubin, serum lactate, international normalized ratio, prothrombin time, encephalopathy, ascites, pleural effusion, varix, and time and delay of occurrence.
Bile leak was defined by the association of many parameters including level of bilirubin and delay in occurrence.
Some studies analysed one or more endpoints of interest.
Table 2 also summarizes the definitions of these endpoints. Mortality was defined in 27 of 29 studies (93%), but six distinct definitions were used. Overall morbidity was defined (at least vaguely) in 33 of 43 studies (77%), but only 16 used a severity grading scale. Specific complications were usually defined, but the definitions were highly variable from one study to another. For example, liver failure was defined in 18 of the 21 studies that used this as an endpoint, but 11 different definitions were used. Similarly, of the five studies that used biliary leak as an endpoint, one did not define this complication and the other four employed different definitions. When stated, the timeframe for the occurrence of an endpoint was short: for example, only two studies and one study reported 90-day mortality and morbidity, respectively.
Predictors
In the 29 studies that used postoperative mortality as the outcome measure, a total of 43 distinct independent predictors were identified (Table 3). However, most overlapped and were related to patient age, comorbidities, operation type, and blood loss or intraoperative transfusion. These variables were not independently associated with mortality in all studies and, when they were, the associated ORs were highly variable.
Table 3.
Variables predictive of overall postoperative mortality in studies performing a risk prediction analysis in patients submitted to liver resection
| Variable | Studies including this variable in multivariable analysis, n | Studies showing this variable to be independent, n | Odds ratio Median (range) |
|---|---|---|---|
| Preoperative variables | |||
| Demographics | |||
| Sex | 22 | 3 | 1.61 (1.44–1.69) |
| Age | 27 | 12 | 1.80 (1.03–4.83) |
| Race | 1 | 1 | 2.15 |
| Insurance status | 3 | 2 | 2.35 (1.80–2.90) |
| Coexisting conditions | |||
| Number/presence of comorbidities | 10 | 2 | 1.28 (1.25–1.31) |
| Comorbidity | 2 | 2 | 2.47 (1.38–3.56) |
| Charlson score | 4 | 4 | 5.20 (1.50–7.17) |
| Chronic renal disease | 5 | 1 | 24.00 |
| Chronic pulmonary disease | 2 | 1 | 2.70 |
| Obesity | 1 | 1 | 1.83 |
| Admission (elective/not elective) | 8 | 2 | 3.40 (3.04–3.75) |
| Indication or stage | |||
| Biliary cancer | 1 | 1 | 3.02 |
| HCC on cirrhosis | 1 | 1 | 11.55 |
| Primary cancer | 21 | 3 | 1.81 (1.61–3.00) |
| Growth pattern of HCC | 1 | 1 | 2.22 |
| Liver function or biological tests | |||
| Galactose eliminating capacity | 1 | 1 | 1.23 |
| ICG of future remnant liver | 1 | 1 | 8.06 |
| Cirrhosis | 12 | 4 | 6.50 (3.28–54.09) |
| Underlying liver disease | 3 | 3 | 7.00 (2.00–7.20) |
| Child–Pugh grade | 4 | 1 | 2.42 |
| Thrombopoenia | 9 | 4 | 5.92 (0.99–12.50) |
| Hypoalbuminaemia | 6 | 2 | 3.22 (2.15–4.3) |
| Increased bilirubin | 9 | 2 | 1.20 (1.10–1.30) |
| Increased creatinine | 5 | 2 | 2.44 (1.01–3.86) |
| Study period | 1 | 1 | 4.58 |
| Hospital factors | |||
| Low hospital volume | 4 | 4 | 1.80 (1.66–3.10) |
| Non-teaching hospital | 3 | 3 | 1.43 (1.42–1.95) |
| Liver transplant unit | 1 | 1 | 1.70 |
| Intraoperative variables | |||
| Operation type | |||
| Extent of resection | 5 | 2 | 11.67 (6.43–16.90) |
| Major hepatectomy | 7 | 3 | 1.82 (1.57–2.26) |
| Percentage of volume resected | 1 | 1 | 1.04 |
| Number of segments resected | 4 | 2 | 1.44 (1.42–1.45) |
| One lobe or more resected | 6 | 5 | 3.90 (2.20–4.90) |
| Complex hepatectomy | 1 | 1 | 2.18 |
| Associated procedures | |||
| Associated procedure | 4 | 1 | 2.35 |
| Associated colon resection | 6 | 1 | 5.40 |
| Surgical factors | |||
| Blood transfusion | 14 | 6 | 3.36 (2.05–10.22) |
| Blood loss | 9 | 4 | 1.55 (1.18–1.89) |
| Postoperative variables | |||
| Liver function | |||
| Increased INR | 2 | 1 | 3.68 |
| Increased bilirubin | 2 | 1 | 10.80 |
| 50/50 day 5 | 2 | 2 | 122.50 (29.40–215.60) |
| 50/50 day 3 | 1 | 1 | 12.70 |
| Sepsis | 1 | 1 | 37.58 |
HCC, hepatocellular carcinoma; ICG, indocyanine green clearance test; INR, international normalized ratio.
50/50 defined by the association of a prothrombin time of <50% and serum bilirubin of >50 μmol/l on postoperative day 5.
From the 43 studies that used overall postoperative morbidity as the outcome measure, 51 distinct independent predictors were identified (Table 4). As with predictors of mortality, most overlapped and were related to patient age, comorbidities, operation type, and blood loss or intraoperative transfusion. Results were inconsistent in terms of independent associations with morbidity and ORs.
Table 4.
Variables predictive of overall postoperative morbidity in studies performing a risk prediction analysis in patients submitted to liver resection
| Variable | Studies including the variable in multivariable analysis, n | Studies showing this variable to be independent, n | Odds ratio Median (range) |
|---|---|---|---|
| Preoperative variables | |||
| Demographics | |||
| Sex | 22 | 2 | 1.72 (1.38–2.06) |
| Age | 23 | 5 | 1.02 (1.02–1.66) |
| Coexisting conditions | |||
| ASA score | 4 | 2 | 5.15 (1.53–8.77) |
| Number/presence of comorbidities | 5 | 3 | 1.65 (1.40–3.95) |
| Obesity | 3 | 1 | 1.24 |
| Cardiovascular disease | 2 | 1 | 1.25 |
| Previous cardiac operation | 1 | 1 | 2.82 |
| Severe chronic obstruction pulmonary disease | 2 | 1 | 2.41 |
| Indication or stage | |||
| HCC on cirrhosis | 1 | 1 | 2.30 |
| Malignancy | 14 | 2 | 5.90 (3.33–8.47) |
| Liver function or biological tests | |||
| Galactose elimination capacity | 1 | 1 | 2.05 |
| Underlying liver disease | 3 | 1 | 1.60 |
| Cirrhosis | 6 | 2 | 3.43 (3.37–3.49) |
| Steatosis | 4 | 2 | 1.36 (1.24–1.47) |
| Child–Pugh grade B/C | 9 | 1 | 5.00 |
| Portal hypertension | 1 | 1 | 2.02 |
| Increased ICG-R15 value | 11 | 1 | 6.03 |
| Thrombopoenia | 5 | 4 | 2.64 (1.67–4.65) |
| Hypoalbuminaemia | 12 | 5 | 1.58 (1.15–3.72) |
| Increased creatinine | 2 | 1 | 1.78 |
| Increased GGT | 3 | 1 | 8.59 |
| Increased liver enzymes | 8 | 2 | 1.81 (1.63–1.99) |
| Decreased prothrombin time | 10 | 1 | 7.50 |
| Increased hepatocyte growth factor | 1 | 1 | 12.56 |
| Increased hyaluronic acid level | 1 | 1 | 1.80 |
| Study period | 3 | 1 | 2.45 |
| Previous medical history | |||
| Chemotherapy | 5 | 1 | 2.40 |
| Cholangitis | 1 | 1 | 9.08 |
| Previous hepatic resection | 1 | 1 | 3.84 |
| Preoperative nutrition | |||
| Sarcopoenia | 1 | 1 | 3.12 |
| Intraoperative variables | |||
| Operation type | |||
| Extent of resection | 2 | 2 | 1.37 (1.17–1.56) |
| Major hepatectomy | 15 | 4 | 3.20 (1.43–5.88) |
| Number of segments resected | 4 | 4 | 1.51 (1.20–10.94) |
| One lobe or more resected | 2 | 2 | 2.16 |
| Resected liver volume | 1 | 1 | 7.00 |
| Right/extended right hepatectomy | 2 | 1 | 2.78 |
| Repeat hepatectomy | 3 | 1 | 2.42 |
| Palliative resection | 1 | 1 | 2.57 |
| Associated procedures | |||
| Associated procedure | 4 | 1 | 2.14 |
| Associated biliary procedures | 2 | 1 | 2.84 |
| Associated vascular resection | 2 | 1 | 2.10 |
| Synchronous bowel procedures | 1 | 1 | 16.11 |
| Extrahepatic procedures | 2 | 1 | 3.45 |
| Surgical factors | |||
| Blood loss | 18 | 14 | 1.83 (1.22–17.10) |
| Blood transfusion | 16 | 13 | 2.52 (1.2–10.78) |
| Length of surgery | 13 | 5 | 1.85 (1.01–5.22) |
| Pringle manoeuvre | 10 | 4 | 2.28 (1.40–2.90) |
| Abdominal drainage | 4 | 1 | 4.44 |
| Thoracoabdominal incision | 2 | 1 | 1.96 |
| Postoperative variables | |||
| Increased bilirubin | 2 | 1 | 83.30 |
| Decreased cholinesterase B | 1 | 1 | 2.20 |
ASA, American Society of Anesthesiologists; GGT, gamma-glutamyl transferase; HCC, hepatocellular carcinoma; ICG-R15, indocyanine green retention rate at 15 min.
For both mortality and morbidity, variables relating to nutritional status or intraoperative events were hardly investigated, or not at all.
Independent predictors of 25 distinct endpoints are summarized in Table S4 (online).
Model presentation
Although ORs (rather than regression coefficients) for the predictors were reported, none of the studies provided the intercept of the regression equation and thus readers are unable to calculate risk predictions for their own patients. Six of the 91 studies (7%), originating from three groups, presented their prognostic model as a simplified prediction score by transforming the regression coefficient into points.23–28
Model development
Nine studies (10%) assessed the predictive performance of their models in terms of calibration (agreement between observed and predictive endpoints) or discrimination (between patients with or without the outcome).24–32 Seven (8%) of the 91 studies carried out some form of validation of a risk prediction model, including six with internal validation23–28 and one with temporal validation.33 In the latter study, however, patient selection differed somewhat from that in the development sample. A summary of these models is provided in Table S5 (online).
There was no impact study quantifying the effect of using a prognostic model on doctor behaviour, patient outcome or the cost-effectiveness of care in comparison with not using such a model.
Quality assessment
The median QUIPS score for the included studies was 34 (range: 24–41) of a maximum score of 50 (Table 5). The highest risks for bias were observed for the domains of confounding factor measurement and study participation.
Table 5.
Assessment of quality score of studies performing a risk prediction analysis in patients submitted to liver resection
| Quality score Median (range) | Maximum score | |
|---|---|---|
| Study participation | 6 (2–9) | 10 |
| Population described for key characteristics | 1 | 2 |
| Sampling and recruitment described | 2 | 2 |
| Inclusion/exclusion criteria described | 1 | 2 |
| Adequate participation | 2 | 2 |
| Baseline study sample described | 1 | 2 |
| Prognostic factor measurement | 9 (4–10) | 12 |
| Prognostic factors are clearly described or defined | 2 | 2 |
| Continuous variables reported, or appropriate cut-off points used | 1 | 2 |
| Prognostic factors are valid and reliable | 2 | 2 |
| Study sample has complete data for prognostic factors | 2 | 2 |
| Same method and setting of measurement for all study participants | 2 | 2 |
| Appropriate methods used for missing prognostic data | 0 | 2 |
| Outcome measurement | 4 (0–6) | 6 |
| Definition of the outcome described | 2 | 2 |
| Outcome measure and method are valid and reliable | 1 | 2 |
| Same method and setting of measurement for all study participants | 1 | 2 |
| Confounding factor measurement | 8 (4–10) | 14 |
| Important confounders are measured | 1 | 2 |
| Clear definitions of confounders described | 2 | 2 |
| Measurement of all confounders is valid and reliable | 1 | 2 |
| Same method and setting of measurement for all study participants | 2 | 2 |
| Appropriate methods used for missing confounder data | 0 | 2 |
| Important confounders accounted for in the study design | 1 | 2 |
| Important confounders accounted for in the analysis | 1 | 2 |
| Analysis | 7 (4–8) | 8 |
| Sufficient presentation of data to assess the adequacy of the analysis | 1 | 2 |
| Strategy for model building appropriate and based on a conceptual model | 2 | 2 |
| Adequate selection of model for the design of the study | 2 | 2 |
| No selective reporting of results | 2 | 2 |
| Median quality score of studies | 34 (24–41) | 50 |
Discussion
Statement of principal findings
Liver resection is a high-risk treatment.3 Risk is a subjective concept used to determine the nature, likelihood and acceptability of the harm associated with a choice or an intervention.34 The aim of this systematic review was to amalgamate the literature on how the risk associated with liver resection has been perceived and evaluated. A large number of studies were identified, but their quality and transferability were globally poor because of a number of methodological shortcomings.
Strengths and limitations of the review
There is increasing interest in the reporting of early outcomes following liver resection.35 Recent systematic reviews have summarized current rates of mortality, total morbidity and specific complications,36 or surrogate endpoints that could be used to substitute for a clinically meaningful endpoint.37 However, to the best of the present authors' knowledge, this is the first study to review the full range of endpoints analysed, their predictors, and the quality of published series.
This study has inherent limitations. Firstly, some articles may have been missed, although the search strategy used has been validated.19 Secondly, the criteria used to assess the quality of studies are semi-quantitative and, in the present study, are occasionally subject to interpretation as these were seldom clearly addressed in the Methods sections of the included articles. Thirdly, standardized definitions of liver failure, biliary leak and haemorrhage after liver resection were published in 2011;38–40 it is therefore possible that, over the past 2 years, definitions of at least some endpoints have improved. However, these definitions have either not yet been validated or already appear controversial.41
Limitations of studies included in the review
Study population
Data used to develop a prognostic model should ideally come from a prospective cohort that has been designed to resolve a specific research question.42 In this review, although data were generally described as having been collected prospectively, only 12% of studies were designed prospectively, only two appeared to have been registered, and none described precisely the full range of variables recorded. Most were retrospective studies, in which the control that investigators have over data collection, which may be incomplete, inaccurate or inconsistently measured, is inherently limited. The accrual period was longer than 10 years or undefined in 40% of the studies. This is of concern as the study period was independently correlated with outcome in several studies,43–45 which suggests the influence of unidentified confounders, such as improved patient selection, instrumentation or management.
Starting point
Submission to liver resection was the starting point of all studies. This excludes from prognostic analysis patients who were considered unfit for surgery or those in whom surgery was aborted. The reasons for this selection (bias) were not addressed and are at least partly subjective, rather than merely objective.46 Population-based or multicentre-based studies may, to some extent, overcome this limitation, but these were rare.
Endpoints and predictors
Endpoints frequently had imprecise definitions, which were inconsistent among studies; for example, mortality was defined as operative, in-hospital, or as 30-, 60- or 90-day mortality. This is a concern as in-hospital or 30-day mortality captures only approximately half the events obtained using 90-day mortality.16,47 There was similarly a lack of standardized definition of predictors. For example, the extent of surgery was expressed in six different ways, which may indicate that the classical definition of a major hepatectomy (resection of three or more segments) is not considered appropriate.48 Similarly, definitions of comorbidities were quite general and subjective, rather than relying on tests of function or physiological reserve. The handling of continuous variables was also of concern. It was generally assumed that they were linearly correlated with the outcome. This was, however, not checked, and continuous variables frequently require modelling.49 When transformed into categorical variables, the threshold value (defining the groups) was frequently chosen arbitrarily and not defined according to best sensitivity and specificity.
Model development
A number of studies were excluded from analysis because they provided only P-values and not regression coefficients (or ORs) linking predictors to outcome. Assessment of the performance of models was infrequent and, as none of the models underwent external validation, their generalizability is unknown.50 No study investigated the impact of a prognostic model on clinical practice or outcome.
Interpretation with reference to other studies
Endpoints
The outcome measures in the included studies essentially referred to postoperative overall morbidity, overall mortality and liver failure. Postoperative mortality is of particular relevance at a time when less invasive treatment strategies are becoming increasingly effective. In terms of risk prediction, however, reported mortality rates were low in the specialized centres that contributed most to this review (i.e. the number of events for statistical analysis was limited), were captured inconsistently (i.e. they were usually assessed as in-hospital or 30-day events),15 and causes of death were not detailed (postoperative death is multifactorial and reflects adverse events, inappropriate indications or failure to cure). This may explain why, except for patients with severe underlying cirrhosis, high-risk groups are still poorly defined, although they do exist.51
The outcome of postoperative morbidity is also potentially relevant as it may result in sequelae or increased expenditure,52 or may adversely impact on survival53,54 or QoL.55 However, morbidity was assessed mainly in terms of overall occurrence, whereas the spectrum of adverse events is wide56 and does not necessarily correlate with common predictors. There was also considerable interest in postoperative liver failure, although its definition was not standardized and it has become extremely rare.57 Recently, it has been suggested that the severity of complications should be graded objectively,58 that composite endpoints might be used,36 and that complications should be reported more comprehensively,59 which was infrequently the case. The performance of root cause analysis of adverse events, as initiated recently in pancreatic surgery,60 as well as a focus on the direct and indirect consequences of complications rather than on complications themselves, are also required.
By contrast with postoperative mortality and morbidity, a number of other clinically relevant endpoints were not or were only seldom addressed. These included, in particular, LoS, success of the operation, ability to receive adjuvant treatment in due course, ability to return to work, and QoL. Provided it is assessed in conjunction with hospital readmission, which is a major concern,61 LoS is highly relevant in the current context of laparoscopic resections, enhanced recovery after surgery (ERAS) protocols62 and, more broadly, day case surgery, for which there is increasing administrative demand.63 Quality of life is coming to represent the main endpoint in some medical or low-risk surgical trials, and there is little reason why high-risk surgery should escape this trend.
These results suggest that the investigators in these studies, as surgeons, focused on technique and perioperative misadventure, but failed, as doctors, to take account of patients' or families' expectations, or of the environment in which they work as surgeons, which includes other specialist physicians (such as anaesthetists and oncologists) and administrators.64 The ensuing risks are those of surgeon isolation, which inevitably results in the performance of a ‘more comprehensive’, ‘objective’ assessment of risk by third parties.
Predictors
Most of the variables tested for their possible association with endpoints focused on readily available preoperative and intraoperative data, particularly comorbid conditions and extent of liver resection. As currently characterized, coexisting conditions failed to predict outcome reliably. This was particularly the case for the American Society of Anesthesiologists (ASA) grade, which underlines the subjectivity of this score.65 The present study also failed to provide evidence that indocyanine green (ICG) clearance predicts outcome and thus does not elucidate why this test is used routinely in Eastern countries and sparingly in the West.66,67
There was a surprising deficit in the evaluation of some preoperative or intraoperative variables. Preoperative nutritional status, for example, was rarely evaluated, although it has been linked consistently to an increase in postoperative complications.68 Intraoperative variables other than extent of surgery (with the limitations addressed above) were restricted to blood loss and its prevention by vascular clamping, whereas the patient's haemodynamic profile, body temperature, receipt of pharmaceutical agents and other anaesthetic-related parameters were largely overlooked.
The high number of predictors identified in this review, most of which overlap, also suggests that the investigators' policy was to design new models, rather than using their data to test or modify existing models; this strategy is not recommended.42
Recommendations
This review shows that prognostic model research in the context of liver resection is still at the developmental stage,69 which does not allow for reliable patient counselling, the design and assessment of surgical innovation, the development of trials or decision models, or the comparison of performance between centres or alternative treatment options.70 This is of concern at a time of emphasis on the use of surgical decision aids17 and evaluation of innovations,66 of evolving vision of primary care and research,71 and of increasing mandatory public reporting of hospital and surgeon outcomes.72
Steps for improving the quality of prognostic research have been updated.21,73 In the context of liver surgery, these involve, in particular, performing prospectively designed and registered studies, using standardized endpoints (either alone or in combination)36 and assessing their severity59 and predictors in a clearly defined, multicentre population, and encouraging the performance of validation and impact studies.
The goal of liver resection has evolved over time. Initially, the aim was for the patient to survive the procedure. With the development of living related-donor liver graft harvesting, the goal has been to minimize complications. Today's ambition for liver surgery should probably be to rephrase its objectives, taking into account patient-reported outcomes74 and the fact that surgery is increasingly part of wider, multidisciplinary management.
Acknowledgments
This study was funded by a Programme Hospitalier de Recherche Clinique grant (PHRC National 2011, AOM 11060) from the French Ministry of Health awarded to OF, and by the Association de Chirurgie Hépato-Biliaire et de Transplantation Hépatique (ACHBT).
Contributors
René Adam (Department of Surgery, Hôpital Paul Brousse, Villejuif, France), Mustapha Adham (Department of Surgery, Hôpital Edouard Herriot, Lyon, France), Beatrice Aussilhou (Department of Surgery, Hôpital Beaujon, Clichy, France; Philippe Bachellier, Department of Surgery, Hôpitaux Universitaires de Strasbourg, France), Louise Barbier (Department of Surgery, Hôpital Beaujon, Clichy, France), Emmanuel Boleslawski (Department of Surgery, Hôpital Hurroez, Lille, France), Denis Castaing (Department of Surgery, Hôpital Paul Brousse, Villejuif, France), Daniel Cherqui (Department of Surgery, Hôpital Paul Brousse, Villejuif, France), Safi Dokmak (Department of Surgery, Hôpital Beaujon, Clichy, France), Federica Dondero (Department of Surgery, Hôpital Beaujon, Clichy, France), Christian Ducerf (Department of Surgery, Hôpital Croix Rousse, Lyon, France), Emilie Gregoire (Department of Surgery, Hôpital Conception, Marseille, France), Jean Hardwigsen (Department of Surgery, Hôpital Conception, Marseille, France), Christophe Laurent (Department of Surgery, Hôpital Saint André, Bordeaux, France), Yves-Patrice Le Treut (Department of Surgery, Hôpital Conception, Marseille, France), Jean-Yves Mabrut (Department of Surgery, Hôpital Croix Rousse, Lyon, France), Gheorghe Petrovai (Department of Surgery, Hôpital Hurriez, Lille, France), François René Pruvot (Department of Surgery, Hôpital Hurriez, Lille, France), Jean-Marc Regimbeau (Department of Surgery, Hôpital Nord, Amiens, France), Antonio Sa Cunha (Department of Surgery, Hôpital Paul Brousse, Villejuif, France), Olivier Scatton (Department of Surgery, Hôpital Pitié Salpétrière, Paris, France), Olivier Soubrane (Department of Surgery, Hôpital Beaujon, Clichy, France), Stéphanie Truant (Department of Surgery, Hôpital Hurriez, Lille, France) and Eric Vibert (Department of Surgery, Hôpital Paul Brousse, Villejuif, France).
Conflicts of interest
None declared.
Supporting Information
Table S1. Search strategy.
Table S2. Methodological quality (based on reference22).
Table S3. Baseline characteristics of included studies.
Table S4. Summary of variables found to be independently predictive of an early outcome following liver resection.
Table S5. Summary of studies that validated a prognostic model.
Appendix S1. References of the 91 studies retained for analysis.
References
- Agrawal S, Belghiti J. Oncologic resection for malignant tumours of the liver. Ann Surg. 2011;253:656–665. doi: 10.1097/SLA.0b013e3181fc08ca. [DOI] [PubMed] [Google Scholar]
- Dindo D, Muller MK, Weber M, Clavien PA. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- Mulligan DC. Living donor safety during the performance of hepatectomy. Liver Transpl. 2012;18:1134–1135. doi: 10.1002/lt.23514. [DOI] [PubMed] [Google Scholar]
- Farges O, Vibert E, Cosse C, Pruvot FR, Le Treut YP, Scatton O, et al. Surgeons' intuition versus prognostic models: predicting the risk of liver resections. Ann Surg. 2014;260:923–930. doi: 10.1097/SLA.0000000000000961. [DOI] [PubMed] [Google Scholar]
- Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- Dokmak S, Fteriche FS, Borscheid R, Cauchy F, Farges O, Belghiti J. 2012 liver resections in the 21st century: we are far from zero mortality. HPB. 2013;15:908–915. doi: 10.1111/hpb.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl RJ, You X, Ghali WA, Kaplan G, Myers R, Dixon E. Recent trends of hepatic resection in Canada: 1995–2004. J Gastrointest Surg. 2008;12:1839–1846. doi: 10.1007/s11605-008-0679-4. [DOI] [PubMed] [Google Scholar]
- Shah BC, Ullrich F, Smith L, Leiphrakpam P, Ly Q, Sasson A, et al. National trends in discharge disposition after hepatic resection for malignancy. HPB. 2011;13:96–102. doi: 10.1111/j.1477-2574.2010.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicherts DA, de Haas RJ, Andreani P, Sotirov D, Salloum C, Castaing D, et al. Impact of portal vein embolization on longterm survival of patients with primarily unresectable colorectal liver metastases. Br J Surg. 2010;97:240–250. doi: 10.1002/bjs.6756. [DOI] [PubMed] [Google Scholar]
- Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicherts DA, Miller R, de Haas RJ, Bitsakou G, Vibert E, Veilhan LA, et al. Longterm results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling two-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomized phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- Dimick JB, Wainess RM, Cowan JA, Upchurch GR, Jr, Knol JA, Colletti LM. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199:31–38. doi: 10.1016/j.jamcollsurg.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Farges O, Goutte N, Bendersky N, Falissard B. Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg. 2012;256:697–704. doi: 10.1097/SLA.0b013e31827241d5. [DOI] [PubMed] [Google Scholar]
- Knops AM, Legemate DA, Goossens A, Bossuyt PM, Ubbink DT. Decision aids for patients facing a surgical treatment decision: a systematic review and meta-analysis. Ann Surg. 2013;257:860–866. doi: 10.1097/SLA.0b013e3182864fd6. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingui BJ, Rogers MA. Searching for clinical prediction rules in MEDLINE. J Am Med Inform Assoc. 2001;8:391–397. doi: 10.1136/jamia.2001.0080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Riley RD, Altman DG. Ten steps towards improving prognosis research. BMJ. 2009;339:b4184. doi: 10.1136/bmj.b4184. [DOI] [PubMed] [Google Scholar]
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- Andres A, Toso C, Moldovan B, Schiffer E, Rubbia-Brandt L, Terraz S, et al. Complications of elective liver resections in a centre with low mortality: a simple score to predict morbidity. Arch Surg. 2011;146:1246–1252. doi: 10.1001/archsurg.2011.175. [DOI] [PubMed] [Google Scholar]
- Breitenstein S, DeOliveira ML, Raptis DA, Slankamenac K, Kambakamba P, Nerl J, et al. Novel and simple preoperative score predicting complications after liver resection in non-cirrhotic patients. Ann Surg. 2010;252:726–734. doi: 10.1097/SLA.0b013e3181fb8c1a. [DOI] [PubMed] [Google Scholar]
- Simons JP, Hill JS, Ng SC, Shah SA, Zhou Z, Whalen GF, et al. Perioperative mortality for management of hepatic neoplasm: a simple risk score. Ann Surg. 2009;250:929–934. doi: 10.1097/SLA.0b013e3181bc9c2f. [DOI] [PubMed] [Google Scholar]
- Simons JP, Ng SC, Hill JS, Shah SA, Bodnari A, Zhou Z, et al. In-hospital mortality for liver resection for metastases: a simple risk score. J Surg Res. 2009;156:21–25. doi: 10.1016/j.jss.2009.03.073. [DOI] [PubMed] [Google Scholar]
- Simons JP, Ng SC, Hill JS, Shah SA, Zhou Z, Tseng JF. In-hospital mortality from liver resection for hepatocellular carcinoma: a simple risk score. Cancer. 2010;116:1733–1738. doi: 10.1002/cncr.24904. [DOI] [PubMed] [Google Scholar]
- Slankamenac K, Breitenstein S, Held U, Slankamenac K, Kambakamba P, Nerl J, et al. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg. 2009;250:720–728. doi: 10.1097/SLA.0b013e3181bdd840. [DOI] [PubMed] [Google Scholar]
- Bolder U, Brune A, Schmidt S, Tacke J, Jauch KW, Löhlein D. Preoperative assessment of mortality risk in hepatic resection by clinical variables: a multivariate analysis. Liver Transpl Surg. 1999;5:227–237. doi: 10.1002/lt.500050302. [DOI] [PubMed] [Google Scholar]
- Dimick JB, Cowan JA, Jr, Knol JA, Upchurch GR., Jr Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg. 2003;138:185–191. doi: 10.1001/archsurg.138.2.185. [DOI] [PubMed] [Google Scholar]
- Dimick JB, Pronovost PJ, Cowan JA, Jr, Lipsett PA. Postoperative complication rates after hepatic resection in Maryland hospitals. Arch Surg. 2003;138:41–46. [PubMed] [Google Scholar]
- Nguyen GC, Thuluvath NP, Segev DL, Thuluvath PJ. Volumes of liver transplant and partial hepatectomy procedures are independently associated with lower postoperative mortality following resection for hepatocellular carcinoma. Liver Transpl. 2009;15:776–781. doi: 10.1002/lt.21767. [DOI] [PubMed] [Google Scholar]
- Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, et al. Prospective validation of the ‘fifty-fifty’ criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124–128. doi: 10.1097/SLA.0b013e31819279cd. [DOI] [PubMed] [Google Scholar]
- Wilson R, Crouch EA. Risk assessment and comparisons: an introduction. Science. 1987;236:267–270. doi: 10.1126/science.3563505. [DOI] [PubMed] [Google Scholar]
- Leppaniemi A, Clavien PA. Reporting complications and outcome: are we there yet? Scand J Surg. 2013;102:219–220. doi: 10.1177/1457496913511019. [DOI] [PubMed] [Google Scholar]
- van den Broek MA, van Dam RM, Malago M, Dejong CH, van Brekelen GJ, Olde Damink SW. Feasibility of randomized controlled trials in liver surgery using surgery-related mortality or morbidity as endpoint. Br J Surg. 2009;96:1005–1014. doi: 10.1002/bjs.6663. [DOI] [PubMed] [Google Scholar]
- Mpabanzi L, van Mierlo KM, Malago M, Dejong CH, Lytras D, Olde Damink SW. Surrogate endpoints in liver surgery-related trials: a systematic review of the literature. HPB. 2013;15:327–336. doi: 10.1111/j.1477-2574.2012.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Post-hepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB. 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypczyk C, Truant S, Salleron J, Langlois C, Boleslawski E, Koriche D, et al. Relevance of the ISGLS definition of post-hepatectomy liver failure in early prediction of poor outcome after liver resection: a study on 680 hepatectomies. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000944. [Epub ahead of print; PMID: 25243550] [DOI] [PubMed] [Google Scholar]
- Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606. [DOI] [PubMed] [Google Scholar]
- de Haas RJ, Wicherts DA, Andreani P, Pascal G, Saliba F, Ichai P, et al. Impact of expanding criteria for resectability of colorectal metastases on short- and longterm outcomes after hepatic resection. Ann Surg. 2011;253:1069–1079. doi: 10.1097/SLA.0b013e318217e898. [DOI] [PubMed] [Google Scholar]
- Guckelberger O, Thelen A, Benckert C, Schoebel C, Reuter S, Klupp J, et al. Diabetes mellitus is no independent risk factor for perioperative mortality following hepatic resection. Exp Clin Endocrinol Diabetes. 2006;114:257–261. doi: 10.1055/s-2006-924234. [DOI] [PubMed] [Google Scholar]
- Taketomi A, Kitagawa D, Itoh S, Harimoto N, Yamashita Y, Gion T, et al. Trends in morbidity and mortality after hepatic resection for hepatocellular carcinoma: an institute's experience with 625 patients. J Am Coll Surg. 2007;204:580–587. doi: 10.1016/j.jamcollsurg.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Orri M, Farges O, Clavien PA, Barkun J, Revah-Levy A. Being a surgeon – the myth and the reality: a meta-synthesis of surgeons' perspectives about factors affecting their practice and well-being. Ann Surg. 2014;260:721–729. doi: 10.1097/SLA.0000000000000962. [DOI] [PubMed] [Google Scholar]
- Mayo SC, Shore AD, Nathan H, Edil BH, Hirose K, Anders RA, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB. 2011;13:473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JW, et al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB. 2011;13:494–502. doi: 10.1111/j.1477-2574.2011.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- Cauchy F, Zalinski S, Dokmak S, Fuks D, Farges O, Castera L, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100:113–121. doi: 10.1002/bjs.8963. [DOI] [PubMed] [Google Scholar]
- Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser D, et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg. 2011;254:907–913. doi: 10.1097/SLA.0b013e31821d4a43. [DOI] [PubMed] [Google Scholar]
- Chok KS, Ng KK, Poon RT, Lo CM, Fan ST. Impact of postoperative complications on longterm outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96:81–87. doi: 10.1002/bjs.6358. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kumamoto T, Nojiri K, Matsuyama R, Takeda K, Endo I. Impact of postoperative morbidity on longterm survival after resection for colorectal liver metastases. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1352-1. [Epub ahead of print; doi: 10.1245/s10434-010-1352-1 ] [DOI] [PubMed] [Google Scholar]
- Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on longterm quality of life after curative colorectal cancer surgery. Ann Surg. 2014;259:916–923. doi: 10.1097/SLA.0000000000000407. [DOI] [PubMed] [Google Scholar]
- Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: a systematic review. Liver Transpl. 2002;8:110–117. doi: 10.1053/jlts.2002.31315. [DOI] [PubMed] [Google Scholar]
- Boleslawski E, Vibert E, Pruvot FR, Le Traut YP, Scatton O, Laurent C, et al. Relevance of postoperative peak transaminase after elective hepatectomy. Ann Surg. 2014;260:815–821. doi: 10.1097/SLA.0000000000000942. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1–7. doi: 10.1097/SLA.0b013e318296c732. [DOI] [PubMed] [Google Scholar]
- Vollmer CM, Jr, Sanchez N, Gondek S, McAuliffe J, Kent TS, Christein JD, et al. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg. 2011;16:89–102. doi: 10.1007/s11605-011-1753-x. discussion 102–103. [DOI] [PubMed] [Google Scholar]
- Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369:1134–1142. doi: 10.1056/NEJMsa1303118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Lun-Hing EM, van Dam RM, Heijnen LA, Busch OR, Terkivatan T, van Hillegersberg R, et al. Is current perioperative practice in hepatic surgery based on enhanced recovery after surgery (ERAS) principles? World J Surg. 2014;38:1127–1140. doi: 10.1007/s00268-013-2398-6. [DOI] [PubMed] [Google Scholar]
- Schultz NA, Larsen PN, Klarskov B, Plum LM, Frederiksen HJ, Christensen BM, et al. Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg. 2013;100:138–143. doi: 10.1002/bjs.8996. [DOI] [PubMed] [Google Scholar]
- Boyd O, Jackson N. How is risk defined in high-risk surgical patient management? Crit Care. 2005;9:390–396. doi: 10.1186/cc3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Nishio H, Ebata T, Igami T, Sugawara G, Nagino M. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br J Surg. 2010;97:1260–1268. doi: 10.1002/bjs.7084. [DOI] [PubMed] [Google Scholar]
- Burden S, Todd C, Hill J, Lal S. Preoperative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. 2012;(11) doi: 10.1002/14651858.CD008879.pub2. CD008879. [DOI] [PubMed] [Google Scholar]
- Steyerberg EW, Moons KG, van der Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10:e1001381. doi: 10.1371/journal.pmed.1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RD, Hayden JA, Steyerberg EW, Moons KG, Abrams K, Kyzas PA, et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med. 2013;10:e1001380. doi: 10.1371/journal.pmed.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, Normand SL. Getting the methods right – the foundation of patient-centred outcomes research. N Engl J Med. 2012;367:787–790. doi: 10.1056/NEJMp1207437. [DOI] [PubMed] [Google Scholar]
- Walker K, Neuburger J, Groene O, Cromwell DA, van der Meulen J. Public reporting of surgeon outcomes: low numbers of procedures lead to false complacency. Lancet. 2013;382:1674–1677. doi: 10.1016/S0140-6736(13)61491-9. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Croft P, Perel P, Hayden JA, Abrams K, Timmis A, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ. 2013;346:e5595. doi: 10.1136/bmj.e5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazeby JM, Fayers P, Conroy T, Sezer O, Ramage J, Rees M. Validation of the European Organization for Research and Treatment of Cancer QLQ-LMC21 questionnaire for assessment of patient-reported outcomes during treatment of colorectal liver metastases. Br J Surg. 2009;96:291–298. doi: 10.1002/bjs.6471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategy.
Table S2. Methodological quality (based on reference22).
Table S3. Baseline characteristics of included studies.
Table S4. Summary of variables found to be independently predictive of an early outcome following liver resection.
Table S5. Summary of studies that validated a prognostic model.
Appendix S1. References of the 91 studies retained for analysis.


