Abstract
Objectives
This retrospective review was conducted to compare the efficacy of radiofrequency ablation (RFA) with that of transarterial chemoembolization (TACE) in treating large (5–8 cm) unresectable solitary hepatocellular carcinomas (HCCs).
Methods
Patients with large unresectable solitary HCCs primarily treated by RFA or TACE were reviewed. The primary endpoint was overall survival. Secondary endpoints were tumour response, time to disease progression, and treatment-related morbidity and mortality.
Results
There were 15 patients in the RFA group. Of these, 12 achieved complete ablation, one had ablation site recurrence, and five developed complications. Median disease-free survival in this group was 13.0 months (range: 2.8–38.0 months). The TACE group included 26 patients, of whom four obtained a partial response, none achieved a complete response, and five developed complications. The median time to disease progression in this group was 8.0 months (range: 1.0–68.0 months). There were no hospital deaths in this series. Median survival was 39.8 months in the RFA group and 19.8 months in the TACE group (P = 0.257). Rates of 1-, 2- and 5-year survival were 93.3%, 86.2% and 20.9%, respectively, in the RFA group and 73.1%, 40.6% and 18.3%, respectively, in the TACE group.
Conclusions
Both RFA and TACE are feasible treatments for large unresectable solitary HCCs. Both modes show comparable rates of complications and longterm survival, but RFA achieves better initial tumour control and results in better short-term survival.
Introduction
Rates of resectability in hepatocellular carcinoma (HCC) patients referred to surgical units are limited to only 20–37% because of various factors, such as multifocal or bilobar disease and poor liver reserve resulting from underlying cirrhosis.1,2 Percutaneous radiofrequency ablation (RFA) is an effective curative treatment for solitary HCCs of ≤5 cm in diameter. In a randomized controlled trial (RCT), this treatment modality achieved overall and disease-free survival rates similar to those obtained by hepatic resection.3 In other studies, it achieved complete necrosis in 76–100% of treated small HCCs.4–6
However, the management of unresectable solitary HCCs of >5 cm in size remains controversial. Although liver transplantation offers cure for such tumours,7,8 the majority of patients cannot benefit from it because of the shortage of liver grafts, and thus transarterial chemoembolization (TACE) remains their mainstay of treatment. The role of RFA in treating HCCs of >5 cm is more controversial. Complete ablation rates of <50%, which are far from satisfactory, have been reported.9,10 The present group has previously reported a complete ablation rate of 83% using a technique that allows for multiple overlapping ablations to be made from different angles under intraoperative ultrasound guidance.11
This study was conducted to compare the outcomes of treatment by RFA and TACE, respectively, of unresectable solitary HCCs measuring 5–8 cm because no such comparison could be found in the literature published to date.
Materials and methods
The Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster permits retrospective studies using data prospectively collected in databases.
Patients
From 2003 to 2010, 2750 patients presented to the Queen Mary Hospital with HCC. Clinical data for all patients were collected prospectively. A retrospective study that included all patients with unresectable solitary HCCs sized 5–8 cm treated by RFA (the RFA group) or TACE (the TACE group) as primary treatment was performed. In these patients, HCCs were defined as unresectable as a result of contraindications including significant comorbidities, the close proximity of the tumour to major vasculatures, poor liver reserve as indicated by an indocyanine green clearance test, and Child–Pugh status that precluded major resection.9,10,12 Radiofrequency ablation was offered if the tumour location was considered accessible by interventional radiologists and surgeons.
Assessment of therapeutic efficacy
The primary endpoint was overall survival. Secondary endpoints were tumour response, time to disease progression, disease-free survival, and treatment-related morbidity and mortality. Time to disease progression was calculated from the date of the first treatment to the date of disease progression. Disease-free survival was calculated as the time from the first treatment to tumour recurrence, and was censored at the time of last follow-up or death if at that time there was no evidence of tumour recurrence. Clinical data and treatment outcomes were compared between the RFA and TACE groups.
Response to RFA was assessed by contrast helical computed tomography (CT) at 1 month after treatment. Complete ablation was defined as the absence of any enhancement in the arterial phase at the ablation site. Response to TACE was assessed by CT or magnetic resonance imaging according to the Response Evaluation Criteria in Solid Tumors (Version 1.1) at 1 month after the procedure.
A complication was defined as any adverse event after treatment, excluding pain or a transient febrile response. Treatment mortality was defined as death occurring within 30 days of treatment. All patients with complete ablation after RFA were monitored for intrahepatic recurrence or distant metastasis according to serum alpha-fetoprotein level, chest radiography and CT every 3 months. Ablation site recurrence was defined as the growth of a contrast-enhancing lesion within or directly adjacent to the ablation zone if the largest diameter of the lesion was in direct contact with the ablation zone.13 Distant intrahepatic recurrence was defined as the occurrence of a new tumour in the liver in an area distinct from the ablated area. Extrahepatic metastasis referred to any recurrence outside the liver. Intrahepatic recurrences were treated with RFA or TACE as deemed appropriate according to tumour size, number and position, as well as liver function at the time of recurrence. Patients with extrahepatic metastasis were treated with systemic therapies (chemotherapy or sorafenib) or supportive care, depending on their performance status and liver function.
Ablation procedure
The Cool-tip Radiofrequency System (Covidien, Inc., Mansfield, MA, USA) was used for RFA. Cluster electrodes consisting of three parallel electrodes were used for tumours of >5 cm. Ablation was performed using an impedance control mode in which current output was automatically adjusted according to the impedance at the needle tip. Temperature at the needle tip was monitored. The needle tip was continuously infused with cold saline through a channel inside the needle during the procedure in order to keep the temperature of the device at <20 °C and thus to prevent charring around the needle tip. The RFA procedure consisted of several 12-min cycles. The duration of cycle was shorter for additional margin ablation. For tumours located close to major portal pedicles, intraoperative cooling of the bile duct by cold saline irrigation via a cystic duct cannula was performed to reduce the risk for bile duct thermal injury.14 Ablations were performed using a percutaneous, open or laparoscopic approach and with the intention of achieving a 1.0-cm margin of non-tumorous liver parenchyma. The percutaneous approach was adopted for tumours at locations in which this approach could be applied. An open approach was used when: (i) the tumour was so large that multiple ablations were required even with the use of cluster electrodes; (ii) the tumour was near the liver dome and thus a percutaneous approach might cause pneumothorax or damage to the diaphragm, and (iii) the tumour was near a visceral organ, such as the gallbladder, colon or stomach. In selected patients, a laparoscopic approach was used if the tumour site was accessible. An i.v. infusion of mannitol was routinely administered before and during the procedure to reduce the risk for renal impairment caused by haemoglobinuria.15 Urine colour was monitored and dipstick tests were performed throughout and after the operation to monitor for haemoglobinuria.
Transarterial chemoembolization
The technique of TACE has been described previously.16,17 Superselective cannulation of the artery supplying the tumour was performed whenever possible. An emulsion of cisplatin (1 mg/ml) and lipiodol (Lipiodol Ultrafluide; Laboratoire Guerbet, Aulnay-sous-Bois, France) at a volume ratio of 1:1 was injected up to a maximum of 60 ml, depending on the tumour size. Embolization was performed with 1.0-mm3 particles of gelatin sponge (Spongostan; Johnson & Johnson Medical Ltd, Skipton, UK) mixed with 40 mg of gentamicin sulphate. Following the procedure, oral amoxicillin-clavulanic acid (375 mg, thrice daily) and an H2-blocker were administered for 5 days. Patients, if clinically fit, were discharged the day after the procedure. The TACE procedure was repeated every 8–12 weeks and stopped if significant disease progression or extrahepatic metastasis was seen on imaging.
Statistical analysis
Pearson's chi-squared test or Fisher's exact test were used to compare discrete variables. The Mann–Whitney U-test was used to compare continuous variables. Cumulative survival and disease-free survival were estimated using the Kaplan–Meier survival method. Statistical comparisons of survival distribution data were analysed using the log-rank test. A P-value of <0.05 signified statistical significance. Statistical analyses were performed using pasw Statistics for Windows Version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Among the 2750 patients, 645 (23.5%) received resection, 600 (21.8%) received TACE, and 274 (10.0%) received RFA. Of the 1347 (49.0%) patients who presented with solitary HCCs, 150 (11.1%) had tumours measuring 5–8 cm. Of these 150 patients, 99 (66.0%) submitted to tumour resection and 10 (6.7%) underwent liver transplantation. The remaining 41 (27.3%) patients underwent either TACE (n = 26; the TACE group) or RFA (n = 15; the RFA group) as primary treatment. In the latter group, 10 patients underwent open RFA, one underwent laparoscopic RFA, and four underwent percutaneous RFA (two of them in two sessions). Demographic data for the two groups are summarized in Table 1. At the time of writing, median follow-up in the RFA and TACE groups was 39.5 months (range: 6.1–81.8 months) and 16.5 months (range: 0.7–94.4 months), respectively (P = 0.044). The rate of treatment-related morbidity was higher in the RFA group (33.0% versus 19.2%), but the difference was not significant (P = 0.525) (Table 2). No treatment-associated mortality occurred. The median total hospital stay for the treatment of the primary tumour was 8.0 days (range: 1–41 days) in the RFA group and 8.5 days (range: 1–44 days) in the TACE group (P = 0.616). The median number of treatment sessions for the primary tumour was one and varied between one and two in the RFA group, and 3.5 (range: one to 25) in the TACE group (P < 0.001).
Table 1.
Comparison of characteristics of the two groups of patients
| RFA group (n = 15) | TACE (n = 26) | P-value | |
|---|---|---|---|
| Age, years, median (range) | 63 (32–82) | 64.5 (50–84) | 0.718 |
| Male : female ratio | 13:2 | 21:5 | 0.958 |
| Aetiology: alcohol, n | 0.241 | ||
| No | 7 | 17 | |
| Yes | 8 | 9 | |
| Hepatitis B virus carrier, n | 10 | 18 | 1 |
| Hepatitis C virus carrier, n | 2 | 5 | 0.472 |
| Child–Pugh class, n | 0.410 | ||
| A | 12 | 22 | |
| B | 2 | 4 | |
| C | 1 | 0 | |
| Tumour size, cm, median (range) | 6.0 (5.2–8.0) | 6.7 (5.1–8.0) | 0.242 |
| Tumour staginga, n | 1 | ||
| Stage I | 13 | 23 | |
| Stage IIIb | 2 | 3 | |
| Total bilirubin, μmol/l, median (range) | 14 (5–33) | 12 (4–38) | 0.904 |
| Albumin, g/l, median (range) | 39.0 (31–43) | 36.5 (27–45) | 0.091 |
| INR, median (range) | 1.1 (0.9–1.2) | 1.1 (0.9–2.0) | 0.820 |
| Platelet count, ×109/l, median (range) | 124.0 (62–248) | 134.5 (28–378) | 0.529 |
| Creatinine, μmol/l, median (range) | 86.0 (59–654) | 93.5 (55–533) | 0.779 |
| Alpha-fetoprotein, ng/ml, median (range) | 10.0 (2–97 200) | 109.5 (2–237 900) | 0.369 |
American Joint Committee on Cancer 2002, 6th edition.
INR, international normalized ratio.
Table 2.
Comparison of treatment-related morbidity and mortality in the two groups
| RFA group (n = 15) | TACE group (n = 26) | P-value | |
|---|---|---|---|
| Hospital deaths, n | 0 | 0 | |
| Patients with complication, n | 5 | 5 | 0.525 |
| Liver abscess | 1 | ||
| Bleeding oesophageal varices | 2 | ||
| Liver function derangement | 2 | ||
| Chest infection | 1 | ||
| Pleural effusion | 2 | ||
| Haemobilia | 1 | ||
| Haemoglobinuria | 1 |
Short- and longterm outcomes
The RFA group
In the RFA group, the median ablation time was 60 min (range: 12–102 min). Complete ablation was achieved in 12 patients. In one patient submitted to percutaneous RFA, the procedure was performed in two separate sessions. Three patients (with HCCs sized 6 cm, 6.5 cm and 8 cm, respectively) had residual disease; one of these patients underwent a second RFA procedure. Ablation site recurrence occurred in one patient at 4.0 months after the initial percutaneous RFA. Nine patients developed intrahepatic recurrence, two patients developed extrahepatic metastasis, and another two patients developed both. Six patients received TACE and five patients received further RFA for intrahepatic recurrence. The median disease-free survival was 13.0 months (range: 2.8–38.0 months). Rates of disease-free survival at 1, 3 and 5 years were 56.3%, 10.0% and 0%, respectively. Median overall survival was 39.8 months (range: 6.1–81.8 months). Rates of overall survival at 1, 3 and 5 years were 93.3%, 70.5% and 20.9%, respectively (Fig. 1).
Figure 1.
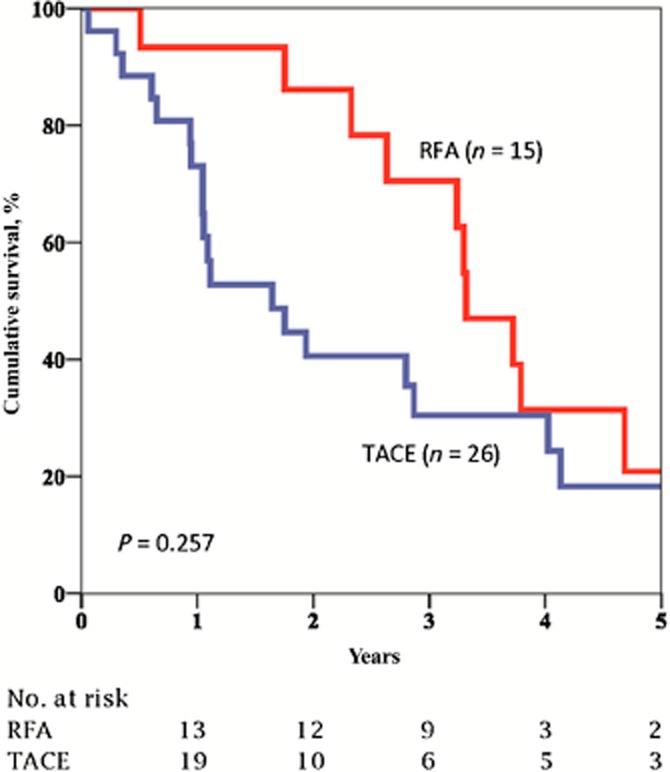
Comparison of overall survival of the two groups of patients
The TACE group
Twenty-six patients received TACE as primary treatment of HCC. The median number of cycles of TACE was three (range: one to 25). No patient achieved a complete response, but four patients obtained a partial response. The median time to disease progression was 8.0 months (range: 1.0–68.0 months). Six patients developed extrahepatic metastasis. Median overall survival was 19.8 months (range: 0.7–94.4 months). Rates of 1-year, 3-year and 5-year overall survival were 73.1%, 30.4% and 18.3%, respectively. The two groups did not differ significantly in terms of overall survival (P = 0.257), although the median duration of overall survival was shorter in the TACE group (19.8 months versus 39.8 months) (Fig. 1).
Discussion
Radiofrequency ablation is considered by some to be as effective as resection for small HCCs. For larger HCCs, however, it is less effective. Reported percentages of patients in whom complete necrosis in larger HCCs was achieved by RFA vary between 29% and 83%.9–11,18 These suboptimal results reflect the fact that coagulative necrosis induced by RFA alone can be limited. However, it has been reported that the multipolar RFA technique can achieve the complete ablation of liver tumours of up to 7.4 cm in size.19–22 Bowles et al.23 also demonstrated RFA to be safe even in large tumours in a series in which tumours of up to 18 cm were ablated and morbidity and recurrence rates were 7% and 9%, respectively. Yin et al.24 reported their experience of treating 109 patients with HCCs sized 3–7 cm by percutaneous thermal ablation using RFA or microwave ablation. The complete ablation rate was 92.6%, local recurrence occurred in 22.0% of patients and the median time to local recurrence was 4.6 months. Yin et al.24 found that patients with tumours measuring 5–7 cm achieved 1-, 2-, 3- and 5-year post-ablation survival rates similar to those of patients with tumours measuring 3–5 cm (P = 0.84).
In the present study, a complete ablation rate of 80.0% was achieved in patients who received RFA as the definitive treatment, and the local recurrence rate was 6.7%. These results are remarkable in comparison with those reported in the literature and can be attributed to the fact that most patients underwent open rather than percutaneous RFA. In a meta-analysis of outcomes in 5224 liver tumours treated by RFA, treatment approach was found to be a significant independent factor associated with local recurrence.25
Transarterial chemoembolization takes advantage of the relatively selective arterial vascularization of liver tumours and is intended to achieve tumour ischaemia. In the treatment, the delivery of chemotherapeutic agents and the embolization of blood vessels are performed simultaneously to increase local chemotherapeutic dwell time. Two randomized trials have shown TACE to improve the survival of patients with unresectable HCCs.16,26 A recent meta-analysis comparing TACE and conservative treatment reported the former to offer a significant survival benefit over the latter.27
A recent study reported that patients who underwent hepatectomy or living-donor liver transplantation as a primary treatment for solitary HCC sized 5–8 cm achieved 5-year overall survival rates of 68.9% and 87.5%, respectively.28 A comparison of the relative efficacy of TACE and RFA in treating patients with unresectable HCCs of ≥5 cm is lacking in the literature. Given the controversy over the management of this group of patients, the findings of the present study are significant. In the present study, RFA was found to surpass TACE in immediate tumour control, which reflects the results of a previous study by this group in patients with multiple and unresectable HCCs of <5 cm.29 In the present study, the median time to disease progression was 8.0 months (range: 1.0–68.0 months) in patients treated with TACE and median disease-free survival was 13.0 months (range: 3.0–38.0 months) in patients treated with RFA. A complete ablation, which reduces the patient's tumour load, may benefit the patient in the short term. Median survival was 39.8 months in the RFA group and 19.8 months in the TACE group and thus did not differ significantly (P = 0.257). This might be explained by the small number of patients in the study. The RFA group achieved a much higher 2-year survival rate than the TACE group (86.2% versus 40.6%), although the two groups had similar rates of 5-year survival (20.9% and 18.3%, respectively). The similarity in 5-year survival reflects the fact that the natural history and biology of HCC cannot be altered by local ablative therapies. No in-hospital mortality occurred in this series, and the two treatment modalities were both well tolerated. The complication rate was insignificantly higher in the RFA group (33.3% versus 19.2%; P = 0.525), and higher than that reported by Mulier et al.,30 probably because more of the procedures (10 of 15) were performed in open surgery.
The present study was not a randomized trial and is inevitably subject to weaknesses such as bias. Nonetheless, it is the first study to demonstrate a probable short- to mid-term advantage of RFA over TACE for solitary unresectable HCCs of 5–8 cm in size, although no significant difference in longterm survival was found.
In the present study, conventional cisplatin-based TACE was used. Whether the use of drug-eluting beads in TACE would produce a different outcome is unknown.31 A recent RCT showed that RFA combined with TACE is more effective than RFA alone in extending the ablated area and decreasing the local tumour progression rate in patients with medium-sized HCCs.32 Some authors believe that TACE can work in synergism with RFA because the heat-sink effect is reduced after embolization and tumour cells are more sensitive to hyperthermia after TACE.33 Whether the combination of these two treatment modalities has a synergistic effect in patients with unresectable large HCCs needs to be investigated further.
Conflicts of interest
None declared.
References
- Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western centre. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: longterm results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radiofrequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic–histologic correlation during follow-up periods. Hepatology. 2002;35:1467–1475. doi: 10.1053/jhep.2002.33635. [DOI] [PubMed] [Google Scholar]
- Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumour size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- Guglielmi A, Ruzzenente A, Battocchia A, Tonon A, Fracastoro G, Cordiano C. Radiofrequency ablation of hepatocellular carcinoma in cirrhotic patients. Hepatogastroenterology. 2003;50:480–484. [PubMed] [Google Scholar]
- Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Effectiveness of radiofrequency ablation for hepatocellular carcinomas larger than 3 cm in diameter. Arch Surg. 2004;139:281–287. doi: 10.1001/archsurg.139.3.281. [DOI] [PubMed] [Google Scholar]
- Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg. 2005;12:31–37. doi: 10.1007/s00534-004-0945-0. [DOI] [PubMed] [Google Scholar]
- Kele PG, van der Jagt EJ, Krabbe PF, de Jong KP. Lack of anatomical concordance between pre-ablation and post-ablation CT images: a risk factor related to ablation site recurrence. Int J Hepatol. 2012;2012:870306. doi: 10.1155/2012/870306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam VW, Ng KK, Chok KS, Cheung TT, Wat J, Fan ST, et al. Safety and efficacy of radiofrequency ablation for periductal hepatocellular carcinoma with intraductal cooling of the central bile duct. J Am Coll Surg. 2008;207:e1–e5. doi: 10.1016/j.jamcollsurg.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Ng KK, Poon RT, Lam CM, Yuen J, Tso WK, Fan ST. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg. 2006;93:440–447. doi: 10.1002/bjs.5267. [DOI] [PubMed] [Google Scholar]
- Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- O'Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325–331. doi: 10.1002/bjs.4045. [DOI] [PubMed] [Google Scholar]
- Seror O, N'Kontchou G, Ibraheem M, Ajavon Y, Barrucand C, Ganne N, et al. Large (≥5.0 cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes – initial experience in 26 patients. Radiology. 2008;248:288–296. doi: 10.1148/radiol.2481071101. [DOI] [PubMed] [Google Scholar]
- Frericks BB, Ritz JP, Roggan A, Wolf KJ, Albrecht T. Multipolar radiofrequency ablation of hepatic tumours: initial experience. Radiology. 2005;237:1056–1062. doi: 10.1148/radiol.2373041104. [DOI] [PubMed] [Google Scholar]
- Ritz JP, Lehmann KS, Reissfelder C, Albrecht T, Frericks B, Zurbuchen U, et al. Bipolar radiofrequency ablation of liver metastases during laparotomy. First clinical experiences with a new multipolar ablation concept. Int J Colorectal Dis. 2006;21:25–32. doi: 10.1007/s00384-005-0781-y. [DOI] [PubMed] [Google Scholar]
- Tacke J, Mahnken A, Roggan A, Günther RW. Multipolar radiofrequency ablation: first clinical results. Rofo. 2004;176:324–329. doi: 10.1055/s-2004-812723. [DOI] [PubMed] [Google Scholar]
- Terraz S, Constantin C, Majno PE, Spahr L, Mentha G, Becker CD. Image-guided multipolar radiofrequency ablation of liver tumours: initial clinical results. Eur Radiol. 2007;17:2253–2261. doi: 10.1007/s00330-007-0626-x. [DOI] [PubMed] [Google Scholar]
- Bowles BJ, Machi J, Limm WM, Severino R, Oishi AJ, Furumoto NL, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumours. Arch Surg. 2001;136:864–868. doi: 10.1001/archsurg.136.8.864. [DOI] [PubMed] [Google Scholar]
- Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: longterm outcome and prognostic factors. Cancer. 2009;115:1914–1923. doi: 10.1002/cncr.24196. [DOI] [PubMed] [Google Scholar]
- Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- Dai WC, Chan SC, Chok KS, Cheung TT, Sharr WW, Chan AC, et al. Good longterm survival after primary living donor liver transplantation for solitary hepatocellular carcinomas up to 8 cm in diameter. HPB. 2014;16:749–757. doi: 10.1111/hpb.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chok KS, Ng KK, Poon RT, Lam CM, Yuen J, Tso WK, et al. Comparable survival in patients with unresectable hepatocellular carcinoma treated by radiofrequency ablation or transarterial chemoembolization. Arch Surg. 2006;141:1231–1236. doi: 10.1001/archsurg.141.12.1231. [DOI] [PubMed] [Google Scholar]
- Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–1250. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC) Eur Radiol. 2006;16:661–669. doi: 10.1007/s00330-005-0029-9. [DOI] [PubMed] [Google Scholar]


