Abstract
Objective
The effects of obesity in liver transplantation remain controversial. Earlier institutional data demonstrated no significant difference in postoperative complications or 1-year mortality. This study was conducted to test the hypothesis that obesity alone has minimal effect on longterm graft and overall survival.
Methods
A retrospective, single-institution analysis of outcomes in patients submitted to primary adult orthotopic liver transplantation was conducted using data for the period from 1 January 2002 to 31 December 2012. Recipients were divided into six groups by pre-transplant body mass index (BMI), comprising those with BMIs of <18.0 kg/m2, 18.0–24.9 kg/m2, 25.0–29.9 kg/m2, 30.0–35.0 kg/m2, 35.1–40.0 kg/m2 and >40 kg/m2, respectively. Pre- and post-transplant parameters were compared. A P-value of <0.05 was considered to indicate statistical significance. Independent predictors of patient and graft survival were determined using multivariate analysis.
Results
A total of 785 patients met the study inclusion criteria. A BMI of >35 kg/m2 was associated with non-alcoholic steatohepatitis (NASH) cirrhosis (P < 0.0001), higher Model for End-stage Liver Disease (MELD) score, and longer wait times for transplant (P = 0.002). There were no differences in operative time, intensive care unit or hospital length of stay, or perioperative complications. Graft and patient survival at intervals up to 3 years were similar between groups. Compared with non-obese recipients, recipients with a BMI of >40 kg/m2 showed significantly reduced 5-year graft (49.0% versus 75.8%; P < 0.02) and patient (51.3% versus 78.8%; P < 0.01) survival.
Conclusions
Obesity increasingly impacts outcomes in liver transplantation. Although the present data are limited by the fact that they were sourced from a single institution, they suggest that morbid obesity adversely affects longterm outcomes despite providing similar short-term results. Further analysis is indicated to identify risk factors for poor outcomes in morbidly obese patients.
Introduction
The national obesity epidemic continues to progress at an alarming rate, affecting 78 million adult Americans. The prevalence of obesity amongst potential liver transplant recipients is also rising; more than half are overweight or obese.1 The full impact of this chronic health condition in the context of liver transplantation is yet to be determined. Non-alcoholic steatohepatitis (NASH), the hepatic manifestation of obesity and metabolic syndrome, is now the fourth leading indication for orthotopic liver transplantation (OLT) in the USA and accounted for 7.4% of OLTs performed in 2010.2 Non-alcoholic steatohepatitis is predicted to surpass hepatitis C as the leading indication for OLT in the next 10 years.3,4 An estimated 25 million Americans will develop NASH by 2025 and as many as five million will suffer from chronic liver failure.2,5 These numbers may further stress a system in which demand for deceased donor livers already exceeds supply.
Previous studies evaluating the outcomes of patients transplanted for NASH cirrhosis have shown conflicting results in terms of the effects of pre-transplant NASH on post-transplant morbidity and mortality.2,6–8 Some suggest that recipients with NASH have higher perioperative rates of cardiovascular events, including myocardial infarction, acute heart failure, arrhythmia and cerebrovascular accident.8 Additionally, pre-transplant NASH may increase the risk for recurrent non-alcoholic fatty liver disease (NAFLD) and allograft cirrhosis post-transplantation.9
The severely and morbidly obese [those with a body mass index (BMI) of >35 kg/m2] are significantly more likely to undergo liver transplantation for NASH cirrhosis than subjects of normal weight.3,10 The effect of BMI on longterm outcomes in liver transplant recipients, as distinct from the effects of NASH, is not well understood. Historically, obesity was considered a relative contraindication to transplantation, largely because of concerns about technical feasibility and worse outcomes. In non-transplant transabdominal surgery, obesity has been associated with increases in blood loss, resource utilization, and perioperative morbidity and mortality.11,12 In liver transplantation, severe obesity (BMI >35 kg/m2) has also been associated with increased rates of perioperative complications, such as wound infection and bleeding.10,13,14 Despite initial studies evaluating its effects on short-term outcomes and complication rates, the impacts of BMI on longterm overall and graft survival are indeterminate. This paper reports the present authors’ institutional experience with liver transplantation in the obese population.
Materials and methods
A retrospective study of outcomes in all patients submitted to primary OLT at Washington University in St Louis between 1 January 2002 and 31 December 2012 was conducted. Recipients aged <18 years were excluded. Liver transplant recipients were divided into six groups based on their pre-transplant BMI in accordance with the World Health Organization classification of obesity: Group 1 (BMI: <18.0 kg/m2); Group 2 (BMI: 18.0–24.9 kg/m2); Group 3 (BMI: 25.0–29.9 kg/m2); Group 4 (BMI: 30.0–35.0 kg/m2); Group 5 (BMI: 35.1–40.0 kg/m2), and Group 6 (BMI: >40.0 kg/m2). Data for BMI were not adjusted for ascites because the volume of ascites drained at the time of transplant did not differ significantly between groups. The mean ± standard deviation (SD) duration of follow-up was 4.5 ± 3.0 years. Pre- and post-transplant parameters were compared among the BMI groups (Table 1). Pre-transplant recipient variables included patient age, race, gender, medical comorbidities [hypertension, coronary artery disease (CAD), non-CAD cardiac disease, diabetes mellitus, renal insufficiency], aetiology of liver disease, presence of hepatocellular carcinoma (HCC), haemodialysis at time of transplant, Model for End-stage Liver Disease (MELD) score (determined by laboratory values), and time on waiting list. Primary outcome measures were graft and overall patient survival at 90 days, 1 year, 3 years, 5 years and 7 years. Secondary outcome measures included operative time, cold and warm ischaemic times, operative transfusion requirement of ≥10 units of packed red blood cells (uPRBC), intensive care unit (ICU) length of stay (LoS), hospital LoS, re-exploration for bleeding, infection, disease recurrence (hepatitis C, NASH and HCC), allograft rejection, aetiology of graft failure, retransplantation, and cause of death.
Table 1.
Population demographics and pre-transplant characteristics in 785 liver transplant recipients
| Variables | Body mass index, kg/m2 | P-value | |||||
|---|---|---|---|---|---|---|---|
| <18.0 (n = 9) | 18.0–24.9 (n = 210) | 25.0–29.9 (n = 294) | 30.0–35.0 (n = 169) | 35.1–40.0 (n = 77) | >40.0 (n = 26) | ||
| Age, years, median | 46 | 55 | 55 | 56 | 56 | 53 | 0.349 |
| Male, % | 4 (44.4) | 134 (63.8) | 214 (72.8) | 119 (70.4) | 47 (61.0) | 10 (37.0) | 0.001 |
| White, % | 7 (0.78) | 171 (81.4) | 249 (84.7) | 148 (87.6) | 68 (88.3) | 13 (73.1) | 0.267 |
| Comorbidities, % | |||||||
| Diabetes | 3 (33.3) | 44 (21.0) | 76 (25.9) | 51 (30.2) | 25 (32.5) | 6 (23.1) | 0.277 |
| Hypertension | 1 (11.1) | 49 (23.3) | 85 (28.9) | 59 (34.9) | 31 (40.3) | 14 (53.8) | <0.0001 |
| Coronary artery disease | 0 (0) | 6 (2.9) | 20 (6.8) | 9 (5.3) | 6 (7.8) | 2 (7.7) | 0.311 |
| Other cardiac disease | 0 (0) | 15 (7.1) | 26 (8.8) | 16 (9.5) | 10 (13.0) | 3 (11.5) | 0.621 |
| Renal insufficiency | 2 (22.2) | 27 (12.9) | 52 (17.7) | 26 (15.4) | 19 (24.7) | 1 (3.8) | 0.089 |
| Renal failure on dialysis | 2 (22.2) | 19 (9.0) | 23 (7.8) | 18 (10.7) | 11 (14.3) | 2 (7.7) | 0.497 |
| Aetiology of liver disease, % | |||||||
| Hepatitis C virus | 3 (33.3) | 80 (38.1) | 149 (50.7) | 78 (46.2) | 31 (40.3) | 12 (46.2) | 0.102 |
| Non-alcoholic steatohepatitis | 0 (0.0) | 2 (0.9) | 19 (6.5) | 14 (8.3) | 17 (22.8) | 6 (23.1) | <0.0001 |
| Alcohol (primary diagnosis) | 4 (44.4) | 32 (15.2) | 35 (11.9) | 21 (12.4) | 12 (15.6) | 2 (7.7) | 0.082 |
| Alcohol (secondary diagnosis) | 0 | 13 (6.2) | 25 (8.5) | 14 (8.3) | 9 (11.7) | 1 (3.8) | 0.543 |
| Primary biliary cirrhosis | 0 | 16 (7.6) | 14 (4.8) | 4 (2.4) | 2 (2.6) | 1 (3.8) | 0.15 |
| Primary sclerosing cholangitis | 1 (11.1) | 18 (8.6) | 11 (3.7) | 4 (2.4) | 1 (1.3) | 0 | 0.012 |
| Other | 1 (11.1) | 62 (29.5) | 66 (22.4) | 48 (28.4) | 14 (18.2) | 5 (19.2) | 0.176 |
| Hepatocellular carcinoma | 0 (0.0) | 81 (38.6) | 110 (37.4) | 61 (36.1) | 29 (37.7) | 7 (26.9) | 0.836 |
| Other | |||||||
| MELD score, lab-based, median | 28 | 18 | 19 | 20 | 24 | 23 | 0.002 |
| Waiting time, days, median | 25 | 59 | 59 | 89 | 82 | 72 | 0.011 |
| Liver and kidney transplant, % | 1 (11.1) | 11 (5.2) | 13 (4.4) | 6 (3.6) | 4 (5.2) | 0 (0) | 0.719 |
MELD, Model for End-stage Liver Disease.
Statistical analysis
Categorical variables were compared using the chi-squared test and continuous variables were compared using Student's t-test; a P-value of <0.05 was considered to indicate statistical significance. Overall patient and graft survival curves were determined using Kaplan–Meier methods and compared using the log-rank test. Independent predictors of patient and graft survival were determined by multivariate Cox regression analysis.
This study was approved by the Washington University School of Medicine Institutional Review Board.
Results
Study population characteristics
A total of 785 patients met the study inclusion criteria (Table 1). Numbers of recipients in each group were: Group 1 (BMI: <18.0 kg/m2): n = 9 (1.2% of study population); Group 2 (BMI: 18.0–24.9 kg/m2): n = 210 (26.8%); Group 3 (BMI: 25.0–29.9 kg/m2): n = 294 (37.5%); Group 4 (BMI: 30.0–35.0 kg/m2): n = 169 (21.5%); Group 5 (BMI: 35.1–40.0 kg/m2): n = 77 (9.8%), and Group 6 (BMI: >40.0 kg/m2): n = 26 (3.3%). There were no significant differences between groups with regard to age (mean ages: 47.8–55.7 years; P = NS) or race. Patients with a BMI of <18 kg/m2 or >40 kg/m2 were more likely to be female (56.6% and 63.0%, respectively) compared with all other groups (P < 0.001). Analysis of comorbid medical conditions demonstrated a significantly increased rate of hypertension in patients with a BMI of >35 kg/m2 (P = 0.001). There were no differences in prevalences of diabetes mellitus, CAD, other (non-CAD) cardiovascular disease, renal insufficiency or dialysis-dependent renal failure.
Liver disease and waiting time
The aetiology of liver disease varied among the groups. Chronic hepatitis C infection was the most common cause of cirrhosis in patients with a BMI of >18 kg/m2, whereas a BMI of <18 kg/m2 was most commonly associated with alcohol cirrhosis. Non-alcoholic steatohepatitis represented the second leading cause of liver disease in patients with a BMI of >35 kg/m2. Recipients (22.8%) with a BMI of >35 kg/m2 were significantly more likely to have NASH as the primary aetiology compared with patients in all other groups (P < 0.0001). Only two patients (0.9%) with a BMI of <25 kg/m2 had NASH. All groups with BMIs of >18 kg/m2 had similar rates of HCC (26.9–38.6%; P = NS). Average MELD scores at the time of transplantation were 24.6 (Group 1), 19.8 (Group 2), 21.1 (Group 3), 22.2 (Group 4), 24.8 (Group 5), and 25.1 (Group 6). The difference in MELD scores between Group 2 and Group 5 (mean MELD scores: 19.8 versus 24.8; P = 0.002) was statistically significant. Patients with BMIs of 30.1–40.0 kg/m2 waited longer for transplant compared with patients with BMIs of 18.0–30.0 kg/m2 (280 days versus 174 days; P = 0.01). There was no difference in need for combined liver and kidney transplantation between groups.
Perioperative outcomes and resource utilization
No significant differences between groups were detected in operative time, intraoperative transfusion requirement (≥10 uPRBC), cold or warm ischaemic time, ICU LoS or hospital LoS (Table 2). No significant differences between groups were detected in rates of postoperative bleeding requiring massive transfusion or re-exploration, hepatic artery thrombosis (HAT), portal vein thrombosis (PVT), biliary complications or infection.
Table 2.
Secondary outcomes after transplantation in 785 liver transplant recipients by patient body mass index
| Body mass index, kg/m2 | P-value | ||||||
|---|---|---|---|---|---|---|---|
| <18.0 (n = 9) | 18.0–24.9 (n = 210) | 25.0–29.9 (n = 294) | 30.0–35.0 (n = 169) | 35.1–40.0 (n = 77) | >40.0 (n = 26) | ||
| Operative time, h, mean | 6.9 | 6.4 | 6.2 | 6.5 | 6.6 | 6.1 | 0.321 |
| Warm ischaemia time, min, mean | 38 | 36 | 37 | 37 | 36 | 33 | 0.786 |
| Cold ischaemia time, h, mean | 6.0 | 6.3 | 6.0 | 6.2 | 6.9 | 6.0 | 0.328 |
| No intraoperative PRBC, % | 0 (0.0) | 53 (25.2) | 65 (22.1) | 51 (30.2) | 15 (19.5) | 4 (15.4) | 0.107 |
| ≥10 units intraoperative PRBC, % | 2 (22.2) | 29 (13.8) | 45 (15.3) | 30 (17.8) | 19 (24.7) | 5 (19.2) | 0.340 |
| ICU LoS, days, median | 6 | 2 | 2 | 2 | 3 | 3 | 0.644 |
| Hospital LoS, days, median | 12 | 7 | 7 | 7 | 9 | 9 | 0.598 |
| Re-exploration for bleeding, % | 0 (0.0) | 15 (7.1) | 20 (6.8) | 15 (8.9) | 10 (13.0) | 2 (7.7) | 0.468 |
| Rejection, % | 3 (33.3) | 51 (24.3) | 73 (24.8) | 28 (16.6) | 21 (27.3) | 7 (26.9) | 0.291 |
ICU, intensive care unit; LoS, length of stay; PRBC, packed red blood cells.
Survival
Rates of overall survival at 90 days averaged 96.5% (ranging from 94.7% in Group 5 to 100% in Groups 1 and 6; P = NS). Mean 1-year overall survival (91.0% in all patients versus 84.6% in patients with a BMI of >40 kg/m2; P = NS) and mean 3-year overall survival (84.6% in all patients versus 76.9% in patients with a BMI of >40 kg/m2; P = NS) did not differ significantly between BMI groups. Rates of overall survival at 5 years and 7 years were significantly decreased in recipients with a BMI of >40 kg/m2 at the time of transplant (5-year survival: 51.3% versus 78.8%; 7-year survival: 38.5% versus 71.5%; P = 0.009) (Fig. 1). No differences in graft survival were detected at 90 days, 1 year or 3 years post-transplant. Graft survival at 5 years and 7 years was significantly decreased for patients with a BMI of >40 kg/m2 in comparison with rates for all groups (49.0% versus 75.8%, and 36.7% versus 68.4%, respectively; P ≤ 0.02) (Fig. 2). Rates of primary disease recurrence of hepatitis C, NASH and HCC did not differ among groups. Secondary graft steatosis occurred in 7.1% of all allografts and trended toward a higher prevalence in patients with a BMI of >40 kg/m2 (26.9% versus a group mean of 7.1%; P = NS). Forty (5.1%) patients required retransplantation for graft failure. Indications for retransplantation included primary non-function (n = 5), HAT (n = 10), PVT (n = 4), cholangiopathy (n = 8), recurrent disease (n = 7), and chronic rejection (n = 6). Indications for retransplantation were not associated with BMI. Cause of death analysis did not identify any significant difference among BMI groups in rates of death caused by cardiovascular, respiratory, renal or multi-organ failure, nor deaths related to sepsis or cancer.
Figure 1.
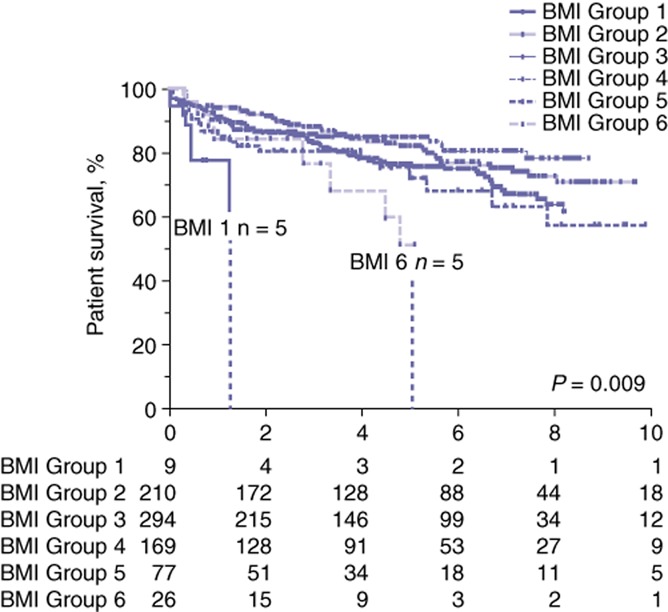
Kaplan–Meier curves for patient survival after liver transplantation in patient groups stratified by body mass index (BMI). Group 1: <18.0 kg/m2; Group 2: 18.0–24.9 kg/m2; Group 3: 25.0–29.9 kg/m2; Group 4: 30.0–35.0 kg/m2; Group 5: 35.1–40.0 kg/m2; Group 6: >40 kg/m2
Figure 2.
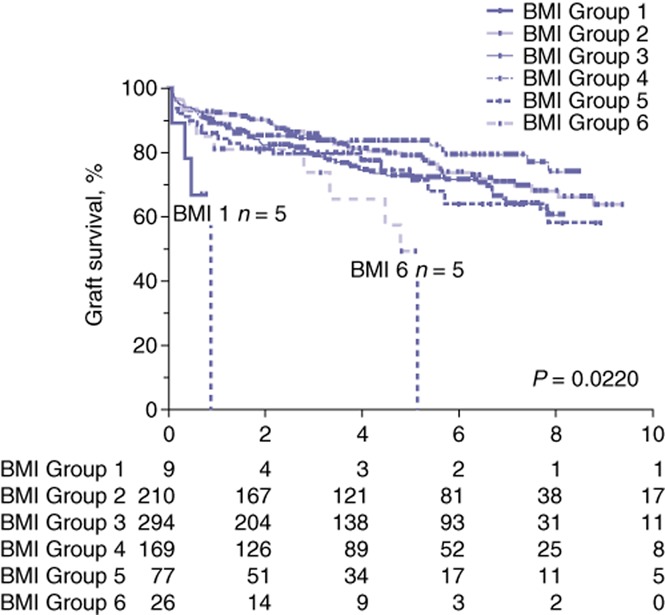
Kaplan–Meier curves for graft survival after liver transplantation in patient groups stratified by body mass index (BMI). Group 1: <18.0 kg/m2; Group 2: 18.0–24.9 kg/m2; Group 3: 25.0–29.9 kg/m2; Group 4: 30.0–35.0 kg/m2; Group 5: 35.1–40.0 kg/m2; Group 6: >40 kg/m2
Multivariate Cox regression analysis of recipient characteristics identified three independent risk factors in patient and graft survival: a BMI >40 kg/m2; hepatitis C as end-stage liver disease aetiology, and non-CAD cardiovascular disease (Table 3). Cox regression analysis confirmed these factors to be independent predictors of survival: a BMI of >40 kg/m2 [hazard ratio (HR) 2.3, 95% confidence interval (CI) 1.2–4.4]; hepatitis C as aetiology of liver disease (HR 1.4, 95% CI 1.1–1.9), and non-CAD cardiovascular disease (HR 1.7, 95% CI 1.1–2.6). Similar results were obtained in a multivariate regression analysis of graft survival. The presence of HCC, although associated with a trend toward diminished survival (HR 3.8), did not reach significance in the univariate analysis (P = 0.051).
Table 3.
Multivariate regression analysis of risk factors for graft and patient survival after liver transplantation
| Cox regression analysis | ||||
|---|---|---|---|---|
| Input variable | Graft survival | Patient survival | ||
| HR | P-value | HR | P-value | |
| Gender | 0.832 | 0.259 | 0.853 | 0.366 |
| Age ≥60 years | 1.120 | 0.518 | 1.184 | 0.365 |
| MELD score >30 | 1.191 | 0.401 | 1.330 | 0.192 |
| Diabetes | 1.235 | 0.199 | 1.317 | 0.112 |
| Hypertension | 0.708 | 0.039 | 0.772 | 0.141 |
| Coronary artery disease | 0.992 | 0.980 | 1.035 | 0.919 |
| Other heart disease | 1.662 | 0.016 | 1.706 | 0.018 |
| Renal disease | 0.902 | 0.667 | 1.065 | 0.797 |
| On dialysis | 1.241 | 0.471 | 1.028 | 0.932 |
| Hepatitis C | 1.341 | 0.065 | 1.432 | 0.035 |
| Alcoholic cirrhosis | 1.043 | 0.817 | 1.222 | 0.286 |
| NASH | 0.913 | 0.788 | 0.853 | 0.669 |
| HCC | 1.108 | 0.525 | 1.264 | 0.173 |
| BMI >40.0 kg/m2 | 2.452 | 0.005 | 2.697 | 0.003 |
P-values in bold indicate statistical significance (P < 0.05).
BMI, body mass index; HCC, hepatocellular carcinoma; HR, hazard ratio; MELD, Model for End-stage Liver Disease; NASH, non-alcoholic steatohepatitis.
Discussion
Available data suggest that the prevalence of obesity will continue to rise in the US population. Consequently, the number of obese transplant candidates is also expected to increase, especially as NASH becomes an increasingly significant cause of end-stage liver disease. Short-term outcomes in obese liver transplant recipients are often conflicting and few data on longterm outcomes in obesity have been published.
Similarly to other studies, this analysis showed that morbidly obese patients (BMI >40 kg/m2) with liver failure are more likely to be female, have hypertension, and have NASH as their primary aetiology for cirrhosis. Previously published data suggested that obesity (BMI >30 kg/m2) increases the risk for HCC in cirrhotic livers, although this finding is not supported by the results of the current study.15 Although it is well established that obesity correlates strongly with metabolic syndrome, patients with high BMIs in the present population did not have higher rates of diabetes or chronic kidney disease. This may be related to selection bias in the evaluation and approval of patients for liver transplantation. All potential recipients undergo rigorous cardiopulmonary testing prior to listing and thus it is possible that only the ‘healthiest’ of the morbidly obese are activated on the waitlist. Because of the perception that obesity alone adds risk, morbidly obese patients, in comparison with their normal-weight counterparts, may be less likely to be approved for transplant if they have other significant medical conditions.
Despite similar comorbidity rates, patients with BMIs of >35 kg/m2 waited longer for transplant and had higher MELD scores at the time of transplantation. Other studies have documented similar findings in time on the waitlist.10,13 In this patient cohort, it is unclear whether the longer wait times resulted in the progression of disease and higher MELD scores or whether severely and morbidly obese patients were evaluated and listed for transplant later in their disease course than non-obese patients.
It is well known that a higher MELD score at the time of transplantation increases morbidity and mortality and results in greater resource utilization. Non-alcoholic steatohepatitis, which is predominantly a disease of the obese, has previously been associated with longer operative times, extended hospital LoS and increased transfusion requirements.3 Interestingly, despite higher rates of NASH and higher MELD scores, obese recipients in this population did not have worse perioperative outcomes or higher rates of complications. The small size of the sample in this previous study may have limited the statistical significance of its findings. Another consideration is that patient obesity is now a common comorbidity and thus care providers (surgeons, anaesthesiologists, intensivists, nurses, coordinators) are more familiar with the challenges associated with these patients and the potential weight-related complications that may occur. Increased surgical exposure and greater experience in managing haemodynamic changes and providing postoperative care to obese recipients can potentially improve outcomes to the extent that a difference between weight groups will no longer exist. Similarities in perioperative outcomes between BMI groups may also be reflected in comparable patient and graft survival curves to 3 years post-transplant.
Although early outcomes in patients with BMIs of >40 kg/m2 were comparable with those in patients in other BMI classes, a BMI of >40 kg/m2 is significantly associated with reduced patient and graft survival at 5 years and 7 years post-transplant. Potential causes for higher rates of delayed graft loss and death include recurrent disease (either greater incidence of recurrence or more rapid disease progression after diagnosis of recurrence), allograft rejection, increased incidence of secondary graft steatosis with progression to NASH cirrhosis, increased risk for recurrent HCC, or increased risk for death from comorbidities associated with obesity and metabolic syndrome (specifically, cardiovascular disease or complications of diabetes).9,16 Each of these risk factors was studied in subgroup analyses, but none were found to significantly differ among BMI groups. Morbidly obese transplant recipients were more likely to develop secondary graft steatosis, but this did not translate into increased rates of post-transplant NASH. Recurrence of hepatitis C and HCC was not affected by BMI. This contrasts with work by Siegel et al., who reported that obesity nearly doubles the risk for HCC recurrence, although their data did not achieve significance.17 In this current analysis, morbid obesity did not predispose individuals to death from a specific cause; the proportions of deaths from sepsis, cancer, and single- and multi-system organ failure did not vary significantly by weight.
Multivariate regression analysis confirmed that morbid obesity itself is an independent risk factor for shortened graft and patient survival. Even after controlling for the factors typically used to explain the effect of BMI on mortality (e.g. NASH, cardiovascular disease), there was a significant impact from BMI alone. Although the present study is not unique in identifying BMI as an independent risk factor for patient and graft survival, it is the first to look at obesity as a risk factor that is distinct from NASH in addition to the other metabolic syndrome conditions. Whether this trend simply reflects the normal life expectancy curve for the morbidly obese in comparison with non-obese individuals must be established. Perhaps morbid obesity puts recipients at risk for death from the same causes as normal-weight recipients, but at an earlier time-point. Multiple prospective cohorts within the general population have demonstrated a life expectancy reduction of 10 years in individuals with BMIs of >40 kg/m2.18 Additionally, another institution previously demonstrated that liver transplant recipients with BMIs of >30 kg/m2 had almost double the risk for death from all causes compared with normal-weight OLT recipients.17
Weight reduction can reverse the longterm sequelae of obesity. However, options for exercise-induced or surgical weight loss in potential transplant recipients have previously been limited by physical deconditioning and weakness, risk for bleeding as a result of portal hypertension and coagulopathy, concerns about post-anaesthesia hepatic decompensation, and risk for postoperative infections. Recently, these theoretical concerns have been challenged and the performance of bariatric surgery before, during and after liver transplantation has been reported. In one series of 20 pre-OLT patients undergoing laparoscopic sleeve gastrectomy, only 15% suffered a serious postoperative complication and only one patient developed hepatic insufficiency.19 A mean excess weight loss of 61% was documented and sustained in the seven patients who subsequently underwent liver transplantation.19 Similarly, Heimbach et al. reported minimal morbidity, sustained weight loss and the resolution of insulin-dependent diabetes in patients undergoing simultaneous OLT and sleeve gastrectomy.20 These early results are promising, but whether bariatric surgery improves longterm outcomes in morbidly obese liver transplant recipients is yet to be determined.
Aggressive management of metabolic syndrome should be a priority in this patient population. Cardiovascular risk reduction can be achieved with antiplatelet therapy and pharmacologic treatment of hypertension, dyslipidaemia and glucose intolerance.21,22 Consideration should be given to early steroid withdrawal or avoidance.23 Diet modification remains an important mechanism by which to reduce weight and obesity-associated risk for mortality.
Although they are limited by being drawn from a single institution, the present data suggest that severe and morbid obesity adversely affect longterm outcomes despite similar short- and medium-term results. Further analysis is indicated to identify risk factors for poor outcomes in morbidly obese patients and to understand the delayed effects on patient and graft survival. With that knowledge, interventions may be taken to improve longterm survival in morbidly obese liver transplant recipients.
Conflicts of interest
None declared.
References
- Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- Afzali A, Berry K, Ioannou GN. Excellent post-transplant survival for patients with non-alcoholic steatohepatitis in the United States. Liver Transpl. 2012;18:29–37. doi: 10.1002/lt.22435. [DOI] [PubMed] [Google Scholar]
- Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, Rana A, et al. Liver transplantation for non-alcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624–633. doi: 10.1097/SLA.0b013e31826b4b7e. [DOI] [PubMed] [Google Scholar]
- Koehler E, Watt K, Charlton M. Fatty liver and liver transplantation. Clin Liver Dis. 2009;13:621–630. doi: 10.1016/j.cld.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Burke A, Lucey MR. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686–693. doi: 10.1111/j.1600-6143.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- Orci LA, Majno PE, Berney T, Morel P, Mentha G, Toso C. The impact of wait list body mass index changes on the outcome after liver transplantation. Transpl Int. 2013;26:170–176. doi: 10.1111/tri.12017. [DOI] [PubMed] [Google Scholar]
- Barritt AS, 4th, Dellon ES, Kozlowski T, Gerber DA, Hayashi PH. The influence of non-alcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol. 2011;45:372–378. doi: 10.1097/MCG.0b013e3181eeaff0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for non-alcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56:1741–1750. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- Yalamanchili K, Saadeh S, Klintmalm GB, Jennings LW, Davis GL. Non-alcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or non-alcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- LaMattina JC, Foley DP, Fernandez LA, Pirsch JD, Musat AI, D'Alessandro AM, et al. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012;26:910–918. doi: 10.1111/j.1399-0012.2012.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawn MT, Bian J, Leeth RR, Ritchie G, Allen N, Bland KI, et al. Impact of obesity on resource utilization for general surgical procedures. Ann Surg. 2005;241:821–826. doi: 10.1097/01.sla.0000161044.20857.24. discussion 826–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TK, Rosato EL, Kennedy EP, Chojnacki KA, Andrel J, Hyslop T, et al. Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2009;208:210–217. doi: 10.1016/j.jamcollsurg.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Dick AA, Perkins JD, Spitzer AL, Lao OB, Healey PJ, Reyes JD. Impact of obesity on children undergoing liver transplantation. Liver Transpl. 2010;16:1296–1302. doi: 10.1002/lt.22162. [DOI] [PubMed] [Google Scholar]
- Ayala R, Grande S, Bustelos R, Ribera C, Garcia-Sesma A, Jimenez C, et al. Obesity is an independent risk factor for pre-transplant portal vein thrombosis in liver recipients. BMC Gastroenterol. 2012;12:114. doi: 10.1186/1471-230X-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–155. doi: 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, et al. Non-alcoholic fatty liver disease in liver transplant recipients: another story of ‘seed and soil’. Am J Gastroenterol. 2010;105:613–620. doi: 10.1038/ajg.2009.717. [DOI] [PubMed] [Google Scholar]
- Siegel AB, Lim EA, Wang S, Brubaker W, Rodriguez RD, Goyal A, et al. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539–543. doi: 10.1097/TP.0b013e31825c58ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MY, Tavakol MM, Sarin A, Amirkiai SM, Rogers SJ, Carter JT, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis. 2013;9:653–658. doi: 10.1016/j.soard.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13:363–368. doi: 10.1111/j.1600-6143.2012.04318.x. [DOI] [PubMed] [Google Scholar]
- Guckelberger O. Longterm medical comorbidities and their management: hypertension/cardiovascular disease. Liver Transpl. 2009;15(Suppl. 2):75–78. doi: 10.1002/lt.21903. [DOI] [PubMed] [Google Scholar]
- Desai S, Hong JC, Saab S. Cardiovascular risk factors following orthotopic liver transplantation: predisposing factors, incidence and management. Liver Int. 2010;30:948–957. doi: 10.1111/j.1478-3231.2010.02274.x. [DOI] [PubMed] [Google Scholar]
- Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, et al. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008;14:512–525. doi: 10.1002/lt.21396. [DOI] [PubMed] [Google Scholar]


