Abstract
Background
Previous studies have shown that liver function is inhomogeneously distributed in diseased livers, and this uneven distribution cannot be compensated for if a global liver function test is used for the prediction of post-operative remnant liver function. Dynamic Gd-EOB-DTPA-enhanced magnetic resonance imaging (MRI) can assess segmental liver function, thus offering the possibility to overcome this problem.
Methods
In 10 patients with liver cirrhosis and 10 normal volunteers, the contribution of individual liver segments to total liver function and volume was calculated using dynamic Gd-EOB-DTPA-enhanced MRI. Remnant liver function predictions using a segmental method and global assessment were compared for a simulated left hemihepatectomy. For the prediction based on segmental functional MRI assessment, the estimated function of the remnant liver segments was added.
Results
Global liver function assessment overestimated the remnant liver function in 9 out of 10 patients by as much as 9.3% [median −3.5% (−9.3–3.5%)]. In the normal volunteers there was a slight underestimation of remnant function in 9 out of 10 cases [median 1.07% (−0.7–2.5%)].
Discussion
The present study underlines the necessity of a segmental liver function test able to compensate for the non-homogeneous nature of liver function, if the prediction of post-operative remnant liver function is to be improved.
Introduction
In spite of improvement in the outcome of liver surgery during the last decades, post-operative liver failure remains a feared complication and is currently the leading cause of post-operative mortality.1–3 In patients with normal liver function, a future liver remnant (FLR) of 25% is usually sufficient for maintenance of immediate post-operative function and regeneration.4,5 In these patients, decision making regarding the FLR is purely volume-based, using computed tomography (CT) or magnetic resonance imaging (MRI) volumetry. In patients with underlying parenchymal disease, for example cirrhosis, the impaired liver function has to be considered in the decision making by increasing the size of the FLR in relation to the degree of dysfunction. Various methods to assess liver functional reserve and predict the remnant function have been proposed and are used.6 These include biochemical serum liver function tests, composite scoring models such as the Child–Pugh and model for end-stage liver disease scores, but also more complex methods for dynamic assessment of liver enzyme systems such as the LiMAx® test and indocyanine green (ICG) clearance are used, yielding a quantitative assessment of global liver function.6,7 In a survey of hepato-pancreato-biliary and transplant centres, large variations in what is regarded as critical liver mass for liver resection were found.8 For healthy livers the critical size was 25% (range 15–40%) and for patients with cirrhosis 50% (range 25–90%). What is particularly striking is the large range in what respondents regard as the critical size in patients with cirrhosis. This could be as a result of the wide spectrum in disease severity in the cirrhosis population, but could also reflect a lack of confidence in and precision of the existing methods to evaluate liver function. The survey also showed large differences in the use of pre-operative metabolic tests to assess liver function.9
A prerequisite for a global liver function test or pure volumetry-based assessment to be accurate in predicting post-operative remnant liver function is that the functional capacity and disease distribution is homogeneous within the liver. Several previous studies have shown that this is not the case in liver cirrhosis.10–14 A way to overcome this problem is to use an imaging-based method that enables sampling from different parts of the liver for functional assessment in a non-invasive fashion. This principle has been applied in scintigraphic methods, either using 99MTc-GSA scintigraphy or iminodiacetic acid (IDA) compounds.15
Gadoxetic acid or Gd-EOB-DTPA (Primovist®/Eovist®; Bayer Healthcare AG, Berlin, Germany) is a gadolinium-based contrast agent for T1-weigthed MRI, used to improve the detection and characterization of liver lesions.16,17 Gadoxetic acid is hepatocyte specific in the sense that it is taken up into hepatocytes by the OATP 1B1/B3 transporter system, and excreted into bile by the ATP-dependent MRP2-complex, these transport pathways being similar to those for the uptake and excretion of ICG and the IDA substances.18–21 Gd-EOB-DTPA has therefore been proposed as a suitable substance for imaging-based liver function assessment using MRI as the imaging modality. Several studies have confirmed a correlation between liver function and various imaging parameters obtained from Gd-EOB-DTPA-enhanced MRI.22–26 In an attempt to quantitatively assess the uptake of Gd-EOB-DTPA, deconvolutional analysis has been proposed and the method previously extensively described.27 Using this algorithm, the hepatic extraction fraction (HEF), as a measurement of parenchymal function, and the input relative blood flow (irBF), as a measurement of perfusion, can be calculated. These parameters have been studied in patients with primary biliary cirrhosis, primary sclerosing cholangitis and liver cirrhosis13,28,29
The aim of this study was to demonstrate, with the use of Gd-EOB-DTPA-enhanced MRI, how non-homogeneous distribution of liver function might impact the prediction of remnant liver function if a global liver function test is used, as opposed to a segment-based functional analysis.
Patients and methods
Study subjects
Ten patients with varying degrees of alcohol- and/or viral hepatitis-induced liver cirrhosis were included. The control group consisted of the 10 normal volunteers. Informed consent was obtained from all participants prior to inclusion, and the study was approved by the Regional Ethical Review Board in Stockholm. Demographic and clinical parameters of the study subjects, including Child–Pugh scores calculated for each patient, are summarized in Table 1.
Table 1.
Study subject characteristics
| Patients (n = 10) | Controls (n = 10) | P-value | Reference | |
|---|---|---|---|---|
| Gender (m/f) | 8/2 | 5/5 | P = 0.18a | |
| Age (median, min-max) | 57 (43–61) | 32 (22–38) | P < 0.05b | |
| Mean (SD) | Mean (SD) | |||
| Bilirubin (μmol/l) | 35 (22.6) | 16 (5.9) | P < 0.05b | (<26) |
| Albumin (g/l) | 32 (7.7) | 42 (2.5) | P < 0.05b | (36–48) |
| Creatinine (μmol/l) | 91 (22.8) | 80 (15.3) | P = 0.22 | [<100 (men); <90 (women)] |
| PK-INR (INR) | 1.4 (0.29) | 1.1 (0.07) | P < 0.05b | (<1.2) |
| ALP (μkat/L) | 2.3 (1.04) | 1.2 (0.61) | P < 0.05b | (<1.9) |
| ALT (μkat/L) | 0.97 (0.69) | 0.51 (0.31) | P = 0.08 | [<1.20 (men); <0.76 (women)] |
| AST (μkat/L) | 1.47 (1.1) | 0.34 (0.09) | P < 0.05b | [<0.76 (men); <0.61 (women)] |
| Disease characteristics of patients | ||||
| Median (min–max) | ||||
| CPS | 7 (5–12) | |||
| Child–Pugh class | (n) | |||
| A | 4 | |||
| B | 5 | |||
| C | 1 |
Fisher's exact test.
Mann–Whitney U-test.
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CPS, Child–Pugh score; PK–INR, prothrombin complex–International normalized ratio.
MRI procedure
All subjects were instructed to fast for at least 4 h prior to the examination. MR imaging was performed using a Philips Intera 1.5 Tesla (T) scanner (Best, The Netherlands), with a Philips four-channel SENSE body coil. The dynamic contrast-enhanced sequence was performed using a T1-weighted 3-D spoiled-gradient-echo sequence [repetition time/echo time/flip angle 4.1 ms/2.0 ms/10°, field of view (FOV) = 415 mm, acquisition matrix resolution 192 × 192, reconstruction matrix 256 × 256, 40 slices, slice thickness 10 mm with 5 mm overlap and SENSE factor R = 2]. These imaging parameters yielded a volume element (voxel) volume of approximately 13 mm3 (1.62 × 1.62 × 5 mm). A voxel is the three-dimensional equivalent of a pixel (a picture element), being the smallest unit in a raster image. The volume was examined in a single breath-hold at 35 different time points (12 s scan time per acquired volume) and the subjects were asked to hold their breath at the same depth during each acquisition. Three volumes were acquired pre-contrast for calculations of baseline signal intensity, followed by repetitive sampling with a step-wise increase in sampling intervals up to a total sampling time of 45 min. The control group had been examined with a study protocol implying a total sampling time of 90 min, but in this study only acquisitions up to 45 min post contrast injection were used, meaning that the sampling time points and imaging parameters were identical in the two groups. A dose of 0.1 ml/kg Gd-EOB-DTPA 0.25 mmol/ml was injected into the anterior cubital vein, coinciding with the start of the fourth acquired volume. The contrast was injected using a power injector (Spectris MR injector System; Medrad, Pittsburgh, PA, USA), at an infusion rate of 2 ml/s, followed immediately by a bolus of 20 ml of saline (NaCl 0.9%) at the same infusion rate.
Liver segmentation and image analysis
From a seed-point placed in the inferior vena cava, lines were drawn in the plane of the right hepatic vein, middle hepatic vein and the falciform ligament/umbilical fissure creating the vertical inter-sectorial and inter-segmental boundaries. Segment I was manually outlined in every slice where it was visible according to the anatomical landmarks as described by Dodds et al.30 The horizontal inter-segmental plane was defined as being in the image slice where the division of the portal vein into the left and right portal branches was identified. Fifty per cent of the voxels in this slice was regarded as representative of segments 2, 4a, 7 and 8, and 50% as part of segments 3, 4b, 5 and 6. The liver contour was manually outlined in every slice, with the major hilar structures being excluded. The volumes of the voxels within the liver boundaries were added to obtain total and segmental liver volumes for each subject. Image analysis and subsequent calculations were performed using in-house software written in MATLAB® (Mathworks, Novi, MI, USA).
Liver function parameters
HEF and irBF were calculated for every voxel within the liver using Fourier transforms as described in a previous study.27 The input function was defined by a region of interest (ROI) placed in the spleen. To ensure that the ROI in the spleen was truly representative of the blood content over the entire acquisition period, it was manually adjusted when needed. A HEF above 0.7 or irBF above 1 was regarded as artefact and omitted from subsequent analysis. Voxels representing vascular structures were expected to have high perfusion and therefore high irBF values, and therefore voxels with an irBF above a user-defined threshold were regarded as representing vessels and not parenchyma and consequently omitted from analysis of parenchymal function and volume. Total liver volumes including vessel volume, as well as parenchymal volumes excluding vessels, were calculated. Total liver parenchymal function and parenchymal volume were obtained by adding the individual HEF and volume of all parenchymal voxels within the liver boundaries and expressed as HEFml. For every segment, the volume and functional capacity were calculated in the same way by adding the volume and function of all parenchymal voxels within the predefined segmental borders. The global median HEF was obtained by calculating the median HEF of all parenchymal voxels within the liver boundaries.
Hepatectomy simulation
A left hemi-hepatectomy implying the removal of segments 2, 3 and 4 was simulated and residual post-operative function as predicted by global assessment versus segmental assessment was compared. The residual function as predicted by an arbitrarily chosen global liver function test was calculated by deducting the percentage of the total hepatocyte extraction capacity (HEFml) equivalent to the percentage of the volume of liver parenchyma resected. For the simulation of the prediction based on segmental functional assessment, the estimated function (in HEFml) of the remaining liver segments was added. For example, if resection implied removal of 45% of the liver volume, the predicted remnant liver function using global assessment was calculated as being 55% of the total liver function, whereas the measured function in HEFml of segments 1, 5, 6, 7, and 8 was added for the segmental function-based prediction.
Statistical analysis
Descriptive statistics were used to present clinical characteristics of the study subjects and the quantitative liver function parameters. Non-parametric tests (Mann–Whitney U-test and Fisher's exact test) were used for group comparisons. The significance threshold was set to α = 0.05. STATA 10 (StataCorp, College Station, TX, USA) was used for the statistical analyses.
Results
The results of liver volume measurements and of the quantitative functional parameters in both groups are shown in Table 2. There were no significant differences regarding total liver volume, but when vascular voxels were not included, the patient group had significantly larger parenchymal volume. In spite of the larger liver volume in the patient group, the overall parenchymal function expressed as HEFml was significantly lower, as was the median HEF, indicating a decreased parenchymal functional capacity.
Table 2.
Results of liver function and volume analysis
| Controls | Patients | P-valuea | |||
|---|---|---|---|---|---|
| Median | (min–max) | Median | (min–max) | ||
| Total liver volume (ml) | 1463.0 | (1076–1724) | 1577.0 | (1357–1886) | P = 0.13 |
| Parenchymal volume (ml) | 1250.0 | (957–1642) | 1435.0 | (1225–1774) | P < 0.05 |
| Total functional capacity (HEFml) | 267.0 | (215–383) | 171.0 | (53–341) | P < 0.05 |
| Standardized functional capacitcy (HEFml/m2)b | 141.0 | (128–222) | 84.0 | (32–172) | P < 0.05 |
| Global median HEF | 0.22 | (0.19–0.28) | 0.10 | (0.02–0.20) | P < 0.05 |
Mann–Whitney U-test.
Total functional capacity in HEFml/body surface area.
The results of the simulated hemi-hepatectomy are presented in Fig. 1 and Table 3. In the normal volunteers there was a slight underestimation of residual function in 9 out of 10 patients [median 1.07% (range −0.7–2.5%)]. Global liver function assessment overestimated the remnant liver function in 9 out of 10 patients by as much as 9.3% in absolute numbers [median −3.5% (range −9.3–3.5%)], and the difference between the groups was statistically significant. If the actual value of the residual function as estimated by the segmental method is used as a reference, the miscalculation or error using the global assessment was as much as 26% (9.3% out of 35.2%), as shown in Table 3. There seemed to be a more pronounced function-to-volume discrepancy in the Child–Pugh B & C patients compared with those with Child–Pugh A, as presented in Fig. 2. Formal statistical testing was not carried out owing to the low number of subjects in each group.
Figure 1.
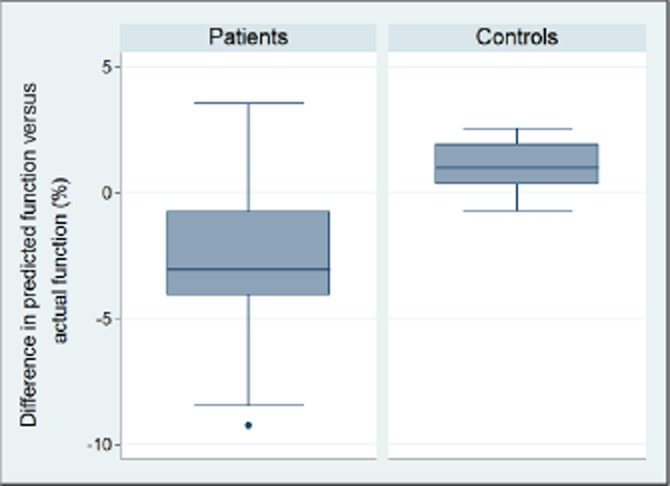
The difference in predicted function versus actual function based on a segmental assessment in the patient and the control group. The difference was significantly larger in the patient group with two patients showing an almost 10% difference
Table 3.
Results of the simulated left-sided hemihepatectomy
| Patient/Child–Pugh class | 1(A) | 2(A) | 3(B) | 4(A) | 5(B) | 6(B) | 7(C) | 8(A) | 9(B) | 10(B) |
|---|---|---|---|---|---|---|---|---|---|---|
| Liver volume (ml) | 1629 | 1331 | 1750 | 1774 | 1410 | 1243 | 1461 | 1225 | 1573 | 1332 |
| Total liver function (HEFml) | 341 | 210 | 174 | 330 | 82 | 169 | 115 | 262 | 53 | 168 |
| Volume of resection (%) | 55 | 52 | 46 | 37 | 56 | 51 | 48 | 46 | 45 | 30 |
| Predicted RLF (HEFml) | 155 | 102 | 93 | 208 | 36 | 83 | 60 | 142 | 29 | 118 |
| Actual RLF (HEFml) | 142 | 94 | 93 | 220 | 29 | 69 | 57 | 140 | 27 | 115 |
| Predicted RLF (%) | 45.4 | 48.5 | 53.6 | 63.1 | 44.4 | 49.3 | 52.4 | 54.3 | 54.7 | 70.3 |
| Actual RLF (%) | 41.6 | 44.7 | 53.3 | 66.6 | 35.2 | 40.8 | 50.0 | 53.5 | 50.6 | 68.6 |
| Difference (%) (actual-predicted) | −3.8 | −3.8 | −0.3 | 3.5 | −9.3 | −8.5 | −2.4 | −0.8 | −4.1 | −1.7 |
| Controls | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Liver volume (ml) | 957 | 1241 | 1259 | 1401 | 1642 | 1053 | 973 | 1474 | 1132 | 1310 |
| Total liver function (HEFml) | 229 | 266 | 353 | 269 | 383 | 219 | 215 | 381 | 260 | 345 |
| Volume of resection (%) | 40 | 26 | 37 | 35 | 28 | 38 | 32 | 32 | 37 | 31 |
| Predicted RLF (HEFml) | 138 | 196 | 220 | 175 | 274 | 137 | 146 | 258 | 165 | 239 |
| Actual RLF (HEFml) | 139 | 201 | 228 | 178 | 275 | 139 | 149 | 259 | 171 | 236 |
| Predicted RLF (%) | 60.2 | 73.8 | 62.5 | 64.9 | 71.5 | 62.4 | 67.7 | 67.7 | 63.2 | 69.3 |
| Actual RLF (%) | 60.8 | 75.7 | 64.6 | 66.0 | 71.8 | 63.2 | 69.5 | 67.9 | 65.7 | 68.5 |
| Difference (%) (actual-predicted) | 0.6 | 1.9 | 2.1 | 1.2 | 0.3 | 0.8 | 1.8 | 0.2 | 2.5 | −0.7 |
RLF, remnant liver function.
Figure 2.
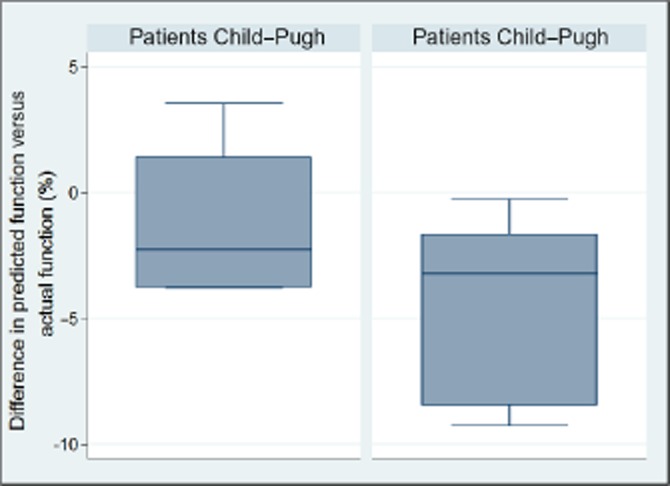
There seemed to be a more pronounced difference in predicted versus actual function in patients with more advanced liver disease
Discussion
In patients with diseased liver considered for liver resection, volume-based decision making alone is not sufficient. The degree of dysfunction has to be accounted for in the decision-making algorithm. All methods currently used in clinical practice for functional analysis give a global assessment of liver function, and these tests are not able to detect and correct for eventual inhomogeneous distribution of liver function.
Certainly, not all the patients included in this study could be candidates for a liver resection, should an indication arise, but the hepatectomy simulation does however demonstrate that relying on a global test in patients with significant inhomogeneity may result in over- or under-estimation of liver function. A difference between the left and right livers regarding the volume/function discrepancy was observed. There seems to be an overestimation of function in a right hemiliver, as opposed to an underestimation of function in a left hemiliver. Both the scenarios have consequences for the patient as overestimating liver function might result in liver failure, whereas underestimation might preclude patients from potential curative treatment by overestimating the risk for post-operative failure. Even if global tests are likely to be become more comprehensive, maybe even giving information on multiple metabolic pathways, the results in this study argue strongly in favour of incorporating data on segmental liver function in the pre-operative assessment of liver functional reserve.
Liver disease in patients considered for a liver resection has previously almost exclusively been limited to cirrhosis in patients with hepatocellular carcinoma, but with the increasing use of neo-adjuvant chemotherapy for colorectal cancer liver metastases, patients presenting with sinusoidal obstruction syndrome and chemotherapy-associated steatohepatitis are frequently encountered in the setting of liver surgery.3,31,32 As obesity is becoming more and more prevalent, the metabolic syndrome-associated conditions of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis, which may in turn lead to liver cirrhosis, have become the most common chronic liver diseases in the western world.33,34 Both chemotherapy-induced liver injury and obesity-associated hepatic disease have been shown to be non-homogeneously distributed in the liver.13,28,35–38
In conclusion, this study demonstrates the non-homogeneous distribution of liver function in cirrhotic patients, and probably not only in this group, regional function assessment has to be added to the equation in order to minimize the risk of post-operative liver failure and possibly death. Patients considered for a liver resection should not be subjected to unnecessary risks by overestimating liver function in the residual liver. Nor should patients that are in fact eligible for surgery be excluded from potential curative treatment owing to underestimation of residual function.
Acknowledgments
The authors wish to express their gratitude towards RT Yvonne Eriksson-Alm for her contributions to the project.
Financial support
Financial support was provided through the regional agreement on medical training and clinical research (ALF) between the Stockholm County Council and the Karolinska Institutet, from the Juhlin Foundation, Karolinska Institutet and from research grants from Karolinska Institutet.
Conflict of interest
Henrik Nilsson, Eduard Jonas and Lennart Blomqvist have received lecturing honoraria and consultancy fees from Bayer Healthcare AG.
References
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50-50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. ; discussion 8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capussotti L, Vigano L, Giuliante F, Ferrero A, Giovannini I, Nuzzo G. Liver dysfunction and sepsis determine operative mortality after liver resection. Br J Surg. 2009;96:88–94. doi: 10.1002/bjs.6429. [DOI] [PubMed] [Google Scholar]
- Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. ; discussion 62-4. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- Morris-Stiff G, Gomez D, Prasad R. Quantitative assessment of hepatic function and its relevance to the liver surgeon. J Gastrointest Surg. 2009;13:374–385. doi: 10.1007/s11605-008-0564-1. [DOI] [PubMed] [Google Scholar]
- Stockmann M, Lock JF, Riecke B, Heyne K, Martus P, Fricke M, et al. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann Surg. 2009;250:119–125. doi: 10.1097/SLA.0b013e3181ad85b5. [DOI] [PubMed] [Google Scholar]
- Breitenstein S, Apestegui C, Petrowsky H, Clavien PA. ‘State of the art’ in liver resection and living donor liver transplantation: a worldwide survey of 100 liver centers. World J Surg. 2009;33:797–803. doi: 10.1007/s00268-008-9878-0. [DOI] [PubMed] [Google Scholar]
- Bennett JJ, Blumgart LH. Assessment of hepatic reserve prior to hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:10–15. doi: 10.1007/s00534-004-0950-3. [DOI] [PubMed] [Google Scholar]
- de Graaf W, van Lienden KP, van Gulik TM, Bennink RJ. (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med. 2010;51:229–236. doi: 10.2967/jnumed.109.069724. [DOI] [PubMed] [Google Scholar]
- Imaeda T, Kanematsu M, Asada S, Seki M, Doi H, Saji S. Utility of Tc-99m GSA SPECT imaging in estimation of functional volume of liver segments in health and liver diseases. Clin Nucl Med. 1995;20:322–328. doi: 10.1097/00003072-199504000-00008. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Onda M, Tajiri T, Kim DY. Hepatic lobar differences in progression of chronic liver disease: correlation of asialoglycoprotein scintigraphy and hepatic functional reserve. Hepatology. 1997;25:828–832. doi: 10.1002/hep.510250407. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Blomqvist L, Douglas L, Nordell A, Janczewska I, Naslund E, et al. Gd-EOB-DTPA-enhanced MRI for the assessment of liver function and volume in liver cirrhosis. Br J Radiol. 2013;86:20120653. doi: 10.1259/bjr.20120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- de Graaf W, Bennink RJ, Vetelainen R, van Gulik TM. Nuclear imaging techniques for the assessment of hepatic function in liver surgery and transplantation. J Nucl Med. 2010;51:742–752. doi: 10.2967/jnumed.109.069435. [DOI] [PubMed] [Google Scholar]
- Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology. 2004;230:266–275. doi: 10.1148/radiol.2301020269. [DOI] [PubMed] [Google Scholar]
- Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci. 2007;6:43–52. doi: 10.2463/mrms.6.43. [DOI] [PubMed] [Google Scholar]
- de Graaf W, Hausler S, Heger M, van Ginhoven TM, van Cappellen G, Bennink RJ, et al. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol. 2011;54:738–745. doi: 10.1016/j.jhep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Keiser M, Oswald S, Kuhn J, Jia J, Grube M, et al. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos. 2010;38:1024–1028. doi: 10.1124/dmd.110.032862. [DOI] [PubMed] [Google Scholar]
- Narita M, Hatano E, Arizono S, Miyagawa-Hayashino A, Isoda H, Kitamura K, et al. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol. 2009;44:793–798. doi: 10.1007/s00535-009-0056-4. [DOI] [PubMed] [Google Scholar]
- Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421–429. doi: 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Bae KE, Kim SY, Lee SS, Kim KW, Won HJ, Shin YM, et al. Assessment of hepatic function with Gd-EOB-DTPA-enhanced hepatic MRI. Dig Dis. 2012;30:617–622. doi: 10.1159/000343092. [DOI] [PubMed] [Google Scholar]
- Sourbron S, Sommer WH, Reiser MF, Zech CJ. Combined quantification of liver perfusion and function with dynamic gadoxetic acid-enhanced MR imaging. Radiology. 2012;263:874–883. doi: 10.1148/radiol.12110337. [DOI] [PubMed] [Google Scholar]
- Utsunomiya T, Shimada M, Hanaoka J, Kanamoto M, Ikemoto T, Morine Y, et al. Possible utility of MRI using Gd-EOB-DTPA for estimating liver functional reserve. J Gastroenterol. 2012;47:470–476. doi: 10.1007/s00535-011-0513-8. [DOI] [PubMed] [Google Scholar]
- Verloh N, Haimerl M, Zeman F, Schlabeck M, Barreiros A, Loss M, et al. Assessing liver function by liver enhancement during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MRI at 3 Tesla. Eur Radiol. 2014;24:1013–1019. doi: 10.1007/s00330-014-3108-y. [DOI] [PubMed] [Google Scholar]
- Yamada A, Hara T, Li F, Fujinaga Y, Ueda K, Kadoya M, et al. Quantitative evaluation of liver function with use of gadoxetate disodium-enhanced MR imaging. Radiology. 2011;260:727–733. doi: 10.1148/radiol.11100586. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Nordell A, Vargas R, Douglas L, Jonas E, Blomqvist L. Assessment of hepatic extraction fraction and input relative blood flow using dynamic hepatocyte-specific contrast-enhanced MRI. J Magn Reson Imaging. 2009;29:1323–1331. doi: 10.1002/jmri.21801. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Blomqvist L, Douglas L, Nordell A, Jacobsson H, Hagen K, et al. Dynamic gadoxetate-enhanced MRI for the assessment of total and segmental liver function and volume in primary sclerosing cholangitis. J Magn Reson Imaging. 2014;39:879–886. doi: 10.1002/jmri.24250. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Blomqvist L, Douglas L, Nordell A, Jonas E. Assessment of liver function in primary biliary cirrhosis using Gd-EOB-DTPA-enhanced liver MRI. HPB. 2010;12:567–576. doi: 10.1111/j.1477-2574.2010.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds WJ, Erickson SJ, Taylor AJ, Lawson TL, Stewart ET. Caudate lobe of the liver: anatomy, embryology, and pathology. AJR Am J Roentgenol. 1990;154:87–93. doi: 10.2214/ajr.154.1.2104732. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860–868. doi: 10.1007/s11605-007-0149-4. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- Pessaux P, Chenard MP, Bachellier P, Jaeck D. Consequences of chemotherapy on resection of colorectal liver metastases. J Visc Surg. 2010;147:e193–e201. doi: 10.1016/j.jviscsurg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- Larson SP, Bowers SP, Palekar NA, Ward JA, Pulcini JP, Harrison SA. Histopathologic variability between the right and left lobes of the liver in morbidly obese patients undergoing Roux-en-Y bypass. Clin Gastroenterol Hepatol. 2007;5:1329–1332. doi: 10.1016/j.cgh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Arun J, Jhala N, Lazenby AJ, Clements R, Abrams GA. Influence of liver biopsy heterogeneity and diagnosis of nonalcoholic steatohepatitis in subjects undergoing gastric bypass. Obes Surg. 2007;17:155–161. doi: 10.1007/s11695-007-9041-2. [DOI] [PubMed] [Google Scholar]
- Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]


