Abstract
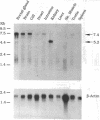
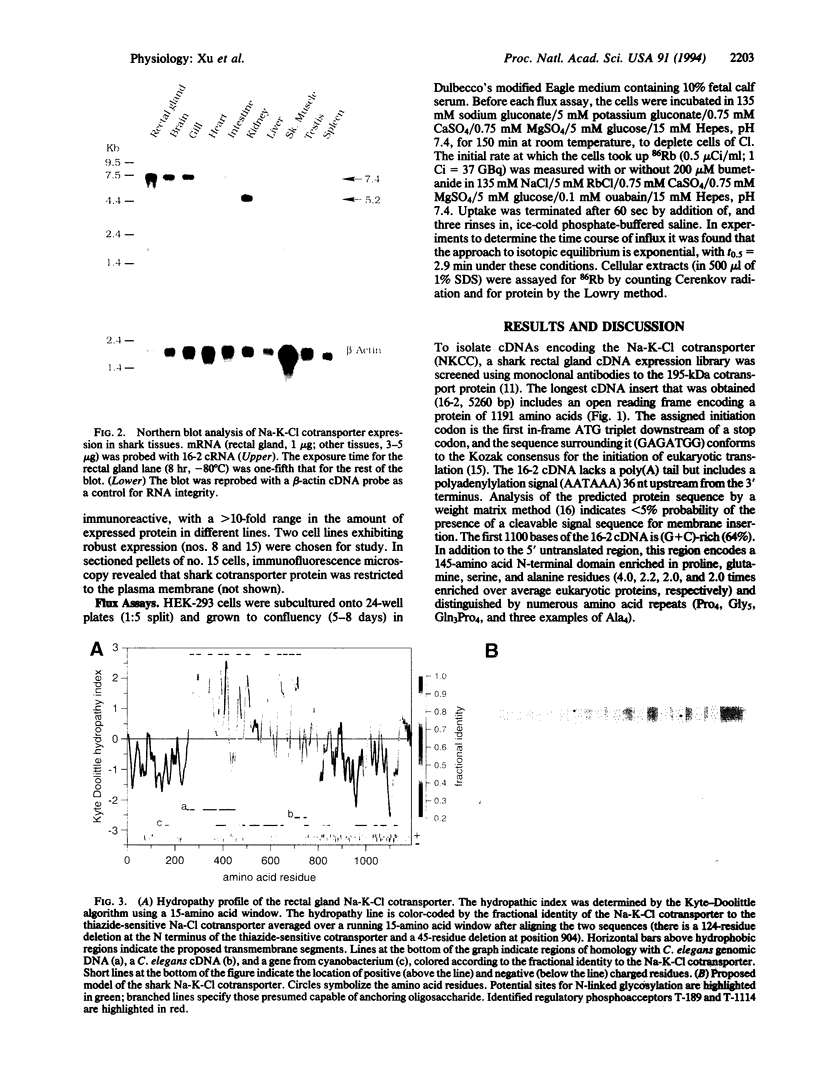
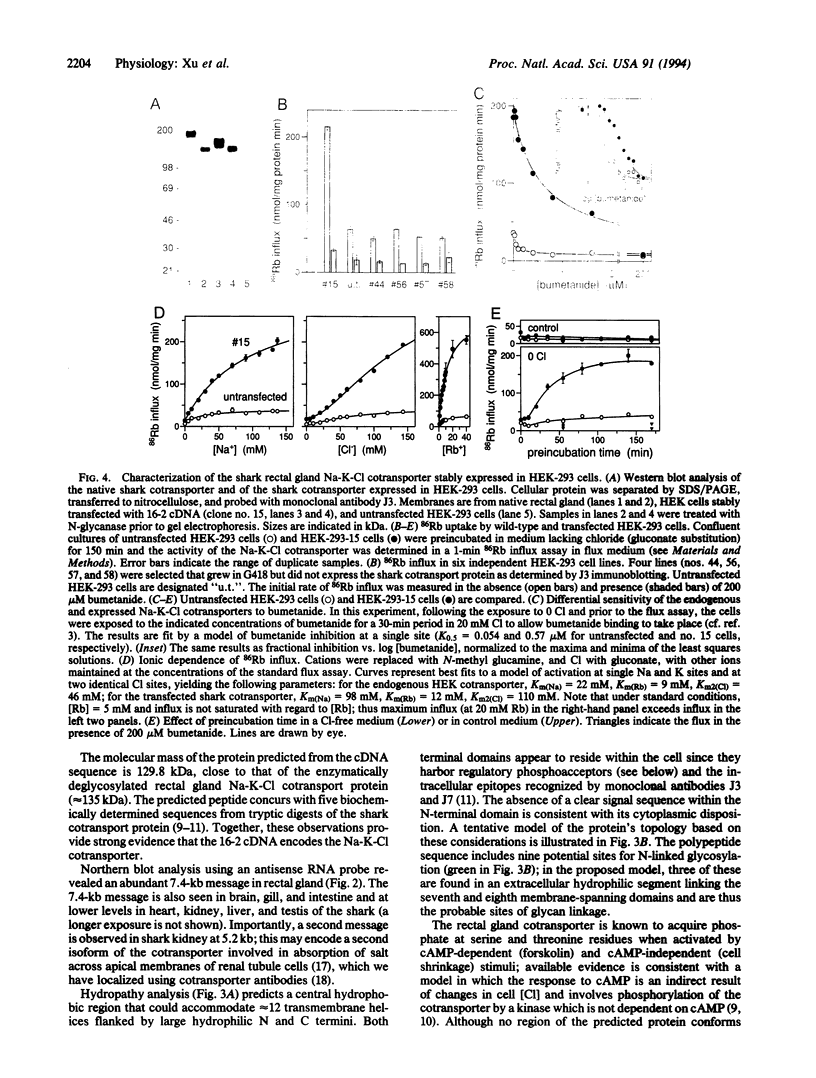
By mediating the coupled movement of Na, K, and Cl ions across the plasma membrane of most animal cells, the bumetanide-sensitive Na-K-Cl cotransporter (NKCC) plays a vital role in the regulation of ionic balance and cell volume. The transporter is a central element in the process of vectorial salt transport in secretory and absorptive epithelia. A cDNA encoding a Na-K-Cl cotransport protein was isolated from a shark rectal gland library by screening with monoclonal antibodies to the native shark cotransporter. The 1191-residue protein predicted from the cDNA sequence has 12 putative transmembrane domains flanked by large cytoplasmic N and C termini. Regulatory phosphoacceptor residues in isolated peptides are identified as Thr-189 and Thr-1114 in the predicted sequence. Northern blot analysis identified a 7.4-kb mRNA in rectal gland and most other shark tissues; a 5.2-kb mRNA was restricted to shark kidney. Homology with an uncharacterized gene from Caenorhabditis elegans and with the thiazide-sensitive Na-Cl cotransporter of flounder urinary bladder was found over most of the coding region; shorter stretches of homology were found with a C. elegans cDNA and with an uncharacterized gene of cyanobacterium. Human HEK-293 cells have been stably transfected with the shark cDNA and shown to express Na-K-Cl cotransport activity with the bumetanide sensitivity of the shark protein. The expressed transporter is functionally quiescent in the host cells and can be activated by depleting the cells of chloride.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Altamirano A. A., Russell J. M. Osmotic stimulation of Na(+)-K(+)-Cl- cotransport in squid giant axon is [Cl-]i dependent. Am J Physiol. 1990 Apr;258(4 Pt 1):C749–C753. doi: 10.1152/ajpcell.1990.258.4.C749. [DOI] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius F., Skou J. C. The effect of cytoplasmic K+ on the activity of the Na+/K(+)-ATPase. Biochim Biophys Acta. 1991 Aug 26;1067(2):227–234. doi: 10.1016/0005-2736(91)90048-d. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Hebert S. C. Diluting segment in kidney of dogfish shark. I. Localization and characterization of chloride absorption. Am J Physiol. 1990 Feb;258(2 Pt 2):R398–R408. doi: 10.1152/ajpregu.1990.258.2.R398. [DOI] [PubMed] [Google Scholar]
- Gamba G., Saltzberg S. N., Lombardi M., Miyanoshita A., Lytton J., Hediger M. A., Brenner B. M., Hebert S. C. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2749–2753. doi: 10.1073/pnas.90.7.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögelein H., Greger R., Schlatter E. Potassium channels in the basolateral membrane of the rectal gland of Squalus acanthias. Regulation and inhibitors. Pflugers Arch. 1987 Jun;409(1-2):107–113. doi: 10.1007/BF00584756. [DOI] [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Lytle C., Forbush B., 3rd Na-K-Cl cotransport in the shark rectal gland. II. Regulation in isolated tubules. Am J Physiol. 1992 Apr;262(4 Pt 1):C1009–C1017. doi: 10.1152/ajpcell.1992.262.4.C1009. [DOI] [PubMed] [Google Scholar]
- Lytle C., Forbush B., 3rd The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem. 1992 Dec 15;267(35):25438–25443. [PubMed] [Google Scholar]
- Lytle C., Xu J. C., Biemesderfer D., Haas M., Forbush B., 3rd The Na-K-Cl cotransport protein of shark rectal gland. I. Development of monoclonal antibodies, immunoaffinity purification, and partial biochemical characterization. J Biol Chem. 1992 Dec 15;267(35):25428–25437. [PubMed] [Google Scholar]
- Marshall J., Martin K. A., Picciotto M., Hockfield S., Nairn A. C., Kaczmarek L. K. Identification and localization of a dogfish homolog of human cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1991 Nov 25;266(33):22749–22754. [PubMed] [Google Scholar]
- Palfrey H. C., Silva P., Epstein F. H. Sensitivity of cAMP-stimulated salt secretion in shark rectal gland to "loop" diuretics. Am J Physiol. 1984 Mar;246(3 Pt 1):C242–C246. doi: 10.1152/ajpcell.1984.246.3.C242. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., Carosi S. L. Properties of single- and double-barreled Cl channels of shark rectal gland in planar bilayers. J Membr Biol. 1992 Feb;126(1):67–73. doi: 10.1007/BF00233461. [DOI] [PubMed] [Google Scholar]
- Sulston J., Du Z., Thomas K., Wilson R., Hillier L., Staden R., Halloran N., Green P., Thierry-Mieg J., Qiu L. The C. elegans genome sequencing project: a beginning. Nature. 1992 Mar 5;356(6364):37–41. doi: 10.1038/356037a0. [DOI] [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A., Hillier L., Durbin R., Green P., Shownkeen R., Halloran N. A survey of expressed genes in Caenorhabditis elegans. Nat Genet. 1992 May;1(2):114–123. doi: 10.1038/ng0592-114. [DOI] [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]