Abstract
A simple and efficient synthesis of novel, D-ring substituted estrone derivatives containing a 16α-triazolyl moiety is described. Two epimeric azido alcohols (16α-azido-17α-hydroxy and 16α-azido-17β-hydroxy) of estra-1,3,5(10)-triene-3-methyl ether were prepared, followed by copper(I)-catalyzed azide-alkyne cycloaddition with various terminal alkynes. The steroidal triazoles were obtained in high yields and their activities against three human cancer cell lines (HeLa, MCF7 and A431) were screened. The most effective analogs were submitted to additional experiments in order to characterize their antiproliferative properties. As evidenced by flow cytometry, the selected steroids induced a disturbance in the HeLa cell cycle in a concentration- and exposure-dependent manner, through an increase of the hypodiploid population (subG1) and a cell cycle arrest in the G2/M phase. A noncancerous human fibroblast cell line (MRC5) was used to determine the selectivities of these compounds. Fluorescent microscopy after Hoechst 33258 - propidium iodide (HOPI) double staining revealed nuclear condensation and disturbed cell membrane integrity. The enhanced activities of caspase-3 and caspase-9 without activation of caspase-8 in the treated cells indicated the activation of the intrinsic pathway of apoptosis. The levels of cell cycle regulators (CDK1, cyclin B1/B2 and cdc25B) were decreased and the ratio Bax/Bcl-2 was increased 24 h after the treatment of HeLa cells (determined at an mRNA level by means of an RT-PCR technique). Under the same conditions, two agents elicited substantially increased degrees of phosphorylation of stathmin, as evidenced by Western blotting. The presented results demonstrate that these steroids can be regarded as appropriate structural scaffolds for the design and synthesis of further steroid analogs as innovative drug candidates with good efficacy.
Introduction
Since cancerous disorders are the second leading cause of death worldwide, following cardiovascular diseases, improvement of their treatment is currently one of the greatest challenges. A survey of epidemiological data from 184 countries suggested that the global burden of cancer will increase to 23.6 million new cases each year by 2030, an increase of 68% compared with 2012 [1].
Steroids are a group of compounds that play versatile roles as anticancer agents. In hormone-dependent tumors such as breast, uterine, ovarian, prostate and endometrial cancers, the overexpression of steroid receptors is involved in enhanced cell proliferation. Different approaches have been devised to reduce the growth-stimulating hormonal response of cancer cells. These compounds are classified as steroidal antihormonal/antiproliferative anticancer agents. Additionally, a broad variety of steroidal molecules have either been isolated from natural sources or rationally designed and synthetized, and have been reported to exhibit efficacy against cancer cells through nonhormonal mechanisms. Cytotoxic steroids exert their actions on various molecular targets (e.g. microtubules or topoisomerase), usually leading to cell cycle blockade and apoptosis [2].
Since the discovery of Cu-catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC) [3, 4], this technique has found numerous applications in a wide range of disciplines, including pharmaceutical sciences [5–8]. Certain advantageous properties, including versatility, regiospecificity, lack of byproducts and high conversions, have made click chemistry an ideal tool for the synthesis of compound libraries available for initial screening and for structure–activity profiling. Not surprisingly, a number of compounds containing a triazole moiety have been reported to exert biological activity, including antibacterial [9], antiallergic [10] and anti-HIV [11] effects. Introduction of a triazole ring at position 3 of the natural triterpene betulinic acid resulted in a set of compounds with considerable antiproliferative potency and proapoptotic capacity [12]. The introduction of a triazole moiety into the podophyllotoxin skeleton yielded conjugates with significant topoisomerase-II-inhibiting activity, and some of these new compounds proved more potent than the clinically used etoposide [13].
The synthesis of steroidal heterocycles has also attracted considerable interest in view of their valuable pharmacological activities [14, 15]. Steroidal azoles have been described as potent inhibitors of 17α-hydroxylase-C17,20-lyase (CYP17), which can block androgen synthesis at an early stage, and may therefore be of use in the treatment of prostatic carcinoma [16, 17]. Furthermore, some heterocyclic derivatives have been found to exert strong inhibitory effects on 5α-reductases [18].
Banday et al. recently reported the syntheses of some 21-triazoles of pregnenolone as potent anticancer agents through a click chemistry approach [19]. In this regard, we have demonstrated that a number of triazolyl androstanes can exert direct cytostatic effects on human cancer cell lines in vitro [20–22]. Although the introduction of substituted triazole rings at position 17 of the estrane skeleton has so far met with only limited success as concerns the antiproliferative activity [23], the synthetic modification of compounds in the estrone series still seems to provide excellent possibilities in the search for novel derivatives with noteworthy biological effects [24].
A triazole ring has been successfully utilized as a linker for the preparation of estradiol-containing agents based on anticancer natural products. The most active conjugate inhibited the growth of cancer cell lines at submicromolar concentrations, exerted disruption of the microtubule network, and disturbed the cell cycle distribution of MCF7 cells and the induction of apoptosis. These properties were explained by the downregulation of cyclin-dependent kinase 1 (CDK1) and the upregulation of crucial tumor suppressors (p21 and p53) [25].
Thus, we set out to devise an effective route for the preparation of estrone-derived triazoles containing the heteroring at position 16, via CuAAC. Although determination of the affinities for the hormonal receptor did not fall within the scope of the present study, the presence of a methoxy group instead of a hydroxy group on C-3 and the steric bulk caused by the incorporated heteroring on C-16 are considered to influence the ability of these molecules to bind to the hormone receptor, thereby preventing the development of a significant estrogenic effect. All of the prepared compounds were screened in vitro for their activities against three human cancer cell lines (HeLa, MCF7 and A431). The most effective four compounds were selected for additional experiments with the aim of characterizing their antiproliferative properties.
Experimental
General methods
Reagents and materials were obtained from commercial suppliers and were used without purification. All solvents were distilled immediately prior to use. Reactions were monitored by TLC on Kieselgel-G (Merck Si 254 F) layers (0.25 mm thick). Spots were detected by spraying with 5% phosphomolybdic acid in 50% aqueous phosphoric acid and observed by illumination at 254 and 365 nm. Flash chromatography: Merck silica gel 60, 40–63 μm. Melting points (mps) were determined on a Kofler block and are uncorrected. Elemental analysis data were obtained with a Perkin Elmer CHN analyzer model 2400. IR spectra were recorded on a Perkin-Elmer FT-IR Spectrum 100. NMR spectra were recorded at room temperature with a Bruker DRX 500 instrument at 500 MHz (1H NMR) or 125 MHz (13C NMR). Chemical shifts are reported in ppm (δ scale), and coupling constants (J) in Hz. For the determination of multiplicities, the J-MOD pulse sequence was used. Automated flow injection analyses were performed by using an HPLC/MSD system. The system comprised an Agilent 1100 micro vacuum degasser, a quaternary pump, a micro-well plate autoinjector and a 1946A MSD equipped with an electrospray ion source (ESI) operated in positive ion mode. The ESI parameters were: nebulizing gas N2, at 35 psi; drying gas N2, at 350°C and 12 L/min; capillary voltage 3000 V; and fragmentor voltage 70 V. The MSD was operated in scan mode with the mass range m/z 60−620. Samples (0.2 μL) were injected with an automated needle wash directly into the solvent flow (0.3 mL/min) of MeCN/H2O 70:30 (v/v) supplemented with 0.1% formic acid. The system was controlled by Agilent LC/MSD Chemstation software.
Chemical syntheses
16α-Azido-3-methoxyestra-1,3,5(10)-trien-17α-ol (2) and 16α-azido-3-methoxyestra-1,3,5(10)-trien-17β-ol (3). Compound 1 (16α-azido-3-methoxyestra-1,3,5(10)-trien-17-one; 9.76 g, 30 mmol) was dissolved in a mixture of CH2Cl2 (60 mL) and MeOH (240 mL), the solution was cooled in an ice-bath to 15 οC, and KBH4 (6.5 g, 120.5 mmol) was added in small portions. The mixture was allowed to stand for 20 min, then poured into water (500 mL), neutralized with concentrated AcOH and extracted with CH2Cl2 (3 × 50 mL). The combined organic layers were washed with water, dried over Na2SO4 and evaporated in vacuo. The residual crude product was chromatographed on silica gel with CH2Cl2 to obtain 2 as a white solid (4.1 g, 42%), mp 95–98°C [97–99°C [26]], 1H NMR (CDCl3): δ H 0.76 (s, 3H, 18-H3), 2.27 (m, 1H, 9-H), 2.86 (m, 2H, 6-H2), 3.75 (d, 1H, J = 5.0 Hz, 17-H), 3.79 (s, 3H, OMe), 4.20 (m, 1H, 16-H), 6.63 (d, 1H, J = 2.0 Hz, 4-H), 6.72 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.21 (d, 1H, J = 8.5 Hz, 1-H); 13C NMR (CDCl3):δ C 17.1 (C-18), 25.7, 28.0, 29.7, 31.0, 31.1, 38.7 (C-8), 43.4 (C-9), 45.6 (C-13), 46.8 (C-14), 55.2 (OMe), 63.4 (C-16), 79.3 (C-17), 111.5 (C-2), 113.8 (C-4), 126.2 (C-1), 132.4 (C-10), 137.8 (C-5), 157.5 (C-3); Anal. calcd. for C19H25N3O2: C, 69.70; H, 7.70. Found: C, 69.56; H, 7.76.
Continued elution with CH2Cl2 resulted in 3 as a white solid (5.3 g, 54%), mp 114–116°C [112–114°C [26]], 1H NMR (CDCl3): δ H 0.83 (s, 3H, 18-H3), 2.24 (m, 1H, 9-H), 2.86 (m, 2H, 6-H2), 3.63 (d, 1H, J = 6.5 Hz, 17-H), 3.78 (s, 3H, OMe), 3.82 (m, 1H, 16-H), 6.64 (d, 1H, J = 2.0 Hz, 4-H), 6.73 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.20 (d, 1H, J = 8.5 Hz, 1-H); 13C NMR (CDCl3): δ C 11.9 (C-18), 25.8, 27.1, 29.6, 30.5, 36.3, 38.2, 43.7, 43.8 (C-13), 48.2, 55.2 (OMe), 67.0 (C-16), 87.2 (C-17), 111.5 (C-2), 113.8 (C-4), 126.2 (C-1), 132.1 (C-10), 137.7 (C-5), 157.5 (C-3); Anal. calcd. for C19H25N3O2: C, 69.70; H, 7.70. Found: C, 69.82; H, 7.55.
General procedure for the preparation of 16α-triazolyl-3-methoxyestra-1,3,5(10)-trien-17-ols (4a–l and 5a–l). Compound 2 or 3 (327 mg, 1.00 mmol) was dissolved in dry CH2Cl2 (10 mL), and a solution of CuSO4·5H2O (12.5 mg, 5 mol%) and sodium ascorbate (30 mg, 15 mol%) in water (10 mL) was poured into the organic phase. The appropriate terminal alkyne (1.00 mmol) was added to the reaction mixture, which was then stirred overnight at ambient temperature. After the consumption of the starting material (TLC monitoring), the two-phase solution was diluted with water (30 mL) and extracted with CH2Cl2 (2 x 30 mL). The combined organic layers were washed with water, dried over Na2SO4 and evaporated in vacuo. The resulting crude product was purified by flash chromatography with EtOAc/CH2Cl2 (5:95).
16α-(4-Phenyl-1H-1,2,3-triazol-1-yl)-3-methoxyestra-1,3,5(10)-trien-17α-ol (4a). According to section 2.2.2, azidoalcohol 2 and phenylacetylene (0.11 mL) were added to the mixture. Product: 4a (385 mg), mp 222–224°C, 1H NMR (CDCl3): δ H 0.97 (s, 3H, 18-H3), 1.46–1.58 (overlapping m, 2H), 1.61 (m, 1H), 1.73 (m, 1H), 1.87 (m, 1H), 2.04 (m, 1H), 2.16 (m, 1H), 2.22–2.31 (overlapping m, 2H), 2.38–2.46 (overlapping m, 2H), 2.88 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 4.22 (d, 1H, J = 5.0 Hz, 17-H), 5.46 (m, 1H, 16-H), 6.66 (d, 1H, J = 2.0 Hz, 4-H), 6.75 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.21–7.27 (overlapping m, 4H, 1-H, 3″-H, 4″-H and 5″-H), 7.52 (d, 2H, J = 8.0 Hz, 2″- and 6″-H), 7.81 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 17.4 (C-18), 25.8 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 32.6 (CH2), 38.8 (CH), 43.4 (CH), 45.9 (C-13), 47.2 (CH), 55.2 (OMe), 62.7 (C-16), 79.4 (C-17), 111.6 (C-2), 113.8 (C-4), 119.9 (C-5′), 125.2 (2C, C-2″ and C-6″), 126.3 (C-1), 127.6 (C-4″), 128.6 (2C, C-3″ and C-5″), 130.1 (C-1″), 132.4 (C-10), 137.7 (C-5), 146.9 (C-4′), 157.5 (C-3); Anal. calcd. for C27H31N3O2: C, 75.49; H, 7.27. Found: C, 75.60; H, 7.33.
16α-[4-(3-Tolyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17α-ol (4b). According to section 2.2.2, azidoalcohol 2 and 3-tolylacetylene (0.13 mL) were added to the mixture. Product: 4b (402 mg), mp 185–187°C, 1H NMR (CDCl3): δ H 0.97 (s, 3H, 18-H3), 1.44–1.54 (overlapping m, 2H), 1.60 (m, 1H), 1.73 (m, 1H), 1.87 (m, 1H), 2.03 (m, 1H), 2.16 (m, 1H), 2.26 (m, 2H), 2.27 (s, 3H, 3″-CH3), 2.37–2.45 (overlapping m, 2H), 2.88 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 4.21 (d, 1H, J = 5.0 Hz, 17-H), 5.44 (m, 1H, 16-H), 6.66 (d, 1H, J = 2.0 Hz, 4-H), 6.75 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.03 (d, 1H, J = 7.5 Hz, 4″-H), 7.19 (t, 1H, J = 7.5 Hz, 5″-H), 7.25 (d, 1H, J = 8.5 Hz, 1-H), 7.40 (bs, 1H, 2″-H), 7.52 (d, 1H, J = 7.5 Hz, 6″-H), 7.79 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 17.3 (C-18), 21.3 (3″-CH3), 25.8 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 32.6 (CH2), 38.7 (CH), 43.4 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (OMe), 62.7 (C-16), 79.4 (C-17), 111.5 (C-2), 113.8 (C-4), 119.9 (C-5′), 122.3 (C-6″), 125.9 (C-2″), 126.3 (C-1), 128.4 and 128.5 (C-4″ and C-5″), 130.0 (C-1″), 132.4 (C-10), 137.7 (C-5), 138.0 (C-3″), 147.0 (C-4′), 157.5 (C-3); Anal. Calcd. For C28H33N3O2: C, 75.81; H, 7.50. Found: C, 75.67; H, 7.46.
16α-[4-(4-Tolyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17α-ol (4c). According to section 2.2.2, azidoalcohol 2 and 4-tolylacetylene (0.13 mL) were added to the mixture. Product: 4c (410 mg), mp 173–175 οC, 1H NMR (CDCl3): δ H 0.95 (s, 3H, 18-H3), 1.44–1.53 (overlapping m, 2H), 1.59 (m, 1H), 1.72 (m, 1H), 1.86 (m, 1H), 2.03 (m, 1H), 2.13 (m, 1H), 2.20–2.29 (overlapping m, 2H), 2.33 (s, 3H, 4″-CH3), 2.36–2.44 (overlapping m, 2H), 2.87 (m, 2H, 6-H2), 3.78 (s, 3H, OMe), 4.19 (d, 1H, J = 5.0 Hz, 17-H), 5.43 (m, 1H, 16-H), 6.65 (d, 1H, J = 2.0 Hz, 4-H), 6.74 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.05 (d, 2H, J = 8.0 Hz, 3″- and 5″-H), 7.24 (d, 1H, J = 8.5 Hz, 1-H), 7.41 (d, 2H, J = 8.0 Hz, 2″-H and 6″-H), 7.76 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 17.3 (C-18), 21.2 (4″-CH3), 25.8 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 32.5 (CH2), 38.7 (CH), 43.4 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (OMe), 62.7 (C-16), 79.5 (C-17), 111.5 (C-2), 113.8 (C-4), 119.6 (C-5′), 125.1 (2C, C-3″ and C-2″), 126.3 (C-1), 127.3 (C-1″), 129.2 (2C, C-2″ and C-6″), 132.4 (C-10), 137.4 (C-4″), 137.8 (C-5), 146.9 (C-4′), 157.5 (C-3); Anal. Calcd. For C28H33N3O2: C, 75.81; H, 7.50. Found: C, 75.99; H, 7.42.
16α-[4-(4-Methoxyphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17α-ol (4d). According to section 2.2.2, azidoalcohol 2 and 4-methoxyphenylacetylene (132 mg) were added to the mixture. Product: 4d (370 mg), mp 132–134οC; 1H NMR (CDCl3): δ H 0.96 (s, 3H, 18-H3), 1.45–1.54 (overlapping m, 2H), 1.60 (m, 1H), 1.72 (m, 1H), 1.87 (m, 1H), 2.03 (m, 1H), 2.14 (m, 1H), 2.21–2.30 (overlapping m, 2H), 2.37–2.45 (overlapping m, 2H), 2.88 (m, 2H, 6-H2), 3.79 (s, 3H, 3-OMe), 3.82 (s, 3H, 4″-OMe), 4.21 (d, 1H, J = 5.0 Hz, 17-H), 5.44 (m, 1H, 16-H), 6.65 (d, 1H, J = 2.0 Hz, 4-H), 6.74–6.78 (overlapping m, 3H, 2-H, 3″-H and 5″-H), 7.27 (d, 1H, J = 8.5 Hz, 1-H), 7.43 (d, 2H, J = 8.5 Hz, 2″-H and 6″-H), 7.71 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 17.3 (C-18), 25.8 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 32.5 (CH2), 38.7 (CH), 43.4 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (2C, 3-OMe, 4″-OMe), 62.7 (C-16), 79.4 (C-17), 111.5 (C-2), 113.7 (C-4), 113.9 (2C, C-3″ and C-5″), 119.1 (C-5′), 122.9 (C-1″), 126.3 (C-1), 126.5 (2C, C-2″ and C-6″), 132.4 (C-10), 137.7 (C-5), 146.6 (C-4′), 157.4 (C-3), 159.1 (C-4″); Anal. calcd. for C28H33N3O3: C, 73.18; H, 7.24. Found: C, 73.29; H, 7.16.
16α-[4-(2-Methoxyphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17α-ol (4e). According to section 2.2.2, azidoalcohol 2 and 2-methoxyphenylacetylene (0.13 mL) were added to the mixture. Product: 4e (365 mg), mp 252–254 οC, 1H NMR (CDCl3): δ H 0.96 (s, 3H, 18-H3), 1.45–1.64 (overlapping m, 3H), 1.71 (m, 1H), 1.89 (m, 1H), 2.10 (m, 1H), 2.19–2.33 (overlapping m, 3H), 2.37–2.44 (overlapping m, 2H), 2.89 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 3.86 (s, 3H, 2″-OCH3), 4.17 (d, 1H, J = 5.0 Hz, 17-H), 5.42 (m, 1H, 16-H), 6.66 (d, 1H, J = 2.0 Hz, 4-H), 6.74 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 6.83 (d, 1H, J = 8.0 Hz, 3″-H), 7.02 (t, 1H, J = 7.5 Hz, 5″-H), 7.22–7.26 (overlapping m, 2H, 1-H and 4″-H), 8.09 (s, 1H, 5′-H), 8.16 (d, 1H, J = 7.5 Hz, 6″-H) 13C NMR (CDCl3): δ C 17.4 (C-18), 25.8 (CH2), 28.1 (CH2), 29.8 (CH2), 31.1 (CH2), 32.2 (CH2), 38.8 (CH), 43.4 (CH), 45.9 (C-13), 47.2 (CH), 55.2 and 55.3 (3-OMe, 2″-OMe), 62.5 (C-16), 80.0 (C-17), 110.5 (C-3″), 111.5 (C-2), 113.8 (C-4), 119.1 (C-1″), 120.8 (C-5″), 123.4 (C-5′), 126.3 (C-1), 127.3 and 128.6 (C-4″ and C-6″), 132.4 (C-10), 137.8 (C-5), 142.7 (C-4′), 155.4 (C-2″), 157.5 (C-3); Anal. calcd. for C28H33N3O3: C, 73.18; H, 7.24. Found: C, 73.22; H, 7.27.
16α-[4-(4-tert-Butylphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17α-ol (4f). According to section 2.2.2, azidoalcohol 2 and 4-tert-butylphenylacetylene (0.18 mL) were added to the mixture. Product: 4f (435 mg), mp 181–183 οC, 1H NMR (CDCl3): δ H 0.96 (s, 3H, 18-H3), 1.34 (s, 9H, 3 tBu-CH3), 1.46–1.55 (overlapping m, 2H), 1.61 (m, 1H), 1.73 (m, 1H), 1.87 (m, 1H), 2.04 (m, 1H), 2.16 (m, 1H), 2.21–2.32 (overlapping m, 2H), 2.38–2.45 (overlapping m, 2H), 2.88 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 4.21 (d, 1H, J = 5.0 Hz, 17-H), 5.46 (m, 1H, 16-H), 6.66 (d, 1H, J = 2.0 Hz, 4-H), 6.75 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.26–7.30 (overlapping m, 3H, 1-H, 3″-H and 5″-H), 7.46 (d, 2H, J = 8 Hz, 2″-H and 6″-H), 7.76 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 17.4 (C-18), 25.9 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 31.3 (3C, 3 tBu-CH3), 32.5 (CH2), 34.5 (tBu-C), 38.8 (CH), 43.4 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (OMe), 62.7 (C-16), 79.4 (C-17), 111.6 (C-2), 113.8 (C-4), 119.6 (C-5′), 125.0 (2C), 125.4 (2C), 126.3 (C-1), 127.4 (C-1″), 132.4 (C-10), 137.8 (C-5), 146.9 (C-4′), 150.6 (C-4″), 157.5 (C-3); Anal. calcd. for C31H39N3O2: C, 76.67; H, 8.09. Found: C, 76.85; H, 8.14.
16α-[4-(4-Ethylphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17α-ol (4g). According to section 2.2.2, azidoalcohol 2 and 4-ethylphenylacetylene (0.14 mL) were added to the mixture. Product: 4g (405 mg), mp 113–115 οC, 1H NMR (CDCl3): δ H 0.97 (s, 3H, 18-H3), 1.26 (t, 3H, J = 7.5 Hz, CH2 CH 3), 1.48–1.55 (overlapping m, 2H), 1.61 (m, 1H), 1.74 (m, 1H), 1.87 (m, 1H), 2.04 (m, 1H), 2.17 (m, 1H), 2.21–2.32 (overlapping m, 2H), 2.38–2.46 (overlapping m, 2H), 2.63 (q, 2H, J = 7.5 Hz, CH 2CH3), 2.89 (m, 2H, 6-H2), 3.80 (s, 3H, OMe), 4.23 (d, 1H, J = 5.0 Hz, 17-H), 5.46 (m, 1H, 16-H), 6.66 (d, 1H, J = 2.0 Hz, 4-H), 6.76 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.06 (d, 2H, J = 8.0 Hz, 3″-H and 5″-H), 7.27 (d, 1H, J = 8.5 Hz, 1-H), 7.41 (d, 2H, J = 8 Hz, 2″-H and 6″-H), 7.76 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 15.5 (CH2 CH 3), 17.4 (C-18), 25.8 (CH2), 28.0 (CH2), 28.6 (CH 2CH3), 29.8 (CH2), 31.2 (CH2), 32.5 (CH2), 38.7 (CH), 43.4 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (OMe), 62.7 (C-16), 79.4 (C-17), 111.5 (C-2), 113.8 (C-4), 119.5 (C-5′), 125.1 (2C, C-2″ and C-6″), 126.3 (C-1), 127.5 (C-1″), 128.0 (2C, C-3″ and C-5″), 132.4 (C-10), 137.8 (C-5), 143.6 (C-4″), 146.9 (C-4′), 157.4 (C-3); Anal. calcd. for C29H35N3O2: C, 76.12; H, 7.71. Found: C, 76.26; H, 7.58.
16α-[4-(4-Propylphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17α-ol (4h). According to section 2.2.2, azidoalcohol 2 and 4-n-propylphenylacetylene (0.16 mL) were added to the mixture. Product: 4h (435 mg), mp 105–107 οC, 1H NMR (CDCl3): δ H 0.96–0.98 (overlapping multiplets, 6H, 18-H3 and CH 3CH2CH2), 1.65 (m, 2H, CH3 CH 2CH2), 1.48–1.54 (overlapping m, 2H), 1.59–1.68 (overlapping m, 3H, CH3 CH 2CH2 and 1H), 1.73 (m, 1H), 1.87 (m, 1H), 2.03 (m, 1H), 2.17 (m, 1H), 2.20–2.31 (overlapping m, 2H), 2.38–2.45 (overlapping m, 2H), 2.56 (t, 2H, J = 7.5 Hz, CH3CH2 CH 2), 2.89 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 4.22 (d, 1H, J = 5.0 Hz, 17-H), 5.45 (m, 1H, 16-H), 6.66 (d, 1H, J = 2.0 Hz, 4-H), 6.76 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.05 (d, 2H, J = 8.0 Hz, 3″-H and 5″-H), 7.27 (d, 1H, J = 8.5 Hz, 1-H), 7.41 (d, 2H, J = 8.0 Hz, 2″-H and 6″-H), 7.76 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 13.8 (CH 3CH2CH2), 17.3 (C-18), 24.4 (CH3 CH 2CH2), 25.8 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 32.5 (CH2), 37.7 (CH3CH2 CH 2), 38.7 (CH), 43.4 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (OMe), 62.7 (C-16), 79.4 (C-17), 111.5 (C-2), 113.8 (C-4), 119.6 (C-5′), 125.0 (2C, C-2″ and C-6″), 126.3 (C-1), 127.5 (C-1″), 128.6 (2C, C-3″ and C-5″), 132.4 (C-10), 137.7 (C-5), 142.1 (C-4″), 146.9 (C-4′), 157.4 (C-3); Anal. calcd. for C30H37N3O2: C, 76.40; H, 7.91. Found: C, 76.52; H, 7.86.
16α-[4-(3-Aminophenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17α-ol (4i). According to section 2.2.2, azidoalcohol 2 and 3-aminophenylacetylene (0.11 mL) were added to the mixture. Product: 4i (334 mg), mp 124–126 οC, 1H NMR (CDCl3): δ H 0.94 (s, 3H, 18-H3), 1.46–1.52 (overlapping m, 2H), 1.58 (m, 1H), 1.70 (m, 1H), 1.86 (m, 1H), 2.04 (m, 1H), 2.11 (m, 1H), 2.20–2.28 (overlapping m, 2H), 2.34–2.43 (overlapping m, 2H), 2.88 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 4.16 (d, 1H, J = 5.0 Hz, 17-H), 5.43 (m, 1H, 16-H), 6.57 (d, 1H, J = 8.0 Hz, 4″-H), 6.65 (d, 1H, J = 2.0 Hz, 4-H), 6.74 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 6.93 (s, 1H, 2″-H), 6.99 (d, 1H, J = 8.0 Hz, 6″-H), 7.09 (t, 1H, J = 8.0 Hz, 5″-H), 7.25 (d, 1H, J = 8.5 Hz, 1-H), 7.77 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 17.0 (C-18), 25.5 (CH2), 27.7 (CH2), 29.4 (CH2), 30.9 (CH2), 32.2 (CH2), 38.4 (CH), 43.0 (CH), 45.5 (C-13), 46.9 (CH), 54.8 (OMe), 62.3 (C-16), 79.1 (C-17), 111.2 (C-2), 111.8 (C-4″), 113.4 (C-4), 114.3 (C-2″), 115.6 (C-6″), 119.8 (C-5′), 126.0 (C-1), 129.1 (C-5″), 130.9 (C-1″), 132.0 (C-10), 137.4 (C-5), 146.3 (C-3″), 146.7 (C-4′), 157.1 (C-3); Anal. calcd. for C27H32N4O2: C, 72.94; H, 7.26. Found: C, 73.05; H, 7.14.
16α-(4-Cyclopropyl-1H-1,2,3-triazol-1-yl)-3-methoxyestra-1,3,5(10)-trien-17α-ol (4j). According to section 2.2.2, azidoalcohol 2 and cyclopropylacetylene (0.09 mL) were added to the mixture. Product: 4j (342 mg), mp 221–223 οC, 1H NMR (CDCl3): δ H 0.82 (m, 2H), 0.91–0.94 (overlapping m, 5H, 18-H3 and 2H), 1.45–1.52 (overlapping m, 2H), 1.57 (m, 1H), 1.67 (m, 1H), 1.86 (m, 1H), 1.91 (m, 1H), 2.08 (m, 1H), 2.13–2.22 (overlapping m, 2H), 2.34 (m, 1H), 2.41 (m, 2H), 2.88 (m, 2H, 6-H2), 3.78 (s, 3H, OMe), 4.06 (d, 1H, J = 5.0 Hz, 17-H), 5.33 (m, 1H, 16-H), 6.64 (d, 1H, J = 2.0 Hz, 4-H), 6.74 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.24 (d, 1H, J = 8.5 Hz, 1-H), 7.35 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 6.7 (CH2), 7.5 (CH2), 7.6 (C), 17.3 (C-18), 25.8 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 32.3 (CH2), 38.7 (CH), 43.3 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (OMe), 62.4 (C-16), 79.5 (C-17), 111.5 (C-2), 113.8 (C-4), 120.1 (C-5′), 126.3 (C-1), 132.4 (C-10), 137.7 (C-5), 149.2 (C-4′), 157.5 (C-3); Anal. calcd. for C24H31N3O2: C, 73.25; H, 7.94. Found: C, 73.33; H, 7.90.
16α-(4-Cyclopentyl-1H-1,2,3-triazol-1-yl)-3-methoxyestra-1,3,5(10)-trien-17α-ol (4k). According to section 2.2.2, azidoalcohol 2 and cyclopentylacetylene (0.12 mL) were added to the mixture. Product: 4k (334 mg), mp 156–158 οC, 1H NMR (CDCl3): δ H 0.93 (s, 3H, 18-H3), 1.45–1.53 (overlapping m, 2H), 1.57–1.74 (overlapping m, 8H), 1.87 (m, 1H), 2.00–2.05 (overlapping m, 4H), 2.15–2.22 (overlapping m, 2H), 2.34–2.43 (overlapping m, 2H), 2.88 (m, 2H, 6-H2), 3.03 (m, 1H, 1″-H), 3.79 (s, 3H, OMe), 4.13 (d, 1H, J = 5.0 Hz, 17-H), 5.44 (m, 1H, 16-H), 6.65 (d, 1H, J = 2.0 Hz, 4-H), 6.74 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.24 (d, 1H, J = 8.5 Hz, 1-H), 7.38 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 17.3 (C-18), 25.0 (CH2), 25.1 (CH2), 25.8 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 32.3 (CH2), 32.8 (CH2), 33.0 (CH2), 36.7 (CH), 38.7 (CH), 43.3 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (OMe), 62.3 (C-16), 79.5 (C-17), 111.5 (C-2), 113.8 (C-4), 120.0 (C-5′), 126.3 (C-1), 132.4 (C-10), 137.7 (C-5), 151.8 (C-4′), 157.4 (C-3); Anal. calcd. for C26H35N3O2: C, 74.07; H, 8.37. Found: C, 74.13; H, 8.29.
16α-(4-Cyclohexyl-1H-1,2,3-triazol-1-yl)-3-methoxyestra-1,3,5(10)-trien-17α-ol (4l). According to section 2.2.2, azidoalcohol 2 and cyclohexylacetylene (0.13 mL) were added to the mixture. Product: 4l (380 mg, 87%), mp 159–161 οC, 1H NMR (CDCl3): δ H 0.93 (s, 3H, 18-H3), 1.22–1.41 (overlapping m, 4H), 1.44–1.62 (overlapping m, 6H), 1.70 (m, 2H), 1.78 (m, 1H), 1.87 (m, 1H), 2.02–2.10 (overlapping m, 4H), 2.15–2.25 (overlapping m, 2H), 2.34–2.43 (overlapping m, 2H), 2.62 (m, 1H, 1″-H), 2.88 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 4.13 (d, 1H, J = 5.0 Hz, 17-H), 5.37 (m, 1H, 16-H), 6.65 (d, 1H, J = 2.0 Hz, 4-H), 6.74 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.24 (d, 1H, J = 8.5 Hz, 1-H), 7.36 (s, 1H, 5′-H); 13C NMR (CDCl3): δ C 17.3 (C-18), 25.8 (CH2), 26.0 (CH2), 26.1 (CH2), 28.0 (CH2), 29.8 (CH2), 31.2 (CH2), 32.3 (CH2), 32.6 (CH2), 33.0 (CH2), 35.1 (CH), 38.7 (CH), 43.3 (CH), 45.8 (C-13), 47.2 (CH), 55.2 (OMe), 62.3 (C-16), 79.5 (C-17), 111.5 (C-2), 113.8 (C-4), 119.8 (C-5′), 126.3 (C-1), 132.4 (C-10), 137.7 (C-5), 152.7 (C-4′), 157.4 (C-3); Anal. calcd. for C27H37N3O2: C, 74.45; H, 8.56. Found: C, 74.61; H, 8.52.
16α-(4-Phenyl-1H-1,2,3-triazol-1-yl)-3-methoxyestra-1,3,5(10)-trien-17β-ol (5a). According to section 2.2.2, azidoalcohol 3 and phenylacetylene (0.11 mL) were added to the mixture. Product: 5a (352 mg), mp 275–277 οC, 1H NMR (DMSO-d 6): δ H 0.85 (s, 3H, 18-H3), 1.30–1.44 (overlapping m, 4H), 1.79–1.87 (overlapping m, 3H), 1.95 (m, 1H), 2.13 (m, 1H), 2.25 (m, 1H), 2.34 (m, 1H), 2.80 (m, 2H, 6-H2), 3.70 (s, 3H, OMe), 3.93 (t, 1H, J = 6.0 Hz, 17-H), 4.80 (m, 1H, 16-H), 5.36 (d, 1H, J = 5.3 Hz, OH), 6.62 (d, 1H, J = 2.0 Hz, 4-H), 6.70 (dd, 1H, J = 8.6 Hz, J = 2.0 Hz, 2-H), 7.20 (d, 1H, J = 8.6 Hz, 1-H), 7.33 (t, 1H, J = 7.4 Hz, 4″-H), 7.45 (t, 2H, J = 7.7 Hz, 3″-H and 5″-H), 7.87 (m, 2H, J = 7.7 Hz, 2″-H and 6″-H), 8.70 (s, 1H, 5′-H); 13C NMR (DMSO-d 6): δ C 11.6 (C-18), 25.6, 26.6, 29.1, 31.6, 35.9, 38.0, 43.3, 43.5 (C-13), 47.6, 54.8 (OMe), 65.9 (C-16), 86.1 (C-17), 111.5 (C-2), 113.4 (C-4), 120.5 (C-5′), 125.0 (2C), 126.1 (C-1), 127.6 (C-4″), 128.8 (2C), 130.9 (C-1″), 131.8 (C-10), 137.3 (C-5), 146.3 (C-4′), 157.0 (C-3); Anal. calcd. for C27H31N3O2: C, 75.49; H, 7.27. Found: C, 75.60; H, 7.23.
16α-[4-(3-Tolyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17β-ol (5b). According to section 2.2.2, azidoalcohol 3 and 3-tolylacetylene (0.13 mL) were added to the mixture. Product: 5b (395 mg), mp 225–228 οC, 1H NMR (CDCl3): δ H 0.97 (s, 3H, 18-H3), 1.43–1.50 (overlapping m, 2H), 1.55 (m, 1H), 1.82–1.89 (overlapping m, 2H), 2.03 (m, 1H), 2.18 (m, 1H), 2.26–2.32 (overlapping m, 2H), 2.36 (m, 1H), 2.39 (s, 3H, 3″-CH3), 2.37–2.45 (overlapping m, 2H), 2.87 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 4.18 (d, 1H, J = 7.0 Hz, 17-H), 4.74 (m, 1H, 16-H), 6.64 (d, 1H, J = 2.0 Hz, 4-H), 6.73 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.15 (d, 1H, J = 7.5 Hz, 4″-H), 7.21 (d, 1H, J = 8.5 Hz, 1-H), 7.30 (t, 1H, J = 7.5 Hz, 5″-H), 7.56 (d, 1H, J = 7.5 Hz, 6″-H), 7.60 (br s, 1H, 2″-H), 7.75 (s, 1H, 5′-H); Anal. calcd. for C28H33N3O2: C, 75.81; H, 7.50. Found: C, 75.92; H, 7.61.
16α-[4-(4-Tolyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17β-ol (5c). According to section 2.2.2, azidoalcohol 3 and 4-tolylacetylene (0.13 mL) were added to the mixture. Product: 5c (403 mg), mp 271–273 οC, 1H NMR (CDCl3): δ H 0.97 (s, 3H, 18-H3), 1.44–1.52 (overlapping m, 2H), 1.55 (m, 1H), 1.82–1.89 (overlapping m, 2H), 2.02 (m, 1H), 2.19 (m, 1H), 2.29–2.35 (overlapping m, 4H), 2.36 (m, 1H), 2.39 (s, 3H, 4″-CH3), 2.88 (m, 2H, 6-H2), 3.78 (s, 3H, OMe), 4.18 (d, 1H, J = 7.0 Hz, 17-H), 4.75 (m, 1H, 16-H), 6.64 (d, 1H, J = 2.0 Hz, 4-H), 6.72 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.20–7.24 (overlapping multiplets, 3H, 1-H, 3″-H and 5″-H), 7.71 (d, 2H, J = 8 Hz, 2″-H and 6″-H), 7.78 (s, 1H, 5′-H); Anal. calcd. for C28H33N3O2: C, 75.81; H, 7.50. Found: C, 75.91; H, 7.39.
16α-[4-(4-Methoxyphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17β-ol (5d). According to section 2.2.2, azidoalcohol 3 and 4-methoxyphenylacetylene (132 mg) were added to the mixture. Product: 5d (405 mg), mp 259–262 οC, 1H NMR (CDCl3): δ H 0.97 (s, 3H, 18-H3), 1.41–1.52 (overlapping m, 2H), 1.54 (m, 1H), 1.82–1.89 (overlapping m, 2H), 2.02 (m, 1H), 2.19 (m, 1H), 2.29–2.40 (overlapping m, 3H), 2.54 (m, 1H), 2.88 (m, 2H, 6-H2), 3.78 (s, 3H, OMe), 3.85 (s, 3H, 4″-OMe), 4.18 (d, 1H, J = 7.0 Hz, 17-H), 4.74 (m, 1H, 16-H), 6.64 (d, 1H, J = 2.0 Hz, 4-H), 6.72 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 6.95 (d, 2H, J = 8.0 Hz, 3″-H and 5″-H), 7.21 (d, 1H, J = 8.5 Hz, 1-H), 7.72 (d, 2H, J = 8.0 Hz, 2″-H and 6″-H), 7.75 (s, 1H, 5′-H); Anal. calcd. for C28H33N3O3: C, 73.18; H, 7.24. Found: C, 73.27; H, 7.18.
16α-[4-(2-Methoxyphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17β-ol (5e). According to section 2.2.2, azidoalcohol 3 and 2-methoxyphenylacetylene (0.13 mL) were added to the mixture. Product: 5e (400 mg), mp 242–244 οC, 1H NMR (CDCl3): δ H 0.98 (s, 3H, 18-H3), 1.41–1.54 (overlapping m, 3H), 1.86–1.92 (overlapping m, 2H), 2.02 (m, 1H), 2.18 (m, 1H), 2.30–2.39 (overlapping m, 3H), 2.53 (m, 1H), 2.87 (m, 2H, 6-H2), 3.79 (s, 3H, OMe), 3.91 (s, 3H, 2″-OCH3), 4.24 (d, 1H, J = 7.0 Hz, 17-H), 4.74 (m, 1H, 16-H), 6.64 (d, 1H, J = 2.0 Hz, 4-H), 6.73 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 6.96 (d, 1H, J = 8.0 Hz, 3″-H), 7.08 (t, 1H, J = 8 Hz, 5″-H), 7.21 (d, 1H, J = 8.5 Hz, 1-H), 7.31 (t, 1H, J = 7.5 Hz, 4″-H), 8.07 (s, 1H, 5′-H), 8.33 (d, 1H, J = 8.0 Hz, 6″-H); Anal. calcd. for C28H33N3O3: C, 73.18; H, 7.24. Found: C, 73.22; H, 7.18.
16α-[4-(4-tert-Butylphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17β-ol (5f). According to section 2.2.2, azidoalcohol 3 and 4-tert-butylphenylacetylene (0.18 mL) were added to the mixture. Product: 5f (450 mg), mp 255–257 οC, 1H NMR (CDCl3): δ H 0.97 (s, 3H, 18-H3), 1.35 (s, 9H, 3 tBu-CH3), 1.41–1.55 (overlapping m, 3H), 1.83–1.89 (overlapping m, 2H), 2.02 (m, 1H), 2.18 (m, 1H), 2.30–2.39 (overlapping m, 3H), 2.76 (m, 1H), 2.88 (m, 2H, 6-H2), 3.78 (s, 3H, OMe), 4.18 (d, 1H, J = 7.0 Hz, 17-H), 4.74 (m, 1H, 16-H), 6.64 (d, 1H, J = 2.0 Hz, 4-H), 6.72 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.21 (d, 1H, J = 8.5 Hz, 1-H), 7.43 (d, 2H, J = 8.0 Hz, 3″-H and 5″-H), 7.71 (d, 2H, J = 8 Hz, 2″-H and 6″-H), 7.75 (s, 1H, 5’-H); 13C NMR (DMSO-d 6): δ 11.6 (C-18), 25.6, 26.6, 29.1, 31.0 (3C, 3 × tBu-CH3), 31.6, 35.9, 38.0, 43.3, 43.5 (C-13), 47.5, 54.8 (OMe), 65.9 (C-16), 86.0 (C-17), 111.5 (C-2), 113.4 (C-4), 120.2 (C-5′), 124.8 (2C), 125.5 (2C), 126.1 (C-1), 128.1 (C-4″), 131.8 (C-1″), 137.3 (C-10), 146.3 (C-5), 150.1 (C-4′), 157.0 (C-3); Anal. calcd. for C31H39N3O2: C, 76.67; H, 8.09. Found: C, 76.75; H, 8.15.
16α-[4-(4-Ethylphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17β-ol (5g). According to section 2.2.2, azidoalcohol 3 and 4-ethylphenylacetylene (0.14 mL) were added to the mixture. Product: 5g (415 mg). mp 250–253 οC, 1H NMR (DMSO-d 6): δ H 0.85 (s, 3H, 18-H3), 1.20 (t, 3H, J = 7.5 Hz, CH 3CH2CH2), 1.31–1.44 (overlapping m, 4H), 1.79–1.87 (overlapping m, 3H), 1.94 (m, 1H), 2.12 (m, 1H), 2.24 (m, 1H), 2.33 (m, 1H), 2.63 (q, 2H, J = 7.5 Hz, CH3 CH 2), 2.78 (m, 2H, 6-H2), 3.69 (s, 3H, OMe), 3.94 (m, 1H, 17-H), 4.78 (m, 1H, 16-H), 5.34 (d, 1H, J = 5.1 Hz, OH), 6.62 (d, 1H, J = 1.8 Hz, 4-H), 6.69 (dd, 1H, J = 8.5 Hz, J = 1.8 Hz, 2-H), 7.19 (d, 1H, J = 8.5 Hz, 1-H), 7.28 (d, 2H, J = 7.9 Hz, 3″-H and 5″-H), 7.79 (d, 2H, J = 7.9 Hz, 2″-H and 6″-H), 8.63 (s, 1H, 5′-H); 13C NMR (DMSO-d 6): δ 11.6 (C-18), 15.4 (CH 3CH2), 25.6, 26.6, 27.8, 29.1, 31.6, 35.9, 38.0, 43.3, 43.5 (C-13), 47.5, 54.8 (OMe), 65.9 (C-16), 86.1 (C-17), 111.4 (C-2), 113.4 (C-4), 120.1 (C-5′), 125.0 (2C), 126.0 (C-1), 128.1 (2C), 128.4 (C-4″), 131.8 (C-1″), 137.3 (C-10), 143.2 (C-5), 146.4 (C-4′), 157.0 (C-3); Anal. calcd. for C29H35N3O2: C, 76.12; H, 7.71. Found: C, 76.23; H, 7.60.
16α-[4-(4-Propylphenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17β-ol (5h). According to section 2.2.2, azidoalcohol 3 and 4-n-propylphenylacetylene (0.16 mL) were added to the mixture. Product: 5h (438 mg). mp 188–190 οC, 1H NMR (DMSO-d 6): δ H 0.85 (s, 3H, 18-H3), 0.90 (t, 3H, J = 7.3 Hz, CH 3CH2CH2), 1.31–1.44 (overlapping m, 4H), 1.61 (m, 2H, CH3 CH 2CH2), 1.78–1.87 (overlapping m, 3H), 1.95 (m, 1H), 2.12 (m, 1H), 2.24 (m, 1H), 2.33 (m, 1H), 2.57 (t, 2H, J = 7.5 Hz, CH3CH2 CH 2), 2.78 (m, 2H, 6-H2), 3.69 (s, 3H, OMe), 3.94 (m, 1H, 17-H), 4.78 (m, 1H, 16-H), 5.34 (d, 1H, J = 5.2 Hz, OH), 6.62 (d, 1H, J = 1.7 Hz, 4-H), 6.69 (dd, 1H, J = 8.5 Hz, J = 1.7 Hz, 2-H), 7.19 (d, 1H, J = 8.5 Hz, 1-H), 7.26 (d, 2H, J = 7.9 Hz, 3″-H and 5″-H), 7.78 (d, 2H, J = 7.9 Hz, 2″-H and 6″-H), 8.63 (s, 1H, 5′-H); 13C NMR (DMSO-d 6): δ 11.6 (C-18), 13.5 (CH 3CH2CH2), 23.9, 25.6, 26.6, 29.1, 31.6, 35.9, 36.9, 38.0, 43.3, 43.5 (C-13), 47.5, 54.8 (OMe), 65.9 (C-16), 86.1 (C-17), 111.4 (C-2), 113.4 (C-4), 120.1 (C-5′), 124.9 (2C), 126.0 (C-1), 128.4 (C-4″), 128.7 (2C), 131.8 (C-1″), 137.3 (C-10), 141.6 (C-5), 146.4 (C-4′), 157.0 (C-3); Anal. calcd. for C30H37N3O2: C, 76.40; H, 7.91. Found: C, 76.52; H, 8.01.
16α-[4-(3-Aminophenyl)-1H-1,2,3-triazol-1-yl]-3-methoxyestra-1,3,5(10)-trien-17β-ol (5i). According to section 2.2.2, azidoalcohol 3 and 3-aminophenylacetylene (0.11 mL) were added to the mixture. Product: 5i (346 mg), oil, 1H NMR (CDCl3): δ H 0.96 (s, 3H, 18-H3), 1.38–1.55 (overlapping m, 3H), 1.79–1.85 (overlapping m, 2H), 2.03 (m, 1H), 2.14 (m, 1H), 2.19–2.23 (overlapping m, 2H), 2.28–2.36 (overlapping m, 2H), 2.86 (m, 2H, 6-H2), 3.78 (s, 3H, OMe), 4.17 (d, 1H, J = 7.0 Hz, 17-H), 4.69 (m, 1H, 16-H), 6.63–6.65 (overlapping multiplets, 2H, 4- and 4″-H), 6.72 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.06–7.08 (overlapping multiplets, 2H, 2″- and 6″-H), 7.16–7.20 (overlapping multiplets, 2H, 1- and 5″-H), 7.64 (s, 1H, 5′-H); Anal. calcd. for C27H32N4O2: C, 72.94; H, 7.26. Found: C, 73.04; H, 7.32.
16α-(4-Cyclopropyl-1H-1,2,3-triazol-1-yl)-3-methoxyestra-1,3,5(10)-trien-17β-ol (5j). According to section 2.2.2, azidoalcohol 3 and cyclopropylacetylene (0.085 mL) were added to the mixture. Product: 5j (350 mg), mp 164–167 οC, 1H NMR (CDCl3): δ H 0.82 (m, 2H), 0.92–0.95 (overlapping multiplets, 5H, 18-H3 and 2H), 1.38–1.58 (overlapping m, 3H), 1.76–1.85 (overlapping m, 2H), 2.00 (m, 1H), 2.13 (m, 1H), 2.18–2.23 (overlapping m, 2H), 2.28–2.37 (overlapping m, 2H), 2.86 (m, 2H, 6-H2), 3.78 (s, 3H, OMe), 4.11 (d, 1H, J = 6.5 Hz, 17-H), 4.65 (m, 1H, 16-H), 6.63 (d, 1H, J = 2.0 Hz, 4-H), 6.72 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.20 (d, 1H, J = 8.5 Hz, 1-H), 7.30 (s, 1H, 5′-H); Anal. calcd. for C24H31N3O2: C, 73.25; H, 7.94. Found: C, 73.34; H, 7.86.
16α-(4-Cyclopentyl-1H-1,2,3-triazol-1-yl)-3-methoxyestra-1,3,5(10)-trien-17β-ol (5k). According to section 2.2.2, azidoalcohol 3 and cyclopentylacetylene (0.12 mL) were added to the mixture. Product: 5k (354 mg), mp 166–168 οC, 1H NMR (CDCl3): δ H 0.95 (s, 3H, 18-H3), 1.42–1.58 (overlapping m, 5H), 1.62–1.69 (overlapping m, 4H), 1.76–1.86 (overlapping m, 4H), 2.00 (m, 1H), 2.10 (m, 1H), 2.21–2.26 (overlapping m, 1H), 2.29–2.38 (overlapping m, 3H), 2.87 (m, 2H, 6-H2), 3.17 (m, 1H, 1″-H), 3.78 (s, 3H, OMe), 4.14 (d, 1H, J = 6.5 Hz, 17-H), 4.68 (m, 1H, 16-H), 6.63 (d, 1H, J = 2.0 Hz, 4-H), 6.72 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.21 (d, 1H, J = 8.5 Hz, 1-H), 7.33 (s, 1H, 5′-H); Anal. calcd. for C26H35N3O2: C, 74.07; H, 8.37. Found: C, 74.17; H, 8.45.
16α-(4-Cyclohexyl-1H-1,2,3-triazol-1-yl)-3-methoxyestra-1,3,5(10)-trien-17β-ol (5l). According to section 2.2.2, azidoalcohol 3 and cyclohexylacetylene (0.13 mL) were added to the mixture. Product: 5l (360 mg), mp 130–132 οC, 1H NMR (CDCl3): δ H 0.95 (s, 3H, 18-H3), 1.42–1.56 (overlapping m, 6H), 1.72–1.86 (overlapping m, 6H), 1.98–2.37 (overlapping m, 8H), 2.49 (m, 1H), 2.73 (m, 1H, 1″-H), 2.87 (m, 2H, 6-H2), 3.78 (s, 3H, OMe), 4.14 (d, 1H, J = 6.5 Hz, 17-H), 4.68 (m, 1H, 16-H), 6.63 (d, 1H, J = 2.0 Hz, 4-H), 6.72 (dd, 1H, J = 8.5 Hz, J = 2.0 Hz, 2-H), 7.21 (d, 1H, J = 8.5 Hz, 1-H), 7.31 (s, 1H, 5′-H); Anal. calcd. for C27H37N3O2: C, 74.45; H, 8.56. Found: C, 74.67; H, 8.38.
Cell culturing and determination of antiproliferative effects of the tested compounds
Human cell lines were purchased from ECACC (Salisbury, UK). HeLa (cervix adenocarcinoma), A431 (skin epidermoid carcinoma), MCF7 (breast adenocarcinoma) and noncancerous MRC-5 fetal lung fibroblast cells were cultivated in minimal essential medium supplemented with 10% fetal bovine serum, 1% non-essential amino acids and an antibiotic–antimycotic mixture. All media and supplements were obtained from PAA Laboratories GmbH, Pasching, Austria. Near-confluent cancer cells were seeded onto a 96-well microplate (5000/well) and attached to the bottom of the well overnight. On the second day, 200 μL of new medium containing the tested compound (at 10 or 30 μM) was added. After incubation for 72 h at 37°C in humidified air with 5% CO2, the living cells were assayed by the addition of 20 μL of 5 mg/mL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution. MTT was converted by intact mitochondrial reductase and precipitated as blue crystals during a 4-h contact period. The medium was then removed and the precipitated crystals were dissolved in 100 μL of DMSO during a 60-min period of shaking at 25°C. Finally, the reduced MTT was assayed at 545 nm, using a microplate reader; wells with untreated cells were utilized as controls [27]. For the most effective compounds, the assays were repeated with a set of dilutions, and sigmoidal dose–response curves were fitted to the measured data in order to determine the IC50 values by means of GraphPad Prism 4.0 (GraphPad Software; San Diego, CA, USA). All in vitro experiments were carried out on two microplates with at least five parallel wells. Cisplatin was used as positive control. Stock solutions of the tested substances (10 mM) were prepared with DMSO. The highest DMSO content of the medium (0.3%) did not have any substantial effect on the cell proliferation.
Cell cycle analysis by flow cytometry
Cellular DNA content was determined by means of flow cytometric analysis, using a DNA- specific fluorescent dye, propidium iodide (PI). The cells were plated in a six-well plate and cultured for 24 h. The cultured cells were treated with various concentrations of the tested compounds for 24 h, after which the medium was removed, and the cells were washed with phosphate-buffered saline (PBS) and trypsinized. The harvested cells were suspended in medium and centrifuged at 1,700 rpm for 15 min at 4°C. The supernatant was then removed and the cells were resuspended in 1 mL of PBS. After the second centrifugation, 1 mL of -20°C 70% EtOH was added dropwise to the cell pellet. The cells were stored at -20°C until the day of DNA staining. On the day of DNA staining, the samples were washed with PBS and suspended in 1 mL of DNA staining buffer containing PI, ribonuclease-A, Triton-X and sodiumcitrate. After incubation for 1 h at room temperature, protected from light, the samples were analyzed by FACStar. For each experiment 20,000 events were counted, and the percentages of the cells in the different cell-cycle phases (subG1, G1, S and G2/M) were determined by means of winMDI 2.8 [28].
Double staining with Hoechst 33258 and PI
Cells were seeded into a 96-well plate and incubated with various concentrations of the tested compounds for 24 h. The medium was then removed and 100 μL of medium with 10 μL of staining solution was added to the cells. The final concentrations of Hoechst 33258 and PI were 5 and 3 μg/mL, respectively. After incubation for 60 min at 37°C, the cells were examined on a Nikon Fluorescence Microscope equipped with a Digital Sight Camera System, including appropriate filters for Hoechst 33258 and PI [29, 30]
Caspase-3 assay
Caspase-3 activity was determined by using a colorimetric assay kit (Sigma-Aldrich Ltd., Budapest, Hungary), Ac-DEVD-pNA serving as substrate. During the assay, the peptide substrate was cleaved by caspase-3, resulting in the release of pNA (p-nitroaniline), which was measured on a microplate reader at an absorbance wavelength of 405 nm. Caspase-3 activity was determined in the presence and absence of a selective inhibitor for caspase-3. HeLa cells were treated with the tested compounds at 3, 10 and 30 μM for 24 h; untreated cells were used as controls. Cells were scraped and incubated on ice with cell lysis buffer in proportion to the cell number for 15 min. The cell lysate was next centrifuged for 15 min at 17,000 g and the supernatant was collected and assayed by means of the microplate reader. Results were expressed in fold increase of caspase-3 activity compared with the control result [31].
Caspase-8 assay
Caspase-8 activity was determined by using a colorimetric assay kit (Sigma-Aldrich Ltd., Budapest, Hungary), Ac-IETD-pNA serving as substrate. During the assay, the peptide substrate was cleaved by caspase-8, resulting in the release of pNA, which was measured on a microplate reader at an absorbance wavelength of 405 nm. All further conditions were identical with those of the caspase-3 assay.
Caspase-9 assay
Caspase-9 activity was determined by using a colorimetric assay kit (Invitrogen; Carlsbad, CA, USA), with Ac-LEHD-pNA as substrate. During the assay, the peptide substrate was cleaved by caspase-9, resulting in the release of pNA, which was measured on a microplate reader at an absorbance wavelength of 405 nm. All further conditions were identical with those of the caspase-3 assay.
Reverse transcription-polymerase chain reaction (RT-PCR) studies
The effects of the tested compounds on the mRNA expression pattern of the markers of apoptosis, such as Bax, Bcl-2, cyclin-dependent kinase 1 (CDK1), cdc25B, cyclin B1 and cyclin B2, which play a crucial role in the transition from the G2 to the M phase, were determined by RT-PCR in HeLa cells. After a 24-h incubation period, the total RNA was isolated from the cells (4×105) through the use of TRIzol Reagent, in accordance with the instructions of the manufacturer (Csertex Ltd; Budapest, Hungary). The pellet was resuspended in 100 μL of DNase- and RNase-free distilled water. The RNA concentrations of the samples were determined from their absorbances at 260 nm. The RNA (0.5 μg) was mixed with DNase- and RNase-free distilled water and 20 μM oligodT (Invitrogen; Carlsbad, CA, USA), in a final reaction volume of 10 μL, and the mixture was incubated at 70°C for 5 min. After the mixture had been cooled to 4°C, 20 U of RNase inhibitor (Promega, Madison, WI, USA), 20 U of MMLV reverse transcriptase (Promega, Madison, USA), 200 μM dNTP (Sigma-Aldrich; Budapest, Hungary) in 50 mM Tris-HCl, pH 8.3, 75 mM KCl and 5 mM MgCl2 in a final reaction volume of 10 μL were added. The mixture was incubated at 37°C for 60 min. The PCR was carried out with 5 μL of cDNA, 12.5 μL of GoTaq Green Master Mix, 2 μL of 20 pM sense and the antisense primers of Bax, Bcl-2, CDK1, cdc25B, cyclin B1, cyclin B2 and 3.5 μL of DNase- and RNase-free distilled water. Human glyceraldehyde 3-phosphate dehydrogenase (hGAPDH) primers were used as internal control in all samples (S1 Table). The PCR was performed with an ESCO SWIFT MAXI thermal cycler (Esco Technologies; Philadelphia, PA, USA) and the products were separated on 2% agarose gels, stained with ethidium bromide and photographed under a UV transilluminator. Semiquantitative analysis was performed by densitometric scanning of the gel with a Kodak IMAGE STATION 2000R (Csertex; Budapest, Hungary).
Western blotting studies
To investigate the actions of the most potent compounds on the functions of phosphorylated and total stathmin, protein expression was determined by using western blot analysis. HeLa cells were harvested in 60-mm dishes at a density of 2 x 105 cells/mL and treated with the tested agents for 48 h. Whole-cell extracts were prepared by washing the cells with PBS and suspending them in lysis buffer (50 mM Tris, 5 mM EDTA, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 1 mM sodium orthovanadate, 100 μg/mL PMSF and protease inhibitors) [32]. 10 μg of protein per well was subjected to electrophoresis on 4–12% NuPAGE Bis–Tris Gel in XCell SureLock Mini-Cell Units (Invitrogen, Carlsbad, CA, USA). Proteins were transferred from gels to nitrocellulose membranes, using the iBlot Gel Transfer System (Invitrogen, Carlsbad, CA, USA). Antibody binding was detected with the WesternBreeze Chemiluminescent Western blot immunodetection kit (Invitrogen, Carlsbad, CA, USA). The blots were incubated on a shaker with stathmin (Op18: rabbit polyclonal antibody raised against amino acids 1–149 representing full-length human protein), phosphorylated stathmin (p-Op18: rabbit polyclonal antibody raised against a short amino acid sequence containing phosphorylated Ser25 of human protein) and β-actin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) 1:200 in the blocking buffer. Each sample was prepared in three parallels and the experiments were repeated twice. Semiquantitative analysis was performed by densitometric scanning of the blot with Kodak IMAGE STATION 2000R (Eastman Kodak Co., Rochester, NY, USA). All determined optical density values were normalized to the optical density value of β-actin. For statistical evaluation, data were analyzed by one-way ANOVA with the Neumann-Keuls post test, using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Synthesis of 16α-triazolylestrone derivatives
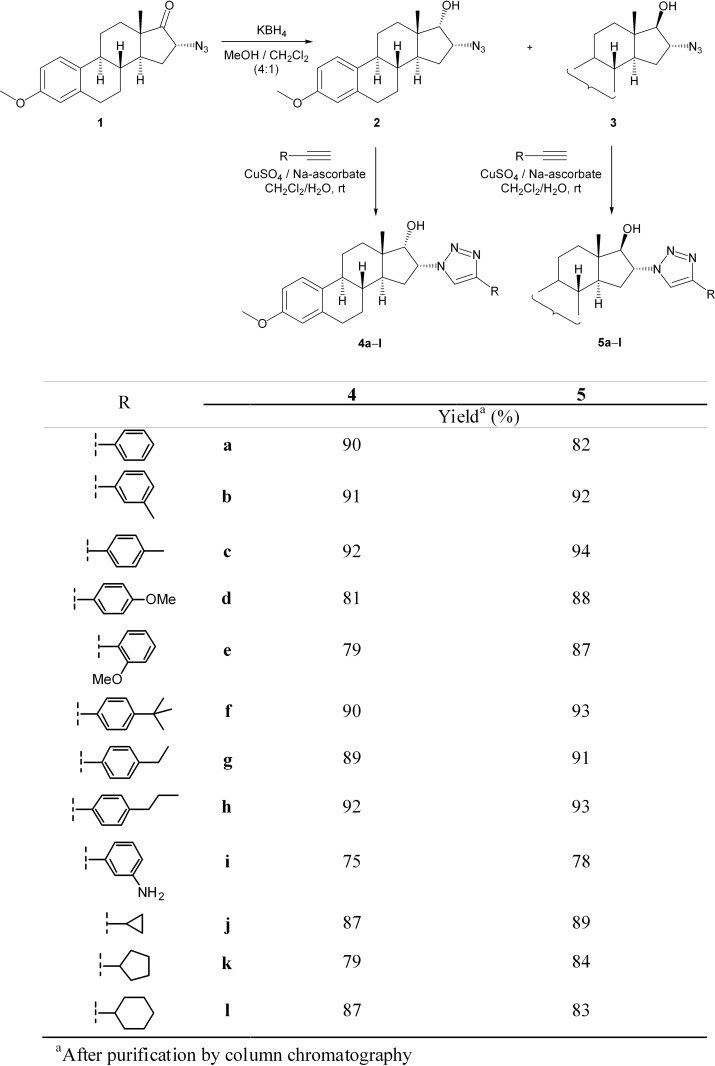
Treatment of 16α-azido-3-methoxyestra-1,3,5(10)-trien-17-one (1) with KBH4 in MeOH/CH2Cl2 (4:1) resulted in two diastereomeric azidoalcohols. The mixture of epimers was separated by flash chromatography to furnish 2 (17α-OH) and 3 (17β-OH) in a ratio of ∼2:3. The similar reduction of 1 with LiBH4 in Et2O was reported earlier to result in the related 16,17-cis and -trans isomers (2 and 3) in a nearly 1:1 ratio [26].
Several 16α-1,2,3-triazolyl derivatives (4a–l and 5a–l) were next synthetized in good to excellent yields through the reactions of 2 or 3 with various terminal alkynes (Fig. 1). Since a Cu(I)-catalyzed process is approximately 107 times faster than the uncatalyzed version, the intermolecular cycloaddition readily occurs at room temperature. As the application of Cu(I) salts in such reactions is known to require high temperature or at least the presence of an amine base additive (DIPEA or Et3N) for sufficient formation of the Cu-acetylide intermediate, and certain chelating ligands (mostly TBTA or bathophenanthroline) are often employed in order to enhance the activity of the catalyst and to protect the Cu(I) from oxidation [4], the Cu(I) species was generated in situ by the reduction of CuSO4 with sodium ascorbate. Furthermore, a two-phase solvent system (CH2Cl2 as a co-solvent with water) was applied in order to facilitate the dissolution of both the steroid and the catalyst system, to eliminate the need for ligands and to simplify the reaction protocol [21].
Fig 1. Synthesis of steroidal 16α-triazoles by CuAAc.
In all cases, full conversion of the starting compound 2 or 3 was observed after overnight stirring at ambient temperature. As expected, the reactions occurred in a regioselective manner, and the triazole products (4a–l and 5a–l) could generally be obtained in yields of 80–94% after chromatographic purification; there were two exceptions: 4i and 5i, in yields of 75% and 78%, respectively.
The structures of the newly synthetized compounds (4a–l and 5a–l) were confirmed by NMR measurements. The 1H NMR spectra of 4a–i and 5a–i revealed the appearance of the new signals of the incorporated aryl groups at 6.8–8.2 ppm as compared with the spectra of the starting materials (2 and 3), while the 5′-H singlet was identified at 7.7–8.0 ppm. Furthermore, the aliphatic region in the spectra of 4j–l and 5j–l containing a cycloalkyl group was enriched by the signals of the appropriate CH2 and CH protons, and the singlet of 5′-H appeared at ∼ 7.3 ppm. As concerns the 16-H and 17-H signals, significant differences were observed between the two epimers. For 4a–l the multiplet of 16-H was identified at ∼ 5.4 ppm, while the signal of 17-H appeared as a doublet (J = 5.0 Hz) at 4.1–4.2 ppm, whereas in the spectra of 5a–l the 16-H multiplet was found at ∼ 4.7 ppm, and the 17-H doublet (J = 7.0 Hz) at 4.1–4.2 ppm. The 13C NMR spectra of 4j–l also contained the signals of the heteroaromatic ring, one for C-5′ at ∼ 120 ppm, and the other for C-4′ at ∼ 147 ppm.
Determination of the antiproliferative properties of 16α-triazolylestrone derivatives
The antiproliferative properties of the prepared 16α-triazolylestrone derivatives were determined on a panel of human cancerous cell lines (HeLa, A431 and MCF7) by means of the MTT assay in a two-step procedure. Two final concentrations (10 and 30 μM) were first applied for all compounds. For agents exhibiting a growth of inhibition at least 60% against any of the cell lines, further assays with lower concentrations were performed and the IC50 values were calculated. The cancer selectivities of these compounds were additionally determined by the same MTT assay against the noncancerous normal lung fibroblast cell line MRC5 (Table 1). The configuration of the OH group at position 17 did not have a consequent effect on the cell growth, although the β configuration seemed to be preferred. Since azidoalcohols 2 and 3 did not exhibit substantial action, the presence of the triazole ring is considered to be essential for the effect. Derivatives with an unsubstituted Ph ring (4a and 5a) and those containing simple substituents (4b–d and 5b–d) or cycloalkyl groups (4j–l and 5j–l) also exerted moderate action. Introduction of a carbon chain (ethyl, propyl or tert-butyl) on the aromatic moiety, however, resulted in increased activities, and these molecular elements combined with 17β-hydroxy groups generated the most potent members of the current set (5f–h). The m-aminophenyl-substituted heteroaromatic ring resulted in another effective compound, but in this case the 17α-hydroxy epimer (4i) proved to be more potent. On the basis of their antiproliferative effects, compounds 4i and 5f–h were selected for further experiments, including characterization of the cancer selectivity. All four steroids exerted limited action on the proliferation of noncancerous fibroblast MRC5. In the cases of 4i and 5h, 50% inhibition was not elicited up to 30 μM.
Table 1. Antiproliferative properties of the synthetized compounds.
| Compound | Conc. (μM) | Inhibition % ± SEM[Calculated IC50 value] a | |||
|---|---|---|---|---|---|
| HeLa | MCF7 | A431 | MRC5 | ||
| 2 | 10 | – b | – | 29.83 ± 2.36 | n.d. c |
| 30 | 30.24 ± 2.25 | – | 51.48 ± 1.87 | ||
| 3 | 10 | – | – | – | n.d. |
| 30 | 24.44 ± 2.51 | – | 47.71 ± 2.22 | ||
| 4a | 10 | 37.44 ± 2.44 | – | 34.92 ± 0.58 | n.d. |
| 30 | 68.28 ± 0.54 | 49.93 ± 0.63 | 47.92 + 1.24 | ||
| [10.21 μM] | [>30 μM] | [>30 μM] | |||
| 4b | 10 | – | – | – | n.d. |
| 30 | – | – | – | ||
| 4c | 10 | – | – | – | n.d. |
| 30 | – | – | – | ||
| 4d | 10 | – | – | 24.68 ± 1.31 | n.d. |
| 30 | 45.45 ± 0.84 | 28.79 ± 1.51 | 26.13 ± 2.30 | ||
| 4e | 10 | 51.34 ± 0.62 | 34.13 ± 2.14 | 41.87 ± 1.92 | n.d. |
| 30 | 71.39 ±1.17 | 64.39 ± 1.15 | 55.47 ± 0.79 | ||
| [14.80 μM] | [17.78 μM] | [22.76 μM] | |||
| 4f | 10 | 47.07 ± 1.06 | 61.87 ± 2.59 | – | n.d. |
| 30 | 97.30 ± 0.49 | 96.36 ± 0.44 | 29.21 ± 2.76 | ||
| [10.68 μM] | [8.07 μM] | [>30 μM] | |||
| 4g | 10 | – | – | – | n.d. |
| 30 | 51.62 ± 2.04 | – | 23.24 ± 1.03 | ||
| 4h | 10 | – | 24.19 ± 2.00 | 23.71 ± 1.03 | n.d. |
| 30 | 92.85 ± 0.41 | 66.63 ± 1.19 | 42.59 ± 1.14 | ||
| [11.68 μM] | [11.58 μM] | [>30 μM] | |||
| 4i | 10 | 47.24 ± 2.13 | – | 26.98 ± 0.87 | – |
| 30 | 98.38 ± 0.15 | 82.48 ± 0.85 | 94.70 ± 0.46 | 25.74 ± 2.94 | |
| [13.85 μM] | [14.88 μM] | [11.75 μM] | [>30 μM] | ||
| 4j | 10 | 23.78 ± 2.27 | 30.96 ± 1.71 | 35.79 ± 1.53 | n.d. |
| 30 | 51.49 ± 1.92 | 43.48 ± 1.30 | 49.96 ± 1.43 | ||
| 4k | 10 | 25.33 ± 2.54 | – | – | n.d. |
| 30 | 38.09 ± 2.03 | 47.94 ± 1.15 | – | ||
| 4l | 10 | – | – | – | n.d. |
| 30 | 34.44 ± 2.14) | 33.29 ± 2.51 | 26.92 ± 1.75 | ||
| 5a | 10 | – | – | – | n.d. |
| 30 | 34.63 ± 2.14 | 39.95 ± 1.96 | 52.94 ± 0.70 | ||
| 5b | 10 | 40.54 ± 0.74 | – | 44.29 ± 0.73 | n.d. |
| 30 | 50.06 ± 1.13 | – | 44.71 ± 1.63 | ||
| 5c | 10 | – | – | – | n.d. |
| 30 | 36.57 ± 1.08 | 44.20 ± 1.54 | 58.09 ± 0.21 | ||
| 5d | 10 | 37.41 ± 1.16 | 26.05 ± 2.73 | – | n.d. |
| 30 | 46.76 ± 2.95 | 37.10 ± 2.35 | 49.14 ± 2.13 | ||
| 5e | 10 | 31.65 ± 2.53 | – | – | n.d. |
| 30 | 40.81 ± 2.35 | – | – | ||
| 5f | 10 | 90.47 ± 0.53 | 73.15 ± 1.39 | 72.94 ± 0.87 | 32.75 ± 2.49 |
| 30 | 95.17 ± 0.27 | 78.94 ± 0.55 | 70.98 ± 0.86 | 68.32 ± 0.76 | |
| [5.08 μM] | [7.88 μM] | [6.77 μM] | [17.64 μM] | ||
| 5g | 10 | 85.62 ± 0.75 | 44.84 ± 2.19 | 47.32 ± 1.02 | 33.74 ± 1.74 |
| 30 | 95.55 ± 0.62 | 60.60 ± 1.97 | 73.60 ± 0.46 | 68.61 ± 1.22 | |
| [8.69 μM] | [10.78 μM] | [10.68 μM] | [17.07 μM] | ||
| 5h | 10 | 75.26 ± 1.57 | 32.80 ± 2.59 | 43.15 ± 1.87 | 21.01 ± 1.58 |
| 30 | 86.44 ± 0.57 | 36.30 ± 1.26 | 51.76 ± 1.52 | 20.44 ± 1.29 | |
| [12.11 μM] | [>30 μM] | [>30 μM] | [>30 μM] | ||
| 5i | 10 | – | – | – | n.d. |
| 30 | 24.55 ± 2.69 | 64.62 ± 1.71 | – | ||
| 5j | 10 | – | – | – | n.d. |
| 30 | 40.47 ± 2.39 | – | – | ||
| 5k | 10 | 26.28 ± 1.23 | – | – | n.d. |
| 30 | 34.55 ± 1.61 | – | – | ||
| 5l | 10 | – | – | – | n.d. |
| 30 | 26.54 ± 2.16 | – | –) | ||
| Cisplatin | 10 | 42.61 ± 2.33 | 53.03 ± 2.29 | 88.54 ± 0.50 | 72.30 ± 2.30 |
| 30 | 99.93 ± 0.26 | 86.90 ± 1.24 | 90.18 ± 1.78 | 70.65 + 1.34 | |
| [12.43 μM] | [9.63 μM] | [2.84 μM] | [4.51 μM] | ||
a Mean value from two independent determinations with five parallel wells, standard deviation less than 15%.
b Inhibition values <20% are not presented for clarity.
c n.d.: not determined.
Cell cycle analysis of HeLa cells treated with triazole-containing estrane
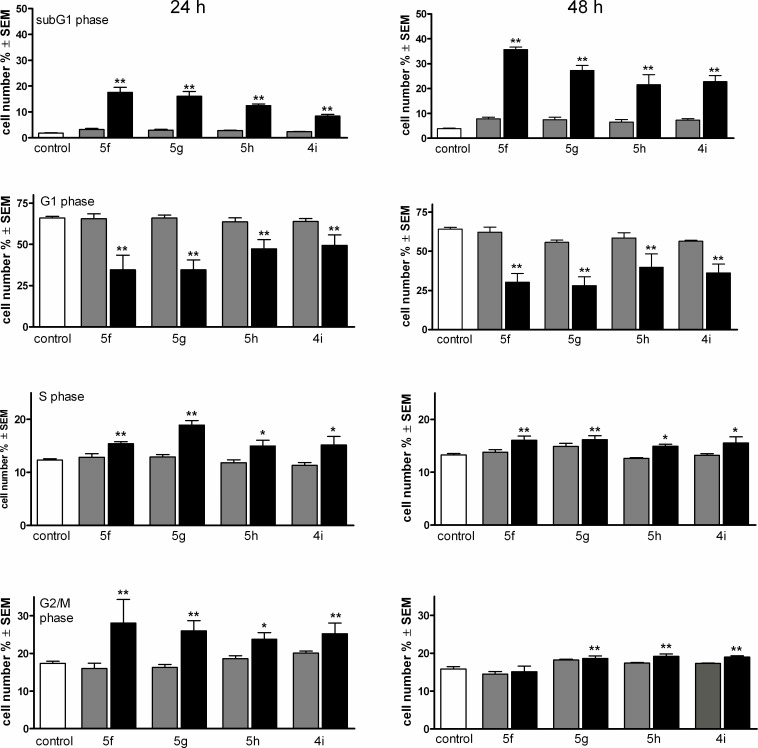
HeLa cells were treated with the tested compounds at 3 and 10 μM for 24 and 48 h, and the phase distribution of the treated cells was determined (Fig. 2). Treatment with the selected estrane analogs resulted in a concentration-dependent increase of subG1 phase cells, which was more pronounced after incubation for 48 h. At the same time, the G1 populations decreased substantially, while the synthetic and G2/M phases exhibited modest, but significant increases. Though compound 5f proved to be the most potent inducer of the hypodiploid cell population, no substantial differences were evidenced in the cell cycle distributions of the treated cells.
Fig 2. Effects of compounds 5f, 5g, 5h and 4i on the HeLa cell cycle distribution after incubation for 24 (left panels) and 48 h (right panels).
Gray and black columns indicate 3 and 10 μM, respectively. * and ** denote p < 0.05 and p < 0.01, respectively, as compared with the control cells.
Double staining with Hoechst 33258 and PI
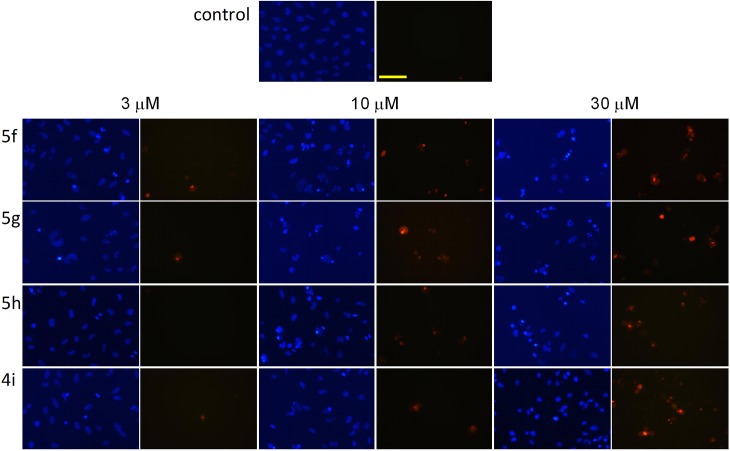
HeLa cells were treated with the steroids in concentrations of 3, 10 and 30 μM and then incubated for 24 h and stained with the fluorescent DNA markers Hoechst 33258 and PI in order to evaluate the morphological markers of the effects induced by the tested compounds. Two separate pictures from the same field were taken for the two fluorescent dyes. Morphological changes such as nuclear condensation, the appearance of apoptotic bodies and increase of the cell membrane permeability were recognized in a concentration-dependent manner as evidence of apoptosis and necrosis (Fig. 3). All four selected steroids (4i, 5f, 5g and 5h) in a concentration of 3 μM induced early apoptosis, as confirmed by nuclear condensation without increased membrane permeability. The gradual impairment of the membrane function was detected by more frequent PI staining on increase of the applied concentration, which may be evidence of late apoptosis or necrosis. Treatment with 30 μM of these compounds resulted in disturbed membrane permeability, without the corresponding nuclear condensation indicating the necrosis-inducing capacity of the agents.
Fig 3. Fluorescent microscopy images of Hoechst 33258-PI double staining.
Two separate pictures from the same field were taken for the two markers. HeLa cells were treated with vehicle (control), or with 5f, 5g, 5h and 4i at the indicated concentrations. The blue fluorescence (left panels) indicates Hoechst 33258, and the red fluorescence (right panels) is a consequence of PI accumulation. The bar in the PI control picture indicates 100 μm.
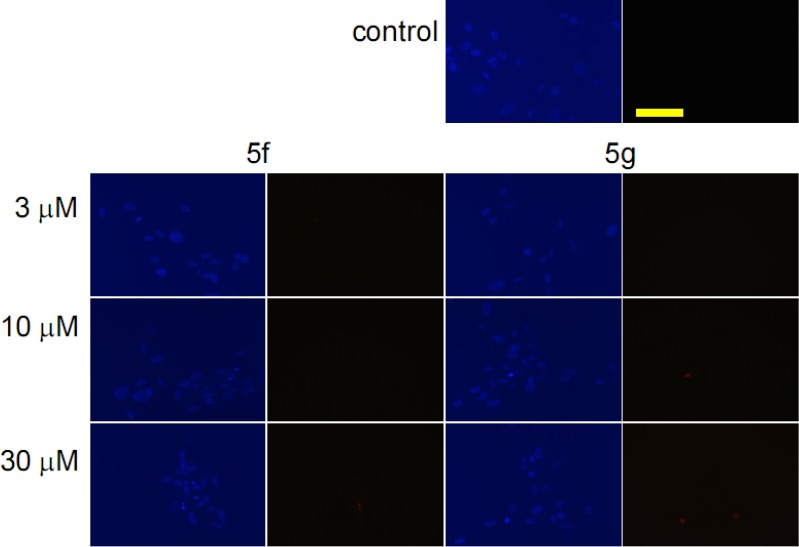
Since two of the selected steroids (5f and 5g) elicited substantial antiproliferative effects on MRC5 cells too, the staining was extended to these noncancerous cells with identical concentrations and incubation period (Fig. 4). Sparse nuclear condensation was evidenced in fibroblast cells treated with higher concentrations (10 or 30 μM), without marked PI staining.
Fig 4. Fluorescent microscopy images of Hoechst 33258-PI double staining.
Two separate pictures from the same field were taken for the two markers. MRC5 cells were treated with vehicle (control), or with 5f and 5g at the indicated concentrations. The blue fluorescence (left panels) indicates Hoechst 33258, and the red fluorescence (right panels) is a consequence of PI accumulation. The bar in the PI control picture indicates 100 μm.
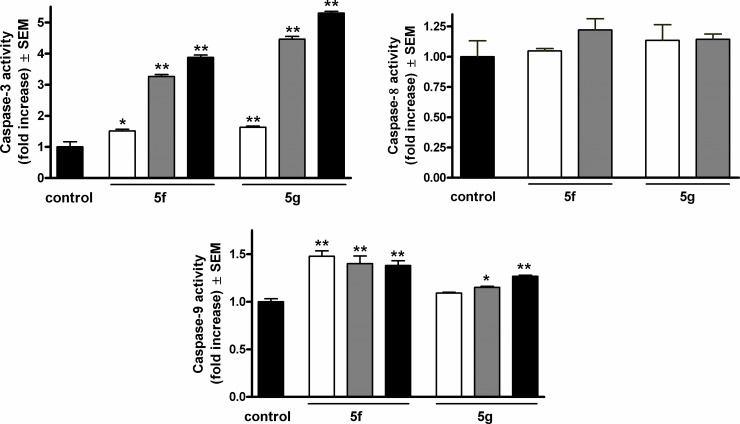
Caspase-3, caspase-8 and caspase-9 assays
On the basis of the results of cell cycle analysis and Hoechst 33258 and PI double staining, the effects of two selected compounds (5f and 5g) on the activities of the apoptotic key enzymes caspase-3, caspase-8 and caspase-9 were determined. Both steroid analogs activated the executive caspase-3 in a concentration-dependent way during a 24-h exposure (Fig. 5). Agent 5g exerted more pronounced action in this respect. The activity of the initiator caspase-9 was also significantly increased by both agents, though the extents were less pronounced. 24 h of exposure to agent 5f resulted in similarly elevated enzyme activities in the concentration range 3–30 μM, while agent 5g caused concentration-dependent caspase-9 activation. On the other hand, none of the tested agents elicited significant activation of caspase-8.
Fig 5. Induction of caspase-3, caspase-8 and caspase-9 activities after incubation with compounds 5f and 5g for 24 h.
White, gray and black columns denote 3, 10 and 30 μM of the given agent. * and ** denote p < 0.05 and p < 0.01, respectively, as compared with the control condition.
RT-PCR studies
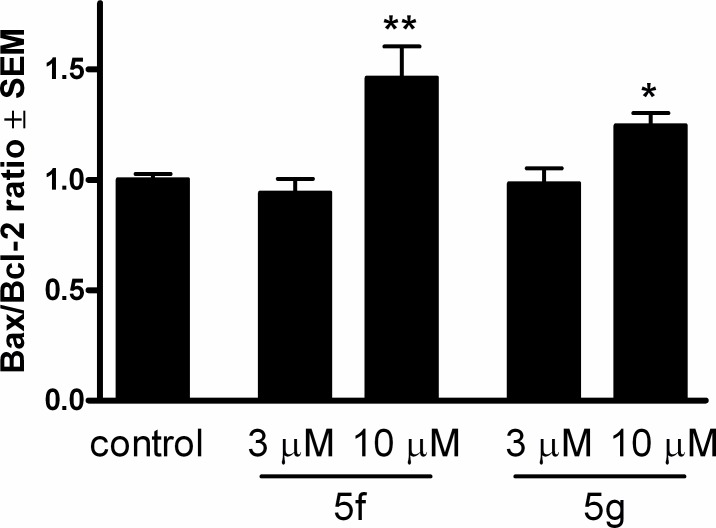
The expressions of the cell cycle regulator factors of the G2–M transition (CDK1, cyclin B1, cyclin B2 and cdc25B) and factors that play key roles in the mitochondrial pathway of apoptosis (Bax and Bcl-2) were determined at the mRNA level by means of RT-PCR. From the results of cell cycle analysis and caspase-3 and caspase-9 assays, the effects of the two most effective compounds at 3 and 10 μM on these mRNA sequences were determined following a 24-h incubation. Two well-characterized proteins responsible for the regulation of outer mitochondrial membrane permeability, Bax and Bcl-2, did not exhibit substantial differences (data not shown). However, under otherwise the same conditions, the ratio Bax/Bcl-2 was significantly higher at the higher concentration for both compounds (Fig. 6). This indicates activation of the intrinsic pathway of apoptosis.
Fig 6. Effects of compounds 5f and 5g on the Bax/Bcl-2 ratio after incubation of HeLa cells for 24 h.
* and ** denote p < 0.05 and p < 0.01, respectively, as compared with the control condition.
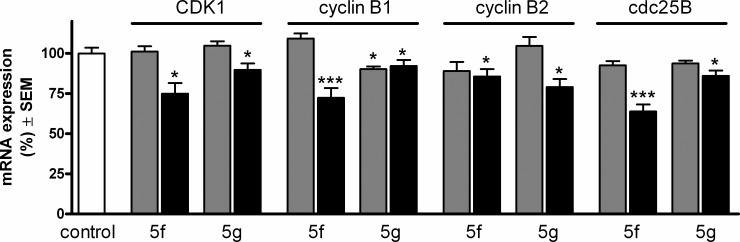
All four selected factors responsible for the G2–M transition were decreased after treatment with the higher concentration (10 μM). Moreover, the expression of cyclin B1 at the mRNA level was significantly reduced even after treatment with 3 μM of 5g (Fig. 7).
Fig 7. Expression of CDK1, cyclin B1, cyclin B2 and cdc25B at the mRNA level after incubation with 3 (gray columns) or 10 (black columns) μM of compounds 5f or 5g.
*, ** and *** denote p < 0.05, p < 0.01 and p < 0.001, respectively, as compared with the control condition.
Western blotting studies
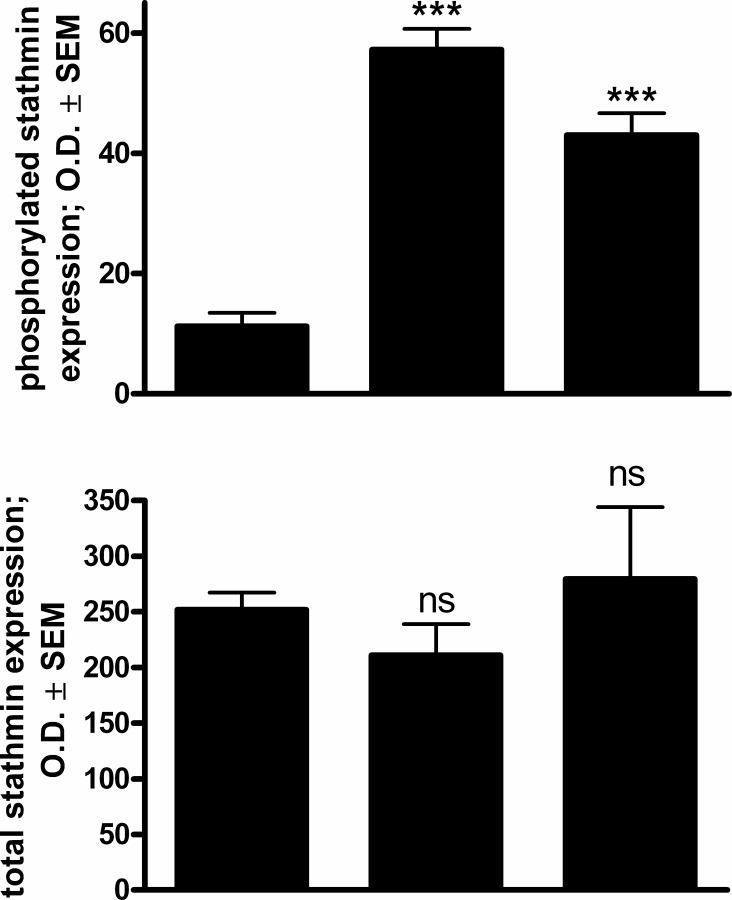
In response to a 48-h exposure to 10 μM 5f or 5g, the protein expression of phosphorylated stathmin, a microtubule destabilizing protein, was significantly increased severalfold as compared to untreated control cells (Fig. 8). On the other hand, the total amount of stathmin did not display any significant alteration indicating a change in the phosphorylation state of the protein.
Fig 8. Effects of 5f and 5g (10 μM) on the expression of phosphorylated (upper panel) and total (lower panel) stathmin protein in HeLa cells after incubation for 48 h, determined by western blot analysis.
Results are mean values ± SEM of the data on two separate measurements, n = 6. ns indicates p > 0.05, *** indicates p < 0.001 as compared with the untreated control cells. Panels below are representative membrane pictures.
Discussion
Compounds with a steroidal skeleton may exert an extremely broad variety of pharmacological activities in spite of the conserved chemical structure. The steroid backbone can therefore be utilized for the design and synthesis of a wide range of drug candidates.
A convincing amount of evidence has accumulated concerning the anticancer efficacy of natural steroids isolated from plants and their synthetic analogs. The cancer-preventing properties of cardiotonics (e.g. digoxin and digitoxin) traditionally used in the treatment of congestive heart failure have been recognized in retrospective epidemiological analyses [33]. These findings suggested the extension of the field of indication of the currently utilized digitaloids, and intensive research has therefore been initiated to develop a digitalis-based, novel class of antitumor agents [34].
The antiproliferative action of a set of solanidine analogs against HeLa cells was reported recently. Some of these steroids substantially inhibited the outflow of rhodamine-123 from murine lymphoma cells, mediated by the ABCB1 transporter [35].
Many steroidal alkaloids, including α-tomatidine and solanidine, inhibited the growth of human cancer cell lines at reasonable concentrations, whereas this action was less pronounced for their aglycones tomatidine and solanidine, respectively, indicating that the attractive properties of the steroid scaffold can be improved by an appropriately selected smaller substituent [36]. This concept was utilized in our current work, in which a substituted triazolyl ring was introduced onto the estrane core.
Estrogens are generally regarded as cellular proliferation-potentiating factors, and this is especially true for some malignancies of gynecological origin. Of the many estrane-related antiproliferative compounds recently described, the most widely investigated (and even subjected to clinical trial) is an endogenous metabolite of estrone, 2-methoxyestradiol, which exerts its anticancer effect by eliciting an imbalance of the microtubule dynamics and the direct inhibition of neoangiogenesis. [37, 38].
A set of estrone-16-oxime ethers were recently synthetized and tested for anticancer properties. The most potent analogs inhibited DNA synthesis in Hela cells, changed the expression of endogenous factors regulating the G1–S transition (retinoblastoma protein, CDK4 and p16) and induced apoptosis [39].
Since most, if not all, of the currently used anticancer agents possess the ability to initiate programmed cell death by modifying the balance between apoptotic and antiapoptotic signaling, the demonstration of apoptosis induction is a critical step in the development of an anticancer drug candidate [40]. Treatment with each of the four selected molecules (4i, 5f, 5g and 5h) for 24 h, even at the lowest concentration (3 μM), resulted in nuclear condensation with minimal or no disruption in membrane permeability, which is a morphological marker of apoptosis. Flow cytometry was utilized for a quantitative description of the cell cycle distribution of the treated cells. The most effective agents increased the hypodiploid (subG1) population in a concentration- and time-dependent manner. The reduced DNA stainability is considered to be a consequence of the progressive loss of DNA due to activation of endonuclease and the elimination fragments as part of the self-decomposition during apoptosis [28].
Since the present compounds contain an estrane skeleton, interaction with estrogenic receptors seems a possible mechanism of action. Additionally, their potential estrogenic activity is a reasonable question. On the basis of a well-established structure–activity relationship, any action mediated through estrogenic receptors can be excluded [41]. The 3-OH group of estradiol is needed as an H-bond donor in its interaction with its receptors, and the 3-methyl ether of estradiol exhibits less than 1% relative binding affinity. A bulky 16-alpha substituent is another structural feature which abolishes the estrogenic activities of estranes.
Caspases are cysteinyl aspartate proteinases present in almost all intact cells as inactive precursors which become activated by proteolytic cleavage upon receiving apoptotic stimuli. In mammals, 18 caspases have been identified and are classified into initiator and executionary caspases according to their role in the apoptotic machinery [42]. In spite of the fact that both caspase-3 and caspase-9 have been implicated in non-apoptotic functions, the activation of these enzymes in cancer cell cultures can still be regarded as an indication of apoptotic execution and initiation of its intrinsic pathway, respectively [43]. From the aspect of the caspase activation pattern of the two selected compounds, it may be pointed out to that they induce apoptosis via the intrinsic pathway. The activation of caspase-9 seems less pronounced, which is not unusual in view of the fact that this enzyme is the first element in a cascade, while caspase-3 is a terminal element and therefore a product of amplification.
Since the mitochondria maintain the capacity to initiate the controlled cellular decomposition upon receiving appropriate signals, they serve as a central hub in the regulation of the apoptosis–survival balance. The mitochondrial pathway of apoptosis is induced by the permeabilization of its outer membrane, resulting in cytochrome c release and the subsequent formation of the apoptosome, a multiprotein complex acting as a scaffold for successive events of apoptosis. The permeabilization of the outer mitochondrial membrane is therefore a crucial event and strictly regulated by the members of the Bcl-2 protein family. Although an antiapoptotic subfamily, including Bcl-2, Bcl-xl and Mcl-1, maintains a balance with some proapoptotic proteins (Bax and Bak), Bcl-2 and Bax are frequently investigated representative members of the two subgroups [44]. Their relation is generally regarded as a marker of the apoptotic–survival balance [45]. The higher concentrations of both selected compounds (5f and 5g) resulted in a significant increase in the ratio Bax/Bcl-2, reinforcing the mitochondrial origin of the detected apoptosis.
Cell division is a highly complex procedure involving an incompletely described array of regulatory steps, most of which are controlled by reversible protein phosphorylation. Specifically, the activity of the cyclin B–CDK1 complex is pivotal in regulating the G2–M phase transition, and especially in the initiation of chromosome condensation [46]. CDK1 is maintained inactive during most of the cell cycle through phosphorylation by Wee1 and Myt1 kinases. When CDK1 activity becomes required for the progression into the M phase, cdc25 phosphatase dephosphorylates the CDK1-containing complex. In mammals, three isoforms of cdc25 have been identified: cdc25A, cdc25B and cdc25C. The overexpression of CDC25A and CDC25B is reported to be involved in carcinogenesis and is associated with poor prognosis [47]. The functional differences between these isoforms have not been fully elucidated, but the normal development of CDC25B and CDC25C double knockout mice indicates that CDC25A is capable of performing all the essential operations [48]. CDC25B was proposed to be responsible for the initial activation of cyclin B–CDK1 complex during G2–M phase transition in HeLa cells [49]. This led us to select the most terminal executive regulators of cell cycle transition for determination of their expression at the mRNA level. Since the activity of the cyclin B–CDK1 complex is regulated in part by phosphorylation, and CDC25B is also phosphorylated by the complex itself, the mRNA level expression appears to be inadequate for a complete description of the tested steroids on the regulatory network. In spite of the limitations of the applied PCR technique, it seems relevant that the expressions of all four regulating factors were significantly decreased, indicating that the intervention in the cell cycle machinery is likely to occur at upstream levels.
Stathmin or oncoprotein 18 is a highly conserved oncoprotein frequently overexpressed in cancer cells which plays a crucial role in the early phase of mitosis, destabilizing the microtubules [50]. It is regulated by phosphorylation on four serine residues after turning off its destabilizing activity, and the cell can enter mitosis. Upon increased phosphorylation of stathmin, therefore, the accumulation of cells in the G2/M phase could be expected. This consideration is in agreement with the presented results and published findings. Treatment with an innovative formulation of paclitaxel resulted in a pronounced increase of gastric cancer cells in the G2/M phase and also the increased phosphorylation of stathmin [51]. Since this phosphorylation can be effected by a broad set of kinases, including CDK1 and CDK2, calmodulin-dependent protein kinase, and cAMP and cGMP dependent protein kinases, the exact enzyme responsible for the action of the presented steroids remains unclear.
Cancer selectivity is one of the most crucial parameters determining the decision as to the further development of a drug candidate. A viability assay and fluorescent staining on intact human fibroblast cells can certainly not be regarded as a complete toxicological evaluation. However, it is clearly promising that two of the four selected molecules (5f and 5g) exhibited higher calculated IC50 values against noncancerous than against malignant cells, and did not elicit substantial membrane damage up to 30 μM. A further one of the selected agents (4i) did not lead to 50% inhibition of fibroblast growth up to 30 μM, and the fourth (5h) proved selective for HeLa cells.
Conclusions
A set of estrone derivatives containing a substituted 16α-triazolyl on ring D were synthetized by click chemistry and investigated for their antiproliferative action on human adherent cell lines. Some of them exhibited in vitro potencies comparable to that of the clinically utilized reference agent cisplatin. The most potent analogs were subjected to additional investigations in order to characterize their pharmacological properties. Activation of the intrinsic pathway of apoptosis was evidenced by biochemical and morphological markers. Cell cycle blockade at the G2–M transition was additionally proved. The presented data demonstrate that estrone may be regarded as a suitable skeleton for the design of innovative antiproliferative drug candidates.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support from the Hungarian Scientific Research Fund (http://www.otka.hu/en) is gratefully acknowledged. Grant numbers: OTKA K-109293 (IZ) and K-101659 (JW). This study was supported by the European Union and co-financed by the European Social Fund (JM, RM, IZ); project number: TÁMOP-4.2.2.A-11/1/KONV-2012-0035 (http://www.u-szeged.hu/tamop422a0035-index). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bray F, Jemal A, Grey N, Ferlay J, Forman D (2012) Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 13: 790–801. 10.1016/S1470-2045(12)70211-5 [DOI] [PubMed] [Google Scholar]
- 2. Gupta A, Kumar BS, Negi AS (2013) Current status on development of steroids as anticancer agents. J Steroid Biochem Mol Biol 137: 242–270. 10.1016/j.jsbmb.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 3. Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew Chem Int Ed Engl 41: 2596–2599. [DOI] [PubMed] [Google Scholar]
- 4. Tornoe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67: 3057–3064. [DOI] [PubMed] [Google Scholar]
- 5. Hein CD, Liu XM, Wang D (2008) Click chemistry, a powerful tool for pharmaceutical sciences. Pharm Res 25: 2216–2230. 10.1007/s11095-008-9616-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krivopalov VP, Shkurko OP (2005) 1,2,3-Triazole and its derivatives. Development of methods for the formation of the triazole ring. Russ Chem Rev 74: 339. [Google Scholar]
- 7. Meldal M, Tornoe CW (2008) Cu-catalyzed azide-alkyne cycloaddition. Chem Rev 108: 2952–3015. 10.1021/cr0783479 [DOI] [PubMed] [Google Scholar]
- 8. Wu P, Fokin VV (2007) Catalytic azide-alkyne cycloaddition: Reactivity and applications. Aldrichim Acta 40: 7–17. [Google Scholar]
- 9. Genin MJ, Allwine DA, Anderson DJ, Barbachyn MR, Emmert DE, et al. (2000) Substituent effects on the antibacterial activity of nitrogen-carbon-linked (azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms Haemophilus influenzae and Moraxella catarrhalis. J Med Chem 43: 953–970. [DOI] [PubMed] [Google Scholar]
- 10. Buckle DR, Rockell CJ, Smith H, Spicer BA (1986) Studies on 1,2,3-triazoles. 13. (Piperazinylalkoxy) [1]benzopyrano[2,3-d]-1,2,3-triazol-9(1H)-ones with combined H1-antihistamine and mast cell stabilizing properties. J Med Chem 29: 2262–2267. [DOI] [PubMed] [Google Scholar]
- 11. Alvarez R, Velazquez S, San-Felix A, Aquaro S, De Clercq E, et al. (1994) 1,2,3-Triazole-[2',5'-bis-O-(tert-butyldimethylsilyl)-beta-D- ribofuranosyl]-3'-spiro-5"-(4"-amino-1",2"-oxathiole 2",2"-dioxide) (TSAO) analogues: synthesis and anti-HIV-1 activity. J Med Chem 37: 4185–4194. [DOI] [PubMed] [Google Scholar]
- 12. Majeed R, Sangwan PL, Chinthakindi PK, Khan I, Dangroo NA, et al. (2013) Synthesis of 3-O-propargylated betulinic acid and its 1,2,3-triazoles as potential apoptotic agents. Eur J Med Chem 63: 782–792. 10.1016/j.ejmech.2013.03.028 [DOI] [PubMed] [Google Scholar]
- 13. Liu JF, Sang CY, Xu XH, Zhang LL, Yang X, et al. (2013) Synthesis and cytotoxic activity on human cancer cells of carbamate derivatives of 4beta-(1,2,3-triazol-1-yl)podophyllotoxin. Eur J Med Chem 64: 621–628. 10.1016/j.ejmech.2013.03.068 [DOI] [PubMed] [Google Scholar]
- 14. Wölfling J, Hackler L, Mernyák E, Schneider G, Tóth I, et al. (2004) Neighboring group participation. Part 15. Stereoselective synthesis of some steroidal tetrahydrooxazin-2-ones, as novel presumed inhibitors of human 5alpha-reductase. Steroids 69: 451–460. [DOI] [PubMed] [Google Scholar]
- 15. Wölfling J, Oravecz EA, Ondre D, Mernyák E, Schneider G, et al. (2006) Stereoselective synthesis of some 17beta-dihydrooxazinyl steroids, as novel presumed inhibitors of 17alpha-hydroxylase-C17,20-lyase. Steroids 71: 809–816. [DOI] [PubMed] [Google Scholar]
- 16.Brodie A, Njar VCO (1999) 17-Azolyl steroids useful as androgen synthesis inhibitors. United States Patent, Patent Number: 5,994,335.
- 17.Hofmeister H, Bittler D, Michna H, Habenicht U, Fritzemeier KH, et al. (1992) Antiandrogenic [3,2c]pyrazole and [3,2-d]triazole steroids. United States Patent, Patent Number: 5,389,624.
- 18. Salvador JA, Pinto RM, Silvestre SM (2013) Steroidal 5alpha-reductase and 17alpha-hydroxylase/17,20-lyase (CYP17) inhibitors useful in the treatment of prostatic diseases. J Steroid Biochem Mol Biol 137: 199–222. 10.1016/j.jsbmb.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 19. Banday AH, Shameem SA, Gupta BD, Kumar HM (2010) D-ring substituted 1,2,3-triazolyl 20-keto pregnenanes as potential anticancer agents: Synthesis and biological evaluation. Steroids 75: 801–804. 10.1016/j.steroids.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 20. Kádár Z, Baji A, Zupkó I, Bartók T, Wölfling J, et al. (2011) Efficient approach to novel 1α-triazolyl-5α-androstane derivatives as potent antiproliferative agents. Org Biomol Chem 9: 8051–8057. 10.1039/c1ob06086d [DOI] [PubMed] [Google Scholar]
- 21. Kádár Z, Kovács D, Frank É, Schneider G, Huber J, et al. (2011) Synthesis and in vitro antiproliferative activity of novel androst-5-ene triazolyl and tetrazolyl derivatives. Molecules 16: 4786–4806. 10.3390/molecules16064786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kádár Z, Molnár J, Schneider G, Zupkó I, Frank É (2012) A facile 'click' approach to novel 15β-triazolyl-5α-androstane derivatives, and an evaluation of their antiproliferative activities in vitro. Bioorg Med Chem 20: 1396–1402. 10.1016/j.bmc.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 23. Frank É, Molnár J, Zupkó I, Kádár Z, Wölfling J (2011) Synthesis of novel steroidal 17α-triazolyl derivatives via Cu(I)-catalyzed azide-alkyne cycloaddition, and an evaluation of their cytotoxic activity in vitro. Steroids 76: 1141–1148. 10.1016/j.steroids.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 24. Frank E, Schneider G (2013) Synthesis of sex hormone-derived modified steroids possessing antiproliferative activity. J Steroid Biochem Mol Biol 137: 301–315. 10.1016/j.jsbmb.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 25. Kamal A, Reddy MK, Ramaiah MJ, Rajender, Reddy JS, et al. (2011) Synthesis and biological evaluation of estradiol linked pyrrolo[2,1-c][1,4]benzodiazepine (PBD) conjugates as potential anticancer agents. Bioorg Med Chem 19: 2565–2581. 10.1016/j.bmc.2011.03.015 [DOI] [PubMed] [Google Scholar]
- 26. Schönecker B, Ponsold K (1975) Steroids. XL. 16,17-Azido and 16,17-amino alcohols of estra-1,3,5-(10)-triene 3-methyl ether. Tetrahedron 31: 1113–1118. [Google Scholar]
- 27. Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 28. Vermes I, Haanen C, Reutelingsperger C (2000) Flow cytometry of apoptotic cell death. J Immunol Methods 243: 167–190. [DOI] [PubMed] [Google Scholar]
- 29. Ribble D, Goldstein NB, Norris DA, Shellman YG (2005) A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol 5: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Minorics R, Szekeres T, Krupitza G, Saiko P, Giessrigl B, et al. (2011) Antiproliferative effects of some novel synthetic solanidine analogs on HL-60 human leukemia cells in vitro. Steroids 76: 156–162. 10.1016/j.steroids.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 31. Molnár J, Ocsovszki I, Puskás L, Ghane T, Hohmann J, et al. (2013) Investigation of the antiproliferative action of the quinoline alkaloids kokusaginine and skimmianine on human cell lines. Curr Signal Transduct Ther 8: 148–155. [Google Scholar]
- 32. Lee CH, Lim H, Moon S, Shin C, Kim S, et al. (2007) Novel anticancer agent, benzyldihydroxyoctenone, isolated from Streptomyces sp. causes G1 cell cycle arrest and induces apoptosis of HeLa cells. Cancer Sci 98: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stenkvist B (1999) Is digitalis a therapy for breast carcinoma? Oncol Rep 6: 493–496. [PubMed] [Google Scholar]
- 34. Mijatovic T, Van Quaquebeke E, Delest B, Debeir O, Darro F, et al. (2007) Cardiotonic steroids on the road to anti-cancer therapy. Biochim Biophys Acta 1776: 32–57. [DOI] [PubMed] [Google Scholar]
- 35. Zupkó I, Molnár J, Réthy B, Minorics R, Frank É, et al. (2014) Anticancer and multidrug resistance-reversal effects of solanidine analogs synthetized from pregnadienolone acetate. Molecules 19: 2061–2076. 10.3390/molecules19022061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee KR, Kozukue N, Han JS, Park JH, Chang EY, et al. (2004) Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J Agric Food Chem 52: 2832–2839. [DOI] [PubMed] [Google Scholar]
- 37. Kambhampati S, Rajewski RA, Tanol M, Haque I, Das A, et al. (2012) A second-generation 2-Methoxyestradiol prodrug is effective against Barrett's adenocarcinoma in a mouse xenograft model. Mol Cancer Ther 12: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peyrat JF, Brion JD, Alami M (2012) Synthetic 2-methoxyestradiol derivatives: structure-activity relationships. Curr Med Chem 19: 4142–4156. [DOI] [PubMed] [Google Scholar]
- 39. Berényi A, Minorics R, Iványi Z, Ocsovszki I, Ducza E, et al. (2013) Synthesis and investigation of the anticancer effects of estrone-16-oxime ethers in vitro. Steroids 78: 69–78. 10.1016/j.steroids.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 40. Tolomeo M, Simoni D (2002) Drug resistance and apoptosis in cancer treatment: development of new apoptosis-inducing agents active in drug resistant malignancies. Curr Med Chem Anticancer Agents 2: 387–401. [DOI] [PubMed] [Google Scholar]
- 41. Anstead GM, Carlson KE, Katzenellenbogen JA (1997) The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 62: 268–303 [DOI] [PubMed] [Google Scholar]
- 42. Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14: 32–43. [DOI] [PubMed] [Google Scholar]
- 43. Connolly PF, Jager R, Fearnhead HO (2014) New roles for old enzymes: killer caspases as the engine of cell behavior changes. Front Physiol 5: 149 10.3389/fphys.2014.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brinkmann K, Kashkar H (2014) Targeting the mitochondrial apoptotic pathway: a preferred approach in hematologic malignancies? Cell Death Dis 5: e1098 10.1038/cddis.2014.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu T, Pang Q, Zhou D, Zhang A, Luo S, et al. (2014) Proteomic investigation into betulinic acid-induced apoptosis of human cervical cancer HeLa cells. PLoS One 9: e105768 10.1371/journal.pone.0105768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lindqvist A, Kallstrom H, Lundgren A, Barsoum E, Rosenthal CK (2005) Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome. J Cell Biol 171: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boutros R, Lobjois V, Ducommun B (2007) CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer 7: 495–507. [DOI] [PubMed] [Google Scholar]
- 48. Ferguson AM, White LS, Donovan PJ, Piwnica-Worms H (2005) Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases. Mol Cell Biol 25: 2853–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gabrielli BG, De Souza CP, Tonks ID, Clark JM, Hayward NK, et al. (1996) Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J Cell Sci 109: 1081–1093. [DOI] [PubMed] [Google Scholar]
- 50. Cassimeris L (2002) The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol 14: 18–24. [DOI] [PubMed] [Google Scholar]
- 51. Zhang C, Awasthi N, Schwarz MA, Hinz S, Schwarz RE (2013) Superior antitumor activity of nanoparticle albumin-bound paclitaxel in experimental gastric cancer. PLoS One 8: e58037 10.1371/journal.pone.0058037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.