Abstract
Background
Controversy persists regarding the use of coronary endarterectomy (CE) in patients with severe coronary artery disease. We compared the comorbidities and perioperative characteristics of patients undergoing coronary artery bypass grafting (CABG) with and without CE.
Methods
This study was performed in two private hospitals in Shiraz, Iran from May 2010 to December 2011 on 967 patients who underwent CABG without CE and 84 patients who underwent CABG with CE (the CE+ group). After follow-up at 9.66±3.65 months post-surgery, 28 patients from the CE+ group underwent angiography to evaluate the patency of grafts and native coronary vessels.
Results
Patients in the CE+ group had a more prevalent history of diabetes (48% vs. 36%) and number of diseased vessels (2.88±0.39 vs. 2.70±0.85). The overall hospital mortality was 1.8%, and no significant difference was observed between the two groups. In the 28 patients who underwent reangiography, 113 vessels were bypassed and 29 endarterectomies were performed, mostly on the left anterior descending artery (12 endarterectomies) and the right coronary artery (8 endarterectomies). In the endarterectomized vessels, a 66% patency rate was found in both the grafts and the native vessels. The native coronary vessels were more likely to be patent when the left internal mammary artery was used as a conduit than when a saphenous vein bypass graft was used.
Conclusion
The lack of a significant difference in postoperative complications in patients who underwent CABG with or without CE may indicate that CE does not expose patients to a higher risk of complications. Since most of the endarterectomized vessels were shown to be patent during the follow-up period, we propose that endarterectomy is a viable option for patients with severely diseased vessels.
Keywords: Coronary artery bypass surgery, Coronary endarterectomy, Comorbidity
INTRODUCTION
Coronary endarterectomy (CE) was introduced more than 40 years ago to improve blood flow to severely diseased coronary arteries in patients with coronary artery disease (CAD). However, it initially resulted in increased morbidity and mortality [1]. Since then, the number of elderly patients with CAD and its associated comorbidities has increased. Moreover, due to advances in non-surgical treatments, patients referred to cardiac surgeons usually suffer from severe CAD [2–5]. As a result, methods that were originally introduced for severely diseased arteries are now used more frequently. Therefore, despite the controversies regarding the efficacy and adverse effects of CE, it is frequently used as an adjunct to coronary artery bypass grafting (CABG). For instance, it has been reported that more than 50,000 coronary endarterectomies were performed in Germany in 2006 [2,3].
Considering the frequent use of CE in recent years, the effect of this procedure on short-term and long-term outcomes should be evaluated carefully. Although some studies, mainly from the 1990s, reported an increase in hospital morbidity and mortality after CE [6–8], others have shown an acceptable perioperative outcome following CE [2–4]. In recent years, CE has mostly been associated with good long-term survival rates [4]. In a study of 107 patients, the three-year survival rate was 91.2% [9]. Ten-year survival rates as high as 74% have been reported in patients undergoing CE [10]. However, the long-term mortality rate among patients who undergo CE has been shown to be slightly higher than among patients who undergo CABG alone, even in recent years [11].
Based on these results, most authors have concluded that CE is a suitable method in the treatment of severely diseased coronary arteries for which complete revascularization cannot be obtained otherwise [2–12]. Prospective randomized trials can provide an accurate and unbiased comparison of outcomes; however, the only well-designed prospective randomized trial so far published comparing CABG with or without CE had a sample size of 50 for each group [13]. The low sample size of this study resulted in a low statistical power, and therefore the study could not show a difference between either the short-term or long-term outcomes associated with CE and CABG [14].
One way of overcoming this methodological problem is to compare the patency of endarterectomized vessels with the patency of non-endarterectomized vessels in the same patient. Therefore, we performed this study with two goals: first, to compare patients who underwent CABG with or without CE and second, to compare the patency of grafts and native endarterectomized vessels with the patency of non-endarterectomized vessels in patients who underwent CE.
METHODS
Patients with CAD who underwent CABG in two private hospitals in Shiraz, Iran from May 2010 to December 2011 were enrolled in this study. All patients were informed about the aims and protocol of the study and written informed consent was obtained. The following baseline characteristics were recorded and entered into the data collection form used in the study: cardiac risk factors (including history of diabetes mellitus [DM], hypertension, hyperlipidemia, smoking, body mass index, and family history of cardiac disease), variables regarding disease severity in the preoperative evaluation (including the number of diseased vessels and the last ejection fraction), operative characteristics (including age at operation, on-pump or off-pump surgery, number of vessels bypassed), postoperative characteristics (including duration of stay in the intensive care unit and the overall duration of the hospital stay), and hospital mortality.
In some patients, CABG was carried out with CE (the CE+ group), while in others the operation was done without CE (the CE- group). During CABG, saphenous vein harvesting was performed using the open technique, and internal mammary conduits were not skeletonized. After CABG, the patients received 160 mg of aspirin once daily, which was changed to 80 mg per day after three months. Patients in the CE+ group received antiplatelet therapy with 80 mg of aspirin per day for three months along with warfarin in order to maintain an international normalized ratio range of 2 to 2.5. After three months, warfarin was discontinued. CE was performed if the coronary lumen was found to be completely occluded or if the lumen was shown to have severe atheroma during the incision of the vessels, making coronary bypass grafting impossible without an endarterectomy. In order to perform the endarterectomy, we made an incision of approximately 1 to 1.5 cm into the coronary vessels, a close endarterectomy was carried out with a dissector, and the atheromas were then excised from the incision site.
In the CE+ group, the patients’ follow-up data were entered into a questionnaire. At the follow-up visits, the presence of chest pain, the final ejection fraction, and the presence of abnormalities in transthoracic echocardiography were recorded and a stress test was done. The patients, especially those with a positive stress test, were then informed about the importance of follow-up angiography. Reangiography of coronary vessels was carried out in patients who consented to undergo assessment of graft patency and the patency of native grafted coronary vessels. In the reangiography, vessels were considered occluded if more than 50% stenosis was observed in the graft or native coronary vessels. Vessels with less than 50% stenosis or with a thrombolysis in myocardial infarction flow score of three were considered patent.
After gathering the data, variables were compared between the CE+ and CE- groups. In the CE+ group, variables were also compared between patients who underwent reangiography and those who did not. Univariate analysis was performed using the chi-squared or Fisher’s exact test for nominal variables and the t-test for quantitative variables. The survival rate for the patients who underwent follow-up was assessed with a Kaplan-Meier curve. Subsequently, in patients who underwent reangiography, the effect of the type of surgery (CABG with or without CE), the type of conduit (arterial versus venous), and the type of the grafted coronary vessel on graft patency and the patency of native coronary vessels was assessed with the log-rank test. All statistical analyses were done using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA).
RESULTS
From May 2010 to December 2011, 1051 patients (736 male and 315 female) with a mean age of 61.29 years underwent CABG procedures performed by a single surgeon at two private hospitals in Shiraz. Concomitant CE for at least one graft was performed on 84 patients (the CE+ group), while the other 967 operations were done without CE (the CE- group). Thus, CE was carried out in 7.99% of the patients.
As shown in Table 1, cardiovascular risk factors, especially hypertension and hyperlipidemia, were frequently found in patients who underwent CABG. DM was present in 37.5% of the patients, while hypertension and hyperlipidemia were present in more than 70% of the patients. A history of smoking and a family history of cardiac disease were found in 36.8% and 53.4% of the patients, respectively. The mean body mass index was 25.57±6.86 kg/m2.
Table 1.
Preoperative, operative, and postoperative characteristics of patients in both case and control groups
| Variable | Total patients (1,051) | Case group (84 patients) | Control group (967 patients) | p-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 70 | 65.47 | 70.42 | 0.342 |
| Cardiac risk factors | ||||
| Diabetes mellitus | 37.5 | 48.19 | 36.62 | 0.037 |
| Hypertension | 76.5 | 75.90 | 76.52 | 0.898 |
| Hyperlipidemia | 72.2 | 67.46 | 72.64 | 0.313 |
| Smoking | 36.8 | 39.50 | 36.61 | 0.605 |
| Family history | 53.4 | 61.33 | 52.74 | 0.151 |
| Body mass index (kg/m2) | 25.57±6.86 | 25.65±4.08 | 25.56±7.05 | 0.905 |
| Preoperative cardiac evaluation | ||||
| No. of diseased vessels | 2.72±0.828 | 2.88±0.395 | 2.70±0.854 | 0.001 |
| Last ejection fraction (%) | 47.12±15.58 | 46.56±14.44 | 47.17±15.68 | 0.732 |
| Operative characteristics | ||||
| Age at operation (yr) | 61.29±10.96 | 61.59±9.27 | 61.29±11.09 | 0.797 |
| Off-pump surgery | 7.1 | 0 | 7.75 | 0.008 |
| No. of bypassed vessels | 4.02±0.963 | 4.11±0.74 | 4.01±0.98 | 0.362 |
| Postoperative characteristics | ||||
| Duration of intensive care unit stay (day) | 2.65±2.87 | 2.78±1.09 | 2.64±2.97 | 0.677 |
| Duration of hospital stay (day) | 5.74±3.54 | 5.49±1.43 | 5.76±3.66 | 0.502 |
| Hospital mortality rate | 1.8 | 1.25 | 1.87 | 1 |
Values are presented as % for nominal variables and mean±standard deviation for quantitative variables. Analyses were done using the chi-square or Fisher’s exact test for nominal variables and the t-test for quantitative variables.
In a univariate analysis, DM was more frequent in the CE+ group than in the CE- group (p=0.037). The mean number of occluded vessels found in preoperative angiography was 2.72. Patients in the CE+ group had a higher number of occluded vessels compared to the CE- group (p=0.001).
Among the surgical variables, the only significant difference between the two groups was connected to the type of surgery. All operations in the CE+ group were on-pump while 7.75% of the operations in the CE- group were performed off-pump (p=0.008).
A total of 4,226 grafts were constructed in 1,051 patients, resulting in a mean of 4.02 bypassed vessels per patient. The mean duration of stay in the intensive care unit after surgery was 2.65±2.87 days, and patients were discharged from the hospital after 5.74±3.54 days. No significant difference was found between the two groups regarding the duration of stay in the intensive care unit or in the hospital (Table 1).
The hospital mortality rate was 1.8% and no significant difference was found between the two groups in this regard. Of the 1,051 patients, 19 (one patient in the CE+ group and 18 patients in the CE- group) died in the hospital after the operation. Higher age at the time of operation was the only significant risk factor for hospital mortality. The mean ages of the surviving and deceased patients were 61.15±10.94 years and 66.95±11.00 years, respectively (p=0.022).
A total of 89 vessels were endarterectomized in the patients belonging to the CE+ group. Endarterectomies were most frequently performed on the left anterior descending artery (LAD) and the right coronary artery, which were endarterectomized in 38 and 22 patients, respectively. Patients in the CE+ group were followed up after a mean of 9.66±3.65 months. During the follow-up visits, 28 patients underwent reangiography. The baseline characteristics, preoperative, operative, and postoperative variables, as well as variables relating to the follow-up visits are summarized in Table 2. As shown in Table 2, the only variable showing a significant difference between the patients who underwent reangiography and those who did not was the duration of hospital stay (4.96±0.43 days vs. 5.75±1.67 days, respectively, p=0.022).
Table 2.
Preoperative, operative, postoperative, and follow-up characteristics of patients who underwent concomitant CABG and CE, comparing those who underwent reangiography with those who did not
| Variable | CE+ group (84 patients) | Reangiography (28 patients) | No reangiography (56 patients) | p-value |
|---|---|---|---|---|
| Gender | ||||
| Male (%) | 65.47 | 71.42 | 62.5 | 0.417 |
| Cardiac risk factors | ||||
| Diabetes mellitus (%) | 48.19 | 50 | 47.27 | 0.814 |
| Hypertension (%) | 75.90 | 71.42 | 78.18 | 0.496 |
| Hyperlipidemia (%) | 67.46 | 71.42 | 69.09 | 0.659 |
| Smoking (%) | 39.50 | 42.85 | 37.73 | 0.654 |
| Family history (%) | 61.33 | 66.67 | 58.82 | 0.515 |
| Body mass index (kg/m2) | 25.65±4.08 | 26.66±2.77 | 25.14±4.55 | 0.108 |
| Preoperative cardiac evaluation | ||||
| No. of diseased vessels | 2.88±0.395 | 2.79±0.49 | 2.93±0.32 | 0.181 |
| Last ejection fraction (%) | 46.56±14.44 | 48.46±14.92 | 45.62±14.24 | 0.399 |
| Preoperative angiography | ||||
| Left main artery occlusion (%) | 8.33 | 3.84 | 10.86 | 0.408 |
| Right coronary artery occlusion (%) | 20.83 | 23.07 | 19.56 | 0.725 |
| Left anterior descending artery occlusion (%) | 98.61 | 100 | 97.82 | 1 |
| Left circumflex artery occlusion (%) | 94.44 | 92.30 | 95.65 | 0.616 |
| Operative characteristics | ||||
| Age at operation (yr) | 61.59±9.27 | 60.41±9.81 | 62.16±9.03 | 0.424 |
| No. of vessels bypassed | 4.11±0.74 | 4.11±0.75 | 4.11±0.75 | 0.982 |
| Postoperative characteristics | ||||
| Duration of intensive care unit stay (day) | 2.78±1.09 | 2.44±0.69 | 2.94±1.22 | 0.052 |
| Duration of hospital stay (day) | 5.49±1.43 | 4.96±0.437 | 5.75±1.67 | 0.022 |
| Hospital mortality (%) | 1.25 | 0 | 1.88 | 1 |
| Follow-up visits | ||||
| Last ejection fraction (%) | 52.06±9.22 | 51.19±12.82 | 52.49±6.92 | 0.560 |
| Follow-up echocardiography | ||||
| Positive (%) | 9.46 | 7.40 | 10.64 | 0.245 |
| Mild positive (%) | 5.41 | 11.11 | 2.13 | |
| Negative (%) | 85.13 | 81.49 | 87.23 | |
Values are presented as % for nominal variables and mean±standard deviation for quantitative variables. Analyses were done using the chi-square or Fisher’s exact test for nominal variables and the t-test for quantitative variables.
CABG, coronary artery bypass grafting; CE, coronary endarterectomy.
A significant difference (p<0.05).
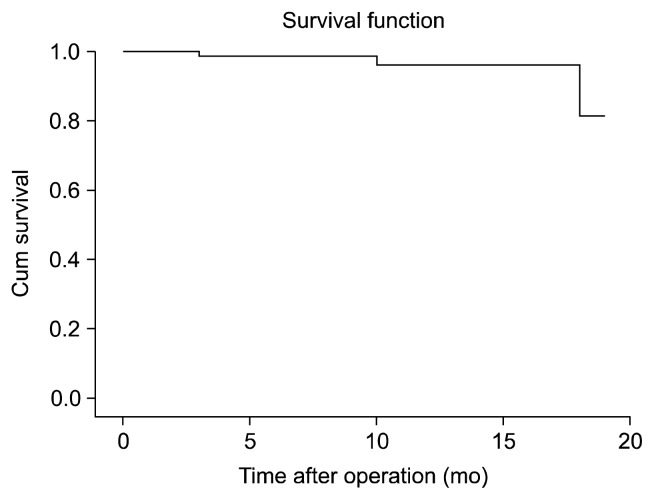
Only three patients in the CE+ group died after the operation: one patient died during the postoperative period and two patients died during the follow-up period. Of the latter two patients, one died from a cerebrovascular accident, whereas the death of the other patient was not related to his cardiac disease (Fig. 1). Because of the high rate of censored data, which reflects surviving patients, assessing the factors affecting survival was impossible.
Fig. 1.
Kaplan-Meier survival curve of patients who underwent coronary artery bypass grafting with coronary endarterectomy, showing a survival rate of nearly 80% after 20 months of follow-up.
Angiography was performed on 28 patients during follow-up visits in order to evaluate graft patency and the patency of native coronary vessels. In these 28 patients, 113 vessels were bypassed and 29 endarterectomies were performed. In 19 of the 29 endarterectomized vessels, both the graft and the native vessels were patent, resulting in a 66% patency rate for both the grafts and native vessels. Of the remaining 10 vessels, five grafts were patent while the native vessels were occluded, and in the other five, both the grafts and the native vessels were occluded. Of the 84 non-endarterectomized vessels, both the grafts and native vessels were patent in 80 vessels, resulting in a patency rate of approximately 95%.
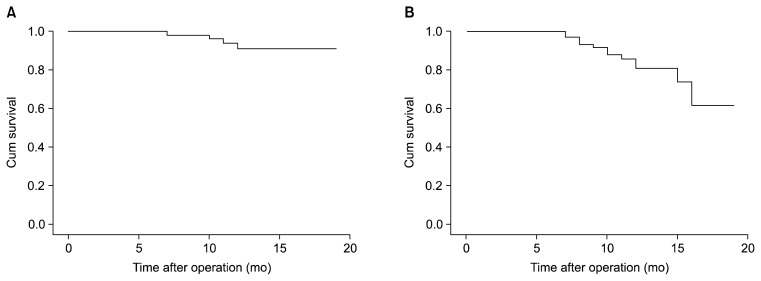
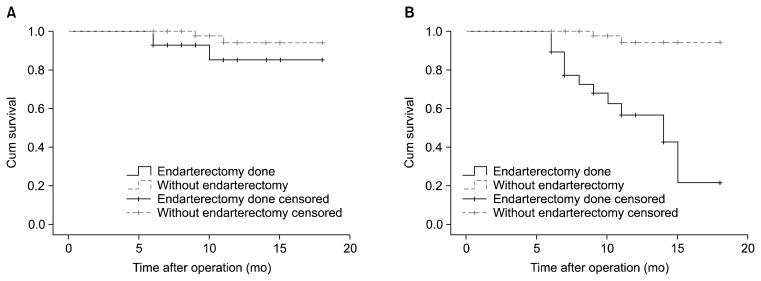
The Kaplan-Meier curve of graft patency and the patency of native coronary vessels is shown in Fig. 2. Log-rank analysis showed that the patency in endarterectomized vessels was significantly lower than in non-endarterectomized vessels (chi-square=25.17, p<0.001). However, graft patency did not differ between the two groups (chi-square=2.86, p=0.090) (Fig. 3).
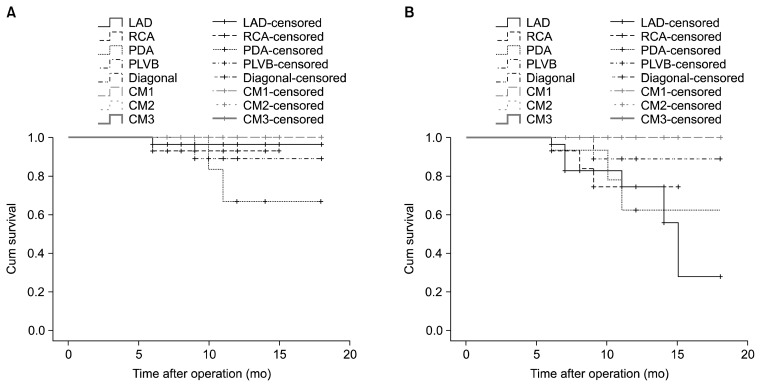
Fig. 2.
Kaplan-Meier curve of (A) graft patency and (B) patency of the grafted coronary vessels for patients who underwent angiography at a follow-up visit, showing relatively better patency in grafts compared to grafted coronary vessels, although no statistical analysis was performed.
Fig. 3.
Kaplan-Meier curve of (A) graft patency and (B) patency of the grafted coronary vessels for the 28 patients who underwent angiography at a follow-up visit for grafts with and without endarterectomy. Log-rank analysis shows that the patency of endarterectomized vessels was significantly lower than that of non-endarterectomized vessels. Graft patency also tended to be higher in the absence of endarterectomy.
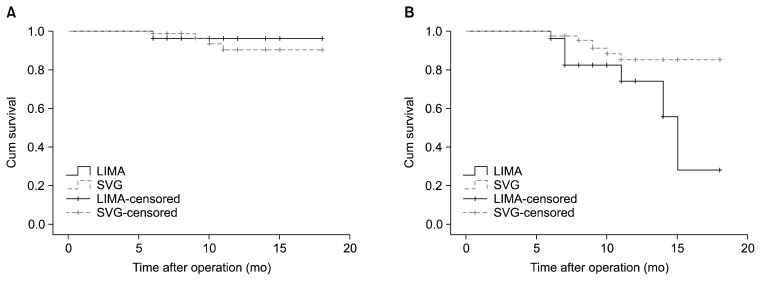
In our patients, the left internal mammary artery (LIMA) was used as the conduit for all grafts on the LAD while a saphenous vein graft was used for all other coronary vessels. Log-rank analysis showed that the type of conduit (the LIMA versus saphenous vein graft) did not affect graft patency (chi-square=0.06, p=0.796). However, the patency of native coronary vessels was higher in patients who underwent endarterectomy when the LIMA was used as the conduit compared with grafts in which a saphenous vein graft was used as the conduit (chi-square=4.51, p=0.034) (Fig. 4).
Fig. 4.
Kaplan-Meier curve of (A) graft patency and (B) patency of the grafted native coronary vessels for patients who underwent angiography at a follow-up visit, based on the type of graft conduit. The type of conduit (the LIMA vs. SVG) did not affect graft patency, but the patency of native vessels was greater when the LIMA was used as a conduit than when the SVG was used. LIMA, left internal mammary artery; SVG, saphenous vein graft.
The effect of the type of the coronary vessel on graft patency and the patency of native coronary vessels was assessed. In a log-rank analysis, graft patency (chi-square=4.89, p=0.672) and patency of native coronary vessels (chi-square=9.82, p=0.199) were not affected by the type of vessel operated upon (Fig. 5).
Fig. 5.
Kaplan-Meier curve of (A) graft patency and (B) the patency of the grafted coronary vessels for patients who underwent angiography at a follow-up visit, based on the type of the grafted coronary vessel. Log-rank analysis shows that neither graft patency nor the patency of the grafted coronary vessel was affected by the type of the grafted coronary vessel. LAD, left anterior descending artery; RCA, right coronary artery; PDA, posterior descending artery; PLVB, posterolateral ventricular branch; CM, circumflex marginal.
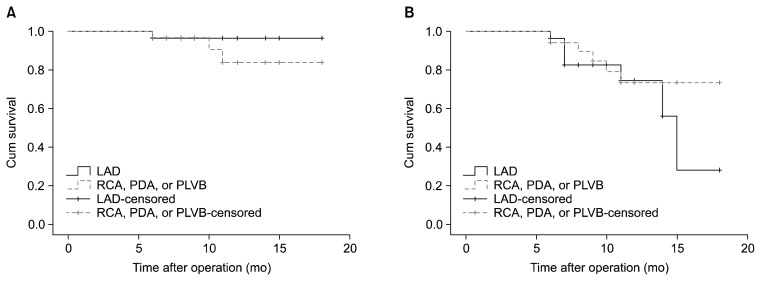
Graft patency and the patency of native coronary vessels were also compared between operations performed on left-sided coronary arteries (the LAD) and right-sided arteries (the right coronary artery, posterior descending artery, or posterolateral ventricular branch). Graft patency (chi-square=4.89, p=0.672) and the patency of native coronary vessels (chi-square=9.82, p=0.199) were not significantly different between the two groups (Fig. 6).
Fig. 6.
Kaplan-Meier curve of (A) graft patency and (B) the patency of the grafted coronary vessels for patients who underwent angiography at a follow-up visit, based on the side of the grafted coronary artery. Operations on the LAD were compared to operations done on the RCA, the branches of the PDA, or the PLVB. Log-rank analysis showed no difference in graft patency and the patency of native coronary vessels. LAD, left anterior descending artery; RCA, right coronary artery; PDA, posterior descending artery; PLVB, posterolateral ventricular branch.
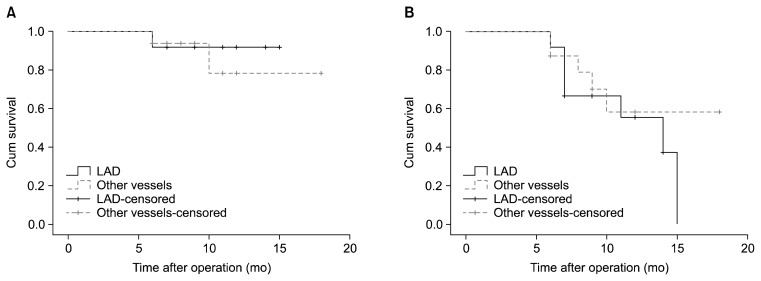
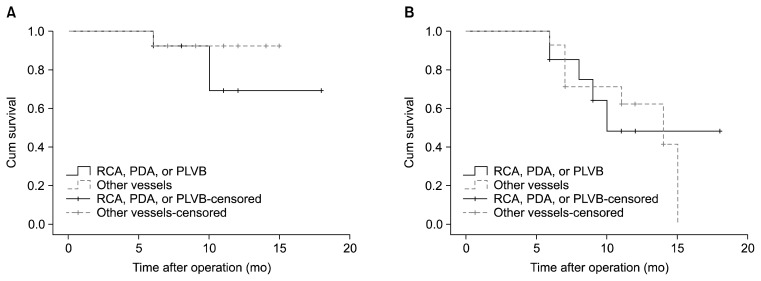
Finally, endarterectomized vessels that underwent reangiography were assessed. When operations on the LAD were compared to operations on the other vessels, neither graft patency (chi-square=0.17, p=0.67) nor the patency of native coronary vessels (chi-square=0.41, p=0.52) showed a significant difference (Fig. 7). Comparing operations on the right coronary artery, the posterior descending artery, or the posterolateral ventricular branch to operations on other vessels also showed no significant difference regarding graft patency (chi-square=0.63, p=0.42) and the patency of native coronary vessels (chi-square=0.00, p=0.99) (Fig. 8).
Fig. 7.
Kaplan-Meier curve of (A) graft patency and (B) the patency of the grafted coronary vessels for endarterectomized vessels that underwent angiography at a follow-up visit, separated into grafts on the LAD and grafts on other vessels. Log-rank analysis showed no difference in graft patency or the patency of native coronary vessels. LAD, left anterior descending artery.
Fig. 8.
Kaplan-Meier curve of (A) graft patency and (B) the patency of the grafted coronary vessels for endarterectomized vessels that underwent angiography at a follow-up visit, separated into grafts on the RCA, the PDA, or the PLVB, and grafts on other vessels. Log-rank analysis showed no difference in graft patency or the patency of native coronary vessels. RCA, right coronary artery; PDA, posterior descending artery; PLVB, posterolateral ventricular branch.
DISCUSSION
In our study, CE was performed on 7.99% of the patients. In previous studies, CE has been performed on 2% to 22% of patients who underwent CABG. In most studies, like ours, this percentage has been less than or equal to 10% [4,12,15–17]. For example, in a large study on 5,782 patients, CE was performed on 8.6% of patients [8]. These low percentages show that CE is performed selectively on a specific group of patients.
In our study, the prevalence of cardiac risk factors was very high. Most of our patients had hypertension and hyperlipidemia. In addition, nearly one third of the patients had DM. The mean number of diseased vessels in our study was 2.72, showing that our patients had severe CAD. The number of occluded vessels was greater in the CE+ group than in the CE- group (p=0.001). This shows that the disease was more severe in the CE+ group compared to the CE- group. However, the increased ejection fraction after surgery shows that CE can improve the cardiac fraction even in this high-risk group.
A univariate analysis showed that in our study, patients who underwent CE had a higher risk profile than patients who underwent CABG without CE. DM was found more frequently in the CE+ group and the patients in this group had more severe disease, as shown by the significantly higher number of diseased vessels in this group. Most previous studies have also shown that patients who underwent CE had a higher risk profile. Schmitto et al. [2] showed that most patients who underwent CE had three or four cardiac risk factors and mainly suffered from three-vessel disease. In another study based on a comparison between 97 patients who underwent CABG with CE and 154 patients who underwent CABG without CE, DM was found in 38% of the patients in the first group and 29% of the patients in the second group. However, the low statistical power and low sample size made these findings statistically insignificant. Nonetheless, patients who underwent CE had a greater number of bypassed vessels and longer ischemic time, which may indicate that the disease severity was significantly higher in this group [12]. In another large study, the prevalence of DM was significantly higher in the CE+ group (49% versus 34%). Three-vessel disease was also found more frequently in endarterectomized patients (90% versus 71%) [17]. Shapira et al. [16] also showed that DM (42% versus 32%), hypertension (80% versus 71%), and three-vessel disease (81% versus 70%) were more frequent in endarterectomized patients than in non-endarterectomized patients. Therefore, it can be concluded that CE is usually performed on patients with a high risk profile, which can affect comparisons between the CE+ and CE- patients. However, since a single surgeon carried out all of the operations in our study, the selection bias in our study was much lower than in other similar studies.
In our study, all operations in the CE+ group were performed on-pump while 7.75% of the surgical procedures in the CE- group were done off-pump. CABG procedures now frequently use the off-pump technique because of the deleterious effects of cardiopulmonary bypass in the on-pump technique [14]. It has been shown that the off-pump technique is feasible for CE with good postoperative outcomes [18,19]. However, the off-pump technique is mostly used for low-risk patients and in cases involving relatively few grafts [14–20]. The extensive and diffuse coronary artery disease of our patients in the CE+ group prevented us from using the off-pump technique.
Hospital mortality has always been a major component of the evaluation of postoperative outcomes. The overall hospital mortality rate was only 1.8% in our study and was insignificantly lower in patients who underwent CE. In recent studies, the hospital mortality rate of such patients has been 1% to 8% [2,4,8,9,12,15–17]. Our results were within the lowest part of this range, indicating an acceptable hospital mortality rate. Despite the higher risk profile of the patients in the CE+ group, we found no significant difference in the hospital mortality rate between the CE+ and CE- groups (1.25% versus 1.87%). Some other studies have found similar results. Shapira et al. [16] showed that CE+ and CE- patients did not have significantly different hospital mortality rates (2.0% versus 1.2%). In another study, the 30-day mortality rate was 2% for patients who underwent CABG without CE, 2.5% for patients who underwent endarterectomy in the right coronary artery, and 3.7% for patients who underwent endarterectomy in the LAD. These differences were not significant [11]. However, other studies have shown higher hospital and short-term mortality rates in patients who underwent CABG with CE. Sirivella et al. [10] compared the short-term and long-term outcomes of patients who underwent CABG with or without CE. The short-term 30-day mortality was not significantly different between the groups, although in contrast to our study, stays in the intensive care unit and in the hospital were significantly longer in patients who underwent CE. Tiruvoipati et al. [8] also reported significantly higher rates of postoperative mortality and complications in the CE+ group. In both of these studies, the patients who underwent CE had a higher risk profile than those who did not. The CE+ group had higher rates of DM, hypertension, and three-vessel disease, as well as greater disease severity as assessed by ejection fraction [8–10].
This discrepancy in outcomes, in which higher mortality rates occur in patients in the CE+ group, may partly be caused by associated comorbidities such as peripheral vascular disease and renal impairment, instead of CE itself [8].
In our study, we found that the only significant risk factor for hospital mortality was higher age at the time of the operation. This finding may reflect the very low rate of overall mortality in our study. Other studies have found multiple factors that predict hospital mortality, such as prolonged cardiopulmonary bypass time, recent acute myocardial infarction, recent operation, poor ventricular function, and female gender [10,11]. However, these studies have also shown that age predicts hospital mortality [11].
We found that patency in endarterectomized vessels was significantly lower than non-endarterectomized vessels (p<0.001), which is consistent with other reports [7–21]. Nonetheless, in light of the 66% patency rate that we observed in both grafts and native vessels in endarterectomized vessels, it seemed best to perform endarterectomies on these highly diseased vessels because the other option was to leave them alone, which would have resulted in a 0% patency rate.
We also found that 95% of both grafts and native coronary vessels were patent among the non-enarterectomized vessels. In a retrospective study, 28 patients who underwent CE were followed up at a mean of two years post-surgery and reangiography was done to evaluate the patency of native coronary vessels. It was found that 71% of the endarterectomized vessels and 93% of the non-endarterectomized vessels were patent, which was a statistically significant difference [22]. In another study with a mean follow-up of 7.1 years, reangiography showed that graft patency was 40% in endarterectomized vessels and 58% in non-endarterectomized vessels, which was likewise a statistically significant difference. As well, the rate of graft patency in patients who underwent CABG without CE was 65% [12]. Since vessel obstruction occurs over time, the lower overall patency was probably caused by the longer follow-up period compared to our study. Graft patency in patients who underwent CABG without CE was higher than in the non-endarterectomized vessels of patients who underwent CE, and patency in both of the above categories was higher than in the endarterectomized vessels of patients who underwent CE. These findings support the idea that both a high risk profile and the CE procedure itself decrease the long-term patency of vessels in patients who undergo CE. Keogh et al. [21] used angioscopy to evaluate intravascular morphology after CE and found intimal flaps or truncation of the side branches in some of the endarterectomized vessels.
Still other studies have shown no difference in graft patency between endarterectomized and non-endarterectomized vessels. Schwann et al. [17] showed that after 30 months, the graft patency rate was 33% in endarterectomized vessels and 25% in non-endarterectomized vessels (p=0.16). We hypothesize that the better graft patency of endarterectomized vessels in this study compared to others is because of their frequent use of arterial conduits for endarterectomized vessels. Instead of saphenous vein bypass grafts, they used the LIMA in grafts on the LAD. Similarly, in another study, significantly higher 10-year patency was reported for patients who underwent LIMA-LAD grafting [23]. Similar results were found in other studies that assessed the use of the LIMA as a conduit [4,11,22,24]. Thus, internal mammary artery grafting for lesions of the anterior descending coronary artery is preferable whenever indicated and technically feasible.
One advantage of our study compared to others [7,12,17,21,22] was that we compared both graft patency and the patency of native coronary vessels of endarterectomized and non-endarterectomized vessels. We showed that there is no significant short-term difference between the two groups of grafts. However, the presence of more severe disease in endarterectomized vessels resulted in lower patency of the native coronary vessels. Graft occlusion is categorized as early and late. Early occlusion takes place within three to six months and is usually caused by thrombosis, whereas late occlusion is a consequence of atherosclerosis. Since most of our patients were followed up for more than six months, we can conclude that CE did not lead to occlusion as a consequence of thrombosis caused by trauma to the endovascular area. In other words, we showed that the poor condition of the vessel wall in endarterectomized vessels does not affect the graft in the short term.
Our study also had some limitations. Although we scheduled follow-up visits for the patients and attempted to convince them that follow-up angiography is necessary to evaluate the grafted and main native vessels, most of the patients who underwent CE did not consent to reangiography. This led to a lower sample size for statistical analysis. Many patients do not consent to reangiography after CABG because they would not like to undergo another invasive procedure, especially if their stress tests were negative.
Another limitation of our study is that we did not perform a propensity score matching analysis to compare patients who underwent CABG with or without CE. The other limitation is that we did not have data about regional wall motion after CE. The reason for this was that most of our patients come from rural areas. After their operations, they return to their place of residence and generally will not come back to the clinic, even for free-of-charge follow-up visits or echocardiography. In conclusion, the lack of a significant difference between postoperative complications in patients who underwent CABG with or without CE may indicate that CE does not expose the patients to a higher level of risk. Therefore, cardiac surgeons should not avoid performing endarterectomies.
Cardiac surgeons may encounter severely diseased coronary vessels with extensive atheroma and choose either to leave such vessels alone or perform an endarterectomy. Since a majority of the endarterectomized vessels in our study were patent during the follow-up period, we recommend endarterectomy as a viable option for treating patients with severely diseased vessels.
ACKNOWLEDGMENTS
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan) (Fund No. KTCS04-014).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Bailey CP, May A, Lemmon WM. Survival after coronary endarterectomy in man. J Am Med Assoc. 1957;164:641–6. doi: 10.1001/jama.1957.02980060017005. [DOI] [PubMed] [Google Scholar]
- 2.Schmitto JD, Kolat P, Ortmann P, et al. Early results of coronary artery bypass grafting with coronary endarterectomy for severe coronary artery disease. J Cardiothorac Surg. 2009;4:52. doi: 10.1186/1749-8090-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gummert JF, Funkat A, Beckmann A, et al. Cardiac surgery in Germany during 2006: a report on behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg. 2007;55:343–50. doi: 10.1055/s-2007-965485. [DOI] [PubMed] [Google Scholar]
- 4.Byrne JG, Karavas AN, Gudbjartson T, et al. Left anterior descending coronary endarterectomy: early and late results in 196 consecutive patients. Ann Thorac Surg. 2004;78:867–73. doi: 10.1016/j.athoracsur.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Tiruvoipati R, Loubani M, Peek G. Coronary endarterectomy in the current era. Curr Opin Cardiol. 2005;20:517–20. doi: 10.1097/01.hco.0000182834.03402.43. [DOI] [PubMed] [Google Scholar]
- 6.Asimakopoulos G, Taylor KM, Ratnatunga CP. Outcome of coronary endarterectomy: a case-control study. Ann Thorac Surg. 1999;67:989–93. doi: 10.1016/S0003-4975(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 7.Taşdemir O, Kiziltepe U, Karagoz HY, Yamak B, Korkmaz S, Bayazit K. Long-term results of reconstructions of the left anterior descending coronary artery in diffuse atherosclerotic lesions. J Thorac Cardiovasc Surg. 1996;112:745–54. doi: 10.1016/S0022-5223(96)70061-2. [DOI] [PubMed] [Google Scholar]
- 8.Tiruvoipati R, Loubani M, Lencioni M, Ghosh S, Jones PW, Patel RL. Coronary endarterectomy: impact on morbidity and mortality when combined with coronary artery bypass surgery. Ann Thorac Surg. 2005;79:1999–2003. doi: 10.1016/j.athoracsur.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Marinelli G, Chiappini B, Di Eusanio M, et al. Bypass grafting with coronary endarterectomy: immediate and long-term results. J Thorac Cardiovasc Surg. 2002;124:553–60. doi: 10.1067/mtc.2002.124670. [DOI] [PubMed] [Google Scholar]
- 10.Sirivella S, Gielchinsky I, Parsonnet V. Results of coronary artery endarterectomy and coronary artery bypass grafting for diffuse coronary artery disease. Ann Thorac Surg. 2005;80:1738–44. doi: 10.1016/j.athoracsur.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamov D, Tamaris M, Guru V, et al. Clinical results of endarterectomy of the right and left anterior descending coronary arteries. J Card Surg. 1999;14:16–25. doi: 10.1111/j.1540-8191.1999.tb00945.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferraris VA, Harrah JD, Moritz DM, Striz M, Striz D, Ferraris SP. Long-term angiographic results of coronary endarterectomy. Ann Thorac Surg. 2000;69:1737–43. doi: 10.1016/S0003-4975(00)01293-5. [DOI] [PubMed] [Google Scholar]
- 13.Abid AR, Farogh A, Naqshband MS, Akhtar RP, Khan JS. Hospital outcome of coronary artery bypass grafting and coronary endarterectomy. Asian Cardiovasc Thorac Ann. 2009;17:59–63. doi: 10.1177/0218492309102609. [DOI] [PubMed] [Google Scholar]
- 14.Halkos ME, Puskas JD. Off-pump versus on-pump coronary artery bypass grafting. Surg Clin North Am. 2009;89:913–922. ix. doi: 10.1016/j.suc.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Sundt TM, 3rd, Camillo CJ, Mendeloff EN, Barner HB, Gay WA., Jr Reappraisal of coronary endarterectomy for the treatment of diffuse coronary artery disease. Ann Thorac Surg. 1999;68:1272–7. doi: 10.1016/S0003-4975(99)00693-1. [DOI] [PubMed] [Google Scholar]
- 16.Shapira OM, Akopian G, Hussain A, et al. Improved clinical outcomes in patients undergoing coronary artery bypass grafting with coronary endarterectomy. Ann Thorac Surg. 1999;68:2273–8. doi: 10.1016/S0003-4975(99)01050-4. [DOI] [PubMed] [Google Scholar]
- 17.Schwann TA, Zacharias A, Riordan CJ, Durham SJ, Shah AS, Habib RH. Survival and graft patency after coronary artery bypass grafting with coronary endarterectomy: role of arterial versus vein conduits. Ann Thorac Surg. 2007;84:25–31. doi: 10.1016/j.athoracsur.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 18.Hussain I, Ghaffar A, Shahbaz A, et al. In hospital outcome of patients undergoing coronary endarterectomy: comparison between off-pump vs on pump CABG. J Ayub Med Coll Abbottabad. 2008;20:31–7. [PubMed] [Google Scholar]
- 19.Vohra HA, Kanwar R, Khan T, Dimitri WR. Early and late outcome after off-pump coronary artery bypass graft surgery with coronary endarterectomy: a single-center 10-year experience. Ann Thorac Surg. 2006;81:1691–6. doi: 10.1016/j.athoracsur.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Lattouf OM, Thourani VH, Kilgo PD, et al. Influence of on-pump versus off-pump techniques and completeness of revascularization on long-term survival after coronary artery bypass. Ann Thorac Surg. 2008;86:797–805. doi: 10.1016/j.athoracsur.2008.04.065. [DOI] [PubMed] [Google Scholar]
- 21.Keogh BE, Bidstrup BP, Taylor KM, Sapsford RN. Angioscopic evaluation of intravascular morphology after coronary endarterectomy. Ann Thorac Surg. 1991;52:766–71. doi: 10.1016/0003-4975(91)91208-D. [DOI] [PubMed] [Google Scholar]
- 22.Dagenais F, Cartier R, Farinas JM, Leclerc Y, Hudon G. Coronary endarterectomy revisited: mid-term angiographic results. Can J Cardiol. 1998;14:1121–5. [PubMed] [Google Scholar]
- 23.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 24.Zacharias A, Habib RH, Schwann TA, Riordan CJ, Durham SJ, Shah A. Improved survival with radial artery versus vein conduits in coronary bypass surgery with left internal thoracic artery to left anterior descending artery grafting. Circulation. 2004;109:1489–96. doi: 10.1161/01.CIR.0000121743.10146.78. [DOI] [PubMed] [Google Scholar]