Abstract
Systemic Lupus Erythematosus is one of the classic examples of autoimmune diseases among human beings and is a rare disease in Pakistani population. Clinically it is a quite diverse and complicated autoimmune disease in a sense that it involves multiple organs of the body and mimics with other diseases as well. This study focused on the distribution of HLA alleles in SLE patients with ACE I/D Polymorphism. A total of 122 individuals were enrolled in this study, 61 were the SLE patients who fulfilled revised ACR criteria and 61 were the healthy controls. Mean age of SLE patients at diagnosis was 30.35 ± 1.687 years (12-68 years). ACE gene I/D polymorphism was performed by nested PCR and DNA based HLA typing technique was used. ACE gene I/D polymorphism of Intron16 was studied and found to be involved in the activity of SLE. There is high frequency of HLA-A*01, HLA-B*40, HLA-DRB1*01 alleles in SLE patients with ACE DD genotype. The distribution of HLA-A, -B, -DRB1 alleles was analyzed in SLE patients with various disease phenotypes. HLA-A*01 and HLA-B*40 was the most common allele found in SLE patients with the involvement of skin. HLA-A*01, -A*03, HLA-B*13 and -B*46 were common in SLE patients with arthritis while HLA-A*26 and -A*69 were commonly found in Lupus nephritis cases. SLE patients involving both skin and kidney had an allele HLA-DRB1*01 common in them.
KEY WORDS: systemic lupus erythematosus, Human leukocyte antigen, polymorphism, angiotensin converting enzyme, Lupus nephritis
INTRODUCTION
Lupus is one of the examples of autoimmune diseases among human beings. There are different types of lupus like cutaneous lupus, neonatal lupus, drug induced lupus but the most important one is Systemic lupus Erythematosus (SLE). SLE is a chronic illness and can prove to be fatal when it involves multiple organs of the body like brain, heart, kidney, skin. This is a multisystem disorder that occurs with the contribution of hormonal, genetic, immunological, and environmental factors. Immunogenetic factors are important in two ways; one by influencing the disease penetration and secondly by the production of Autoantibodies [1]. Angiotensin converting Enzyme (ACE) gene contains a polymorphism based on the presence (Insertion I) or absence (deletion D) of a non-sense fragment. In humans, I/D polymorphism is located in Intron 16 of the angiotensin gene. This polymorphism is responsible for the ACE activity level which increases 2-fold in homozygous deletion carriers (D/D) as compared to homozygous insertion carriers (I/I) while I/D carriers show intermediate ACE activity. The influence of ACE I/D polymorphism on pathological conditions mediated through ACE activity is found to be associated with various diseases and one of them is SLE [2]. Similarly Major Histocompatibility Complex (MHC) also shows a high degree of polymorphism and these polymorphisms are inherited, lead to autoimmune disorders like SLE. This study mainly focused on the distribution of HLA alleles in SLE patients with ACE I/D Polymorphism [3].
MATERIALS AND METHODS
Patients
A total of 122 individuals were enrolled in this study. Of the 122, 61 were the SLE patients who fulfilled revised ACR criteria and 61 were the healthy controls [4].
Procedures
Blood samples from lupus patients were collected from Rheumatology and Nephrology Departments of different hospitals of Lahore. Consents of the patients were obtained on a consent form and Ethical Committee (University of the Punjab), approved the study design. Physical, biochemical and hematological parameters were also collected. Hematological parameters like Complete Blood Count (CBC), prothrombin time and Biochemical parameters like complete urine analysis, serum urea, and creatinine were measured only in those cases where history of the patient suggested. Immunological Parameters (ANA, ds-DNA, Anti-Sm, Anti-SSAo, La, nDNA, Anti-histone) were measured by using Indirect ELISA Technique (Orgentec kits). Nested PCR for ACE I/D Polymorphism and DNA based HLA typing was performed for studying 22 alleles at locus A, 37 alleles at locus B and 17 alleles at locus DRB1.
Statistical analysis
Statistical analysis was done by using SPSS ver 13.
RESULTS
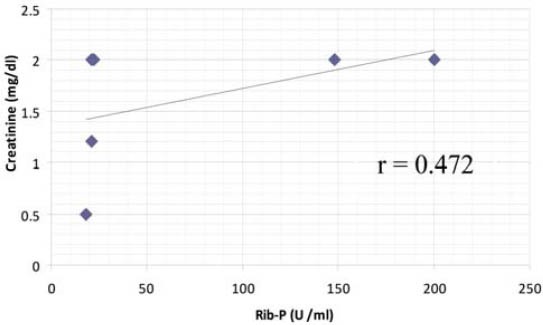
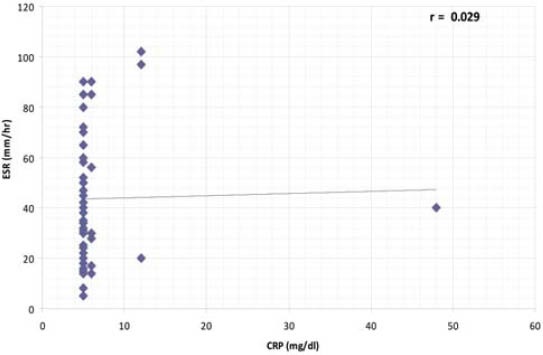
Autoantibodies (anti-dsDNA, anti-Sm, anti-Rib-P, anti-SSA, anti-SSB, anti-histone, anti-MCV), cytokine (IL-15), and immunoglobulins (IgA, IgM, IgG) were found to be correlated with one another in one way or other. Anti-SSB and antiSSA were the autoantibodies that were found to co-exist in the sera of SLE patients, there was a positive correlation between anti-SSA and anti-SSB but it was not found to be statistically significant (r = 0.232, p > 0.05). Interestingly a significant positive correlation was found between anti-histone and anti-SSB (r = 0.321, p > 0.05). A significant positive correlation was found between anti-MCV antibodies and IL-15 indicating that if the amount of anti-MCV antibodies increases, the level of IL-15 also increases (Table 1). SLE patients (9.83%) which were positive for ribosomal P antibodies were also found to be positive for anti-dsDNA antibodies. Of these ribosomal P antibodies positive patients, 66.66% were the patients of lupus nephritis and here lupus nephritis was defined by high creatinine level and proteinuria. In this study, a positive correlation was found between ribosomal P antibodies and lupus nephritis represented by a linear line but statistically it was not found to be significant (r = 0.472) at a level of 0.05 (Figure 1). The relationship between ESR and CRP in SLE patients was linear as a positive correlation was found between ESR and CRP (r = 0.029, p = NS) but it was not significant (Figure 2). A number of syndromes overlapped with SLE like Sjogren’s syndrome, Scleroderma, Rheumatoid arthritis, Antiphospholipid syndrome and Budd Chiari syndrome. Photosensitivity was found to be found in most of the SLE patients overlapping with various syndromes (Figure 3). ACE I/D polymorphism was found to be significantly associated with lupus activity. SLE patients with II genotype had higher serum levels of ANA (100%), anti-SSA (75%), anti-SSB (50%), anti-MCV (75%) and of nDNA (100%). SLE patients with ID genotype had higher serum levels of ANA (100%), anti-dsDNA (100%), anti-Sm (33.33%), and also of nDNA (100%) like that of SLE patients with II genotype. SLE patients with DD genotype were positive for anti-histone (3.7%), anti-Ribosomal P antibodies (11.11%) and also for IL-15 while SLE patients with II and ID genotype were found to be negative for them (Figure 4). Levels of C3 and C4 were divided in to four groups in SLE patients with different ACE genotypes. First group indicated normal C3 and C4 levels, second group represented low C3 but normal C4 level, similarly third group showed normal C3 and low C4 while fourth one represented low C3 as well as low C4 levels. 25% of the SLE patients with II genotype had normal C3 and C4 levels while 75% of them had normal C3 but low C4 levels. 33.33% of the SLE patients with ID genotype had normal complement levels, 33.33% had low C3 and C4 levels and the remaining 33.33% lie in to the group that had normal C3 but low C4 levels. Variations in C3 and C4 levels were found in SLE patients with DD genotype, like 38.88% of such patients had normal C3 and C4 levels, 14.8% had low C3 and C4 levels, 40.74% had normal C3 but low C4 levels. Interestingly, 5.55% of the SLE patients with DD genotype also lie in to the group that had low C3 but normal C4 levels (Figure 5a). 100% of SLE patients with II and ID genotype had high IgG and IgM levels. Similarly, high percentage of SLE patients with DD genotype (96.3%) had high IgG level while 3.7% of such patients showed normal IgG level. Only 25.9% of the SLE patients with DD genotype had high IgM levels and 74.1% of these patients showed normal IgM levels (Figure 5b). HLA alleles found in present SLE subjects with ACE II and ID genotypes are listed in Table-2 and in Table 3. However, there is high frequency of HLA-A*01, HLA-B*40, HLA-DRB1*01 alleles in SLE patients with DD genotype (Figure 6a, Figure 6b, Figure 6c). These are the HLA alleles that showed overall significant association with SLE patients. We also analyzed distribution of HLA-A, -B, -DRB1 alleles in SLE patients with various disease phenotypes like skin involvement, lupus nephritis, rheumatoid arthritis or a combination of them. HLA-A*01 was the most common allele found in SLE patients with the involvement of skin. HLA-A*01 and -A*03 were common in SLE patients with arthritis while HLA-A*26 and -A*69 were commonly found in SLE patients with nephritis (Figure 7a). On the other hand, HLA-B*40 was the most common allele found in SLE patients with the involvement of skin. HLA-B*13 and -B*46 were common in SLE patients with arthritis while -B*37 was commonly found in SLE patients with nephritis. As we know that SLE is a multifactorial disease; the Figure 7b represents that the HLA-B*39 allele is commonly found in SLE patients with the combination skin and nephritis. SLE patients involving both skin and kidney had an allele HLA-DRB1*01 common in them but we can’t comment on other manifestations as the differences were not appreciable (Figure 7c).
TABLE 1.
Correlation between different parameters used in the diagnosis of SLE
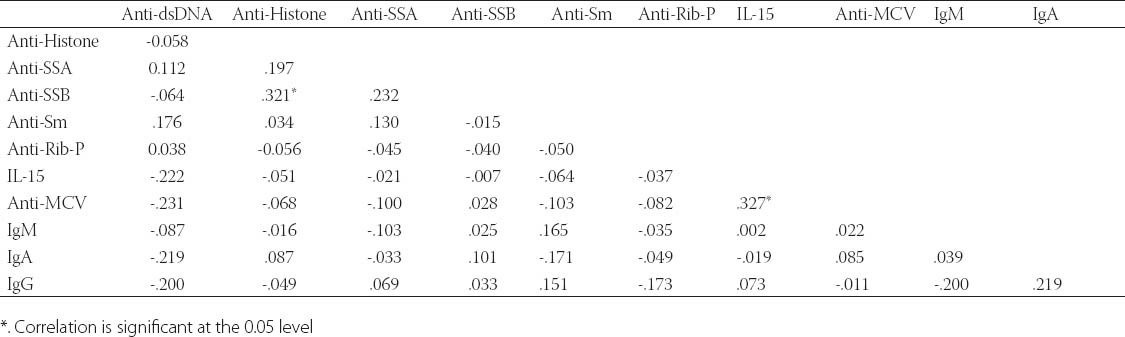
FIGURE 1.
Correlation between Ribosomal P antibodies and lupus nephritis
FIGURE 2.
Correlation between inflammatory markers (ESR and CRP) in SLE patients
FIGURE 3.
Manifestations commonly seen in various syndromes overlapping with SLE
FIGURE 4.
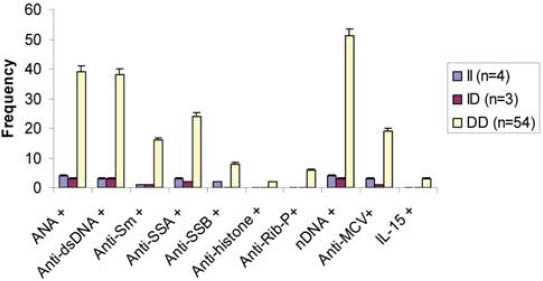
Serum Levels of different parameters in SLE patients with ACE genotypes
ANA: Antinuclear antibodies; dsDNA: Double stranded DNA; Anti-Sm: Anti-Smith; SSA: Soluble Substance A; SSB: Soluble Substance B; Anti-Rib-P: Anti-ribosomal P antibodies; nDNA: nucleosomal DNA; MCV: Mutated Citrullinated Vimentin; IL-15: Interleukin 15.
FIGURE 5.
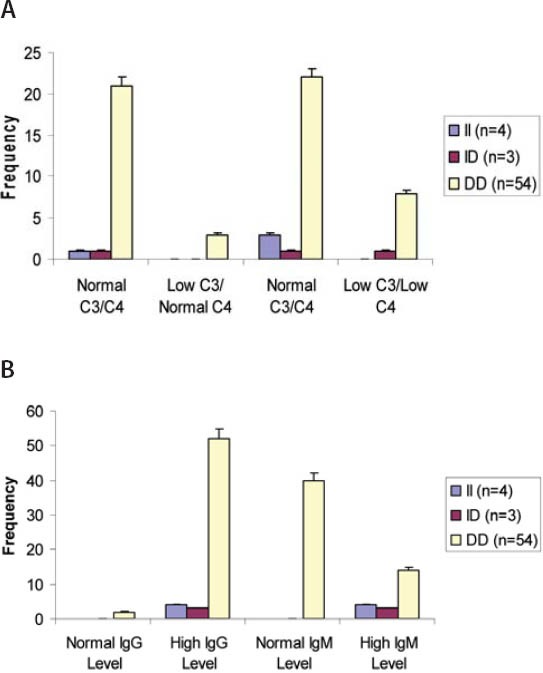
(A) Levels of C3 and C4 in SLE patients with different ACE genotypes (B) Levels of immunoglobulins IgG and IgM in SLE patients with different ACE genotype
TABLE 2.
Distribution of HLA alleles in SLE patients with ACE II genotype (n=4)
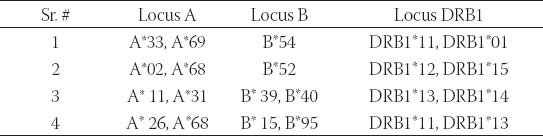
TABLE 3.
Distribution of HLA alleles in SLE patients with ACE ID genotype (n=3)

FIGURE 6.
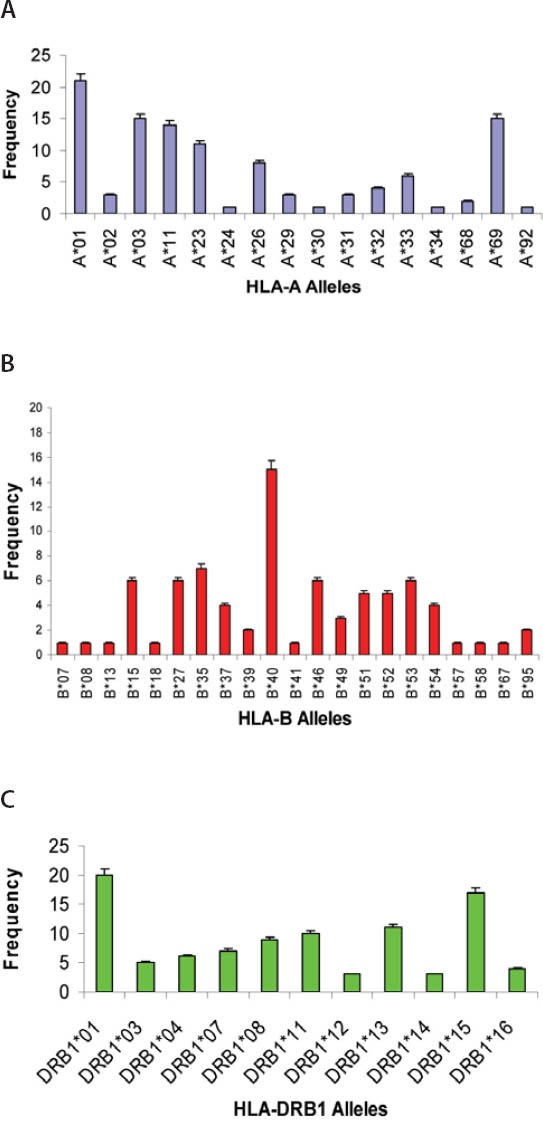
(A) Distribution of HLA-A alleles in SLE patients with ACE DD genotype (n=54) (B) Distribution of HLA-B alleles in SLE patients with ACE DD genotype (n=54) (C) Distribution of HLA-DRB1 alleles in SLE patients with ACE DD genotype (n=54)
FIGURE 7.
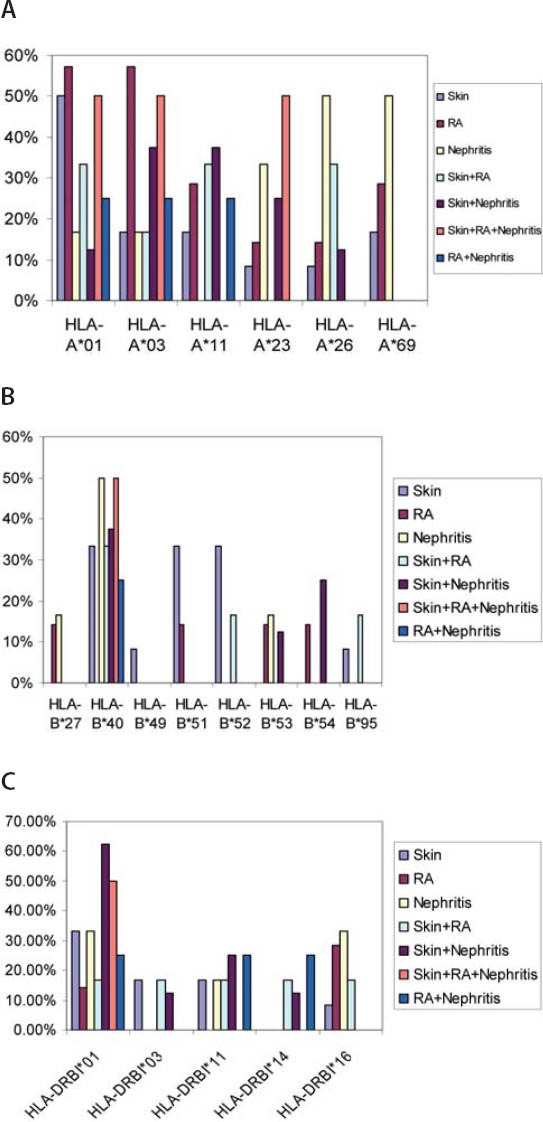
(A) Distribution of HLA-A alleles among SLE Patients (B) Distribution of HLA-B alleles among SLE Patients (C) Distribution of HLA-DRB1 alleles among SLE Patients.
DISCUSSION
Circulating antinuclear antibodies were commonly seen in SLE patients and their presence formed part of the diagnosis. In this study, the most affected organs were skin, kidney and joints while eye, heart were also found to be involved. SLE was a complex cascade of autoantibodies, complement components, and of immunoglobulins. Rheumatoid arthritis was the most common autoimmune inflammatory arthritis found commonly as one of the clinical manifestations of SLE. Circulating autoantibodies like ANA (χ2 = 73.84, p < 0.0001), anti-dsDNA (x2 = 68.82, p < 0.0001), anti-SSA (t= 4.921, p < 0.001), anti-histone (t= 4.921, p < 0.001) and anti-Rib-P (t= 4.880, p = 0.001) were found to be significantly associated with SLE while anti-Sm (t=1.223, p = 0.226) and anti-SSB (t= 1.334, p = 0.187) were found to be insignificantly associated with SLE. Serum levels of immunoglobulins and of complement components were estimated. IgG and IgM levels were significantly high in SLE patients while low level of C4 was found in most of the SLE patients. Interestingly it was noted that anti-MCV autoantibodies (t= 2.870, p = 0.006) were not only associated with rheumatoid arthritis but also with SLE. Cytokines are believed to play an important role in SLE but cytokine IL-15 was found to be associated not significantly with SLE in Pakistani SLE patients (χ2 = 3.076, p = 0.079). A significant positive correlation was found between anti-MCV antibodies and IL-15, one of the reasons is that IL-15 is a cytokine produced by rheumatoid synovium. Gerli et al. [5] as well as Massardo et al. [6] could not find any correlation between ribosomal P antibodies and lupus nephritis while Reichlin and Marianne [7] found a strong association of anti dsDNA and of anti ribosomal P antibodies with lupus nephritis. No doubt autoantibodies are involved in the pathogenicity of lupus as it is an autoimmune disease for example levels of anti-dsDNA antibodies are directly proportional to lupus nephritis severity. But recently Macedo et al. [8] has proved the protective role of ribosomal P antibodies, according to him SLE patients with ribosomal P antibodies alone have a tendency of better renal survival as compared to other lupus patients (anti-Rib P+/anti-dsDNA+; anti-Rib P-/anti-dsDNA). In the present study, SLE patients (9.83%) which were positive for ribosomal P antibodies were also found to be positive for anti-dsDNA antibodies. A positive correlation was found between ribosomal P antibodies and lupus nephritis but it was not significant. A number of syndromes overlapped with SLE like Sjogren’s syndrome, Scleroderma, Rheumatoid arthritis, Antiphospholipid syndrome and Budd Chiari syndrome. Boier et al. [9] found a positive association of photosensitivity, oral ulcer and of Raynaud’s phenomenon with SLE overlapping with Sjogren’s syndrome but a negative association of lupus nephritis with SLE having Sjogren’s syndrome [9]. In the present study, Raynaud’s Phenomenon (100%) and Photosensitivity (50%) were commonly seen in SLE patients with Sjogren’s syndrome while oral ulcer and lupus nephritis were not found in these patients. Eye involvement and Raynaud’s phenomenon are commonly seen in SLE patients with Scleroderma [10]. Similarly, in the present study, SLE patients with Scleroderma, Raynaud’s phenomenon (100%) and Photosensitivity (100%) had common manifestations of lupus but organs like eye, kidneys were not found to be involved in them. However, in addition to vasculitis in SLE patients with secondary antiphospholipid syndrome, photosensitivity was also found in one of such patient. So a common feature was photosensitivity that flares up not in SLE alone but also in Scleroderma, Sjogren’s syndrome and in Raynaud’s phenomenon overlapping with SLE. According to Sato et al. [11], SLE patients homozygous for insertion polymorphism display higher serum level of ds-DNA antibodies and thus showed significant increase in SLE activity. In the present study, 100% of the SLE patients with II and ID genotype showed higher serum levels of ds-DNA antibodies, similarly, 70.3% of the SLE patients homozygous for deletion polymorphism were also positive for dsDNA antibodies, thus indicating lupus activity in all of the subjects with ACE II, ID, and DD genotypes. Uhm et al. [12] found that individuals with DD genotype had a very low level of anti-Sm antibodies and thus found to be less associated with Raynaud’s phenomenon but in this study 29.62 % of the SLE patients with DD genotype had higher levels of anti-Sm antibodies and had the obvious symptoms of Raynaud’s phenomenon. The frequency of DD genotype was found to be higher in SLE patients with Lupus Nephritis, Sjogren’s syndrome, Rayanud’s phenomenon and with Rheumatoid arthritis. So one can say that ACE I/D polymorphism is might be involved in syndromes overlapping with SLE. Complement levels indicate lupus exacerbation especially C3 and C4 levels which are found to be significantly associated with lupus nephritis [13]. In this study, levels of C3 and C4 were studied in SLE patients with different ACE genotypes. Low C3 particularly C4 were found to be more depressed in SLE patients with DD genotype. Hong et al. [14] found IgA mesangial deposition in renal biopsy specimens of lupus nephritis patients. In the present study, all SLE patients with different ACE genotypes had a normal IgA level. There are several studies of ACE I/D polymorphism in relation to HLA-DR in several diseases like Sarcodosis [15] but we could not find any previous data on such relation in SLE patients. The present study was population based, here patient and control samples were collected from the same relatively homogenous population. The main advantage is that such studies are easy to perform and make it possible to establish the risk factors of individuals with certain HLA types. This study confirmed and extended the association of SLE with certain MHC alleles in Pakistani population. This study will open up new opportunities to improve diagnosis and treatment of SLE patients in this population, thus new therapies can be developed.
CONCLUSION
Thus it is concluded that SLE is an interplay of autoantibodies which is found to be fatal when overlapped with various syndromes or when any organ of the body get involved. The differences in the clinical presentation or in the auto-antibody profile of lupus patients were most probably due to genetic differences. ACE I/D Polymorphism was found to be actively involved in the progression of this disease particularly DD genotype. Identification of HLA alleles involved in the development of SLE will provide new insight into the development of disease in susceptible individuals.
DECLARATION OF INTEREST
Authors declare that there is no conflict of interest related to this work.
REFERENCES
- 1.CrispÍn JC, Tsokos GC. IL-17 in Systemic Lupus Erythematosus. J Biomed Biotechnol. 2010;10:1155. doi: 10.1155/2010/943254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parsa A, Peden E, Lum RF, Seligman VA, Olson JL, Li H, et al. Association of angiotensin-converting enzyme polymorphisms with systemic lupus erythematosus and nephritis: analysis of 644 SLE families. Genes Immun. 2002;3 (Suppl 1):S42–S46. doi: 10.1038/sj.gene.6363907. [DOI] [PubMed] [Google Scholar]
- 3.Lee KW, Oh DH, Lee CC, Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 2005;65(5):437–447. doi: 10.1111/j.1399-0039.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith EL, Shmerling RH. The American college of Rheumatology for the classification of systemic lupus erythematosus: Strengths, weaknesses, and opportunities for improvement. Lupus. 1999;8(8):586–595. doi: 10.1191/096120399680411317. [DOI] [PubMed] [Google Scholar]
- 5.Gerli R, Caponi L, Tincani A, Scorza R, Sabbadini MG, Danieli MG, et al. Clinical and serological associations of ribosomal P autoantibodies in systemic lupus erythematosus: prospective evaluation in a large cohort of Italian patients. Rheumatology. 2002;41 (12):1357–1366. doi: 10.1093/rheumatology/41.12.1357. [DOI] [PubMed] [Google Scholar]
- 6.Massardo L, Burgos P, Martinez ME, Pérez R, Calvo M, Barros J, et al. Anti-ribosomal P protein antibodies in Chilean SLE patients: no association with renal disease. Lupus. 2002;11(6):379–383. doi: 10.1191/0961203302lu209oa. [DOI] [PubMed] [Google Scholar]
- 7.Reichlin M, Wolfson-Reichlin M. Correlations of anti-dsDNA and anti-ribosomal P autoantibodies with lupus nephritis. Clin Immunol. 2003;108 (1):69–72. doi: 10.1016/s1521-6616(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 8.De Macedo PA, Borba EF, Santos VD, Leon EP, Testagrossa A, Barros RT, et al. Antibodies to ribosomal P proteins in lupus nephritis: A surrogate marker for a better renal survival. Autoimmun Rev. 2011;10(3):126–130. doi: 10.1016/j.autrev.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Boire G, Barr SG, Zummer M, Fortin PR, Wither J, Rioux JD. A targeted association study in SLE identifies multiple susceptibility alleles. Genes Immun. 2010;10:1038. doi: 10.1038/gene.2010.47. [DOI] [PubMed] [Google Scholar]
- 10.Leber MJ, Lakdawala VS. Systemic lupus erythematosus. Rheumatol. 2009;27:1–23. [Google Scholar]
- 11.Sato H, Akai Y, Iwano M, Kurumatani N, Kurioka H, Kubo A, et al. Association of insertion polymorphism of ACE gene with the activity of SLE. Lupus. 1998;7(8):530–534. doi: 10.1191/096120398678920622. [DOI] [PubMed] [Google Scholar]
- 12.Uhm WS, Lee HS, Chung YH, Kim TH, Bae SC, Joo KB. Angiotensin-converting enzyme gene polymorphism and vascular manifestations in Korean patients with SLE. Lupus. 2002;11(4):227–233. doi: 10.1191/0961203302lu174oa. [DOI] [PubMed] [Google Scholar]
- 13.Birmingham DJ, Irshaid F, Nagaraja HN, Zou X, Tsao BP, Wu H, et al. The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus. 2010;19(11):1272–1280. doi: 10.1177/0961203310371154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu SI, Ramirez SB, Winn MP, Bonventre JP, Owen WF. Evidence for genetic factors in the development and progression of IgA nephropathy. Kidney Int. 2000;57(5):1818–1835. doi: 10.1046/j.1523-1755.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- 15.Planck A, Eklund A, Yamaguchi E, Grunewald J. ACEI/D Polymorphism in relation to HLA-DR in sarcodosis. J Intern Med. 2002;251(3):217–222. doi: 10.1046/j.1365-2796.2002.00946.x. [DOI] [PubMed] [Google Scholar]


