Abstract
The aim of this study was to investigate changes in serum nitric oxide (NO) concentration in inflammatory bowel diseases (IBD) patients and its use as potential biomarker in differential diagnosis of ulcerative colitis (UC) and Crohn’s disease (CD) and in disease activity assessment.
In 60 patients of both genders – 30 with ulcerative colitis and 30 with Crohn’s disease - and 30 controls serum nitric oxide concentration was determined by measuring nitrite concentration, a stable metabolic product of NO with oxygen. Conversion of nitrates (NO3-) to nitrites (NO2-) was done with elementary zinc. The nitrite concentration was determined by classic colorimetrical Griess reaction.
Median serum NO concentration was statistically different (p=0,0005) between UC patients (15.25 μmol/L; 13.47 – 19.88 μmol/L), CD patients (14.54 μmol/L; 13.03 –16.32 μmol/L) and healthy controls (13.29 μmol/L; 12.40 – 13.92 μmol/L). When active UC and CD patients were compared with inactive UC and CD patients respectively a significant difference in serum NO level was found (p=0.0005). With a cut-off level of 17.39 μmol/L NO had a sensitivity of 100% and a specificity of 100% in discriminating between active and inactive UC patients. With cut-off value of 14.01 μmol/L serum NO level had a sensitivity of 88% and a specificity of 69% in distinguishing between patients with active CD and inactive CD.
Serum NO concentration is a minimally invasive and rapid tool for discriminating between active and inactive IBD patients and could be used as useful biomarker in monitoring of disease activity in IBD patients.
KEY WORDS: nitric oxide, inflammatory bowel disease, ulcerative colitis, Crohn’s disease
INTRODUCTION
NO is a free radical gas messenger molecule with both intra- and extracellular regulatory functions for many cells. Endogenous NO is generated from the L-arginine by oxidation of terminal nitrogen in the guanidine group in reaction catalyzed by the enzyme nitric oxide synthase (NOS) [1]. Two distinct functional forms of NOS can be recognized: constitutive and the inducible forms. NO synthesis by the constitutive isoforms, endothelial (eNOS) and neuronal (nNOS), generate low levels of NO under normal physiological conditions [1, 2]. In the gastrointestinal tract (GIT) constitutive isoforms found in the endothelial cells (eNOS) and certain nerve terminals (nNOS) innervating the colon, regulate blood flow and bowel motility by promoting muscle relaxation of the vessels and the bowel, respectively [3]. Third isoform inducible (iNOS) is highly expressed in macrophages, neutrophiles, endothelial and smooth muscle cells upon different stimuli. After induction by endotoxine and/or cytokine, iNOS generates high, sustained levels of NO. These elevated levels of NO may be toxic and may damage healthy tissue. Tissue injury may result from an interaction of NO with superoxide anion, one of the reactive oxygen species, resulting in a formation of peroxynitrite that further contributes to tissue damage and up-regulation of the inflammatory response [3, 4]. Inflammatory bowel disease (IBD), ulcerative colitis (UC) and Crohn’s disease (CD) are idiopathic, chronic and remitting inflammatory diseases of gastrointestinal tract. Clinical symptoms, endoscope findings, hystopathological characteristics and immunopathophysiology differ among them [5]. Despite extensive investigation the etiology and pathogenesis of human IBD remains unknown. Previous experimental and clinical studies have suggested that the initiation and pathogenesis of IBD are multifactorial and involve interaction between genetic, environmental, infective and immunological factors. Various mediators have been suggested as possible participants in the pathogenesis of the inflammatory response. Nitric oxide (NO) is one of the proposed possible etiological factors in the inflammatory process of these diseases [3]. Numerous manifestations of IBD which include: vasodilation in the mucosa, increased vascularand epithelial permeability and motility disorders are related with direct or indirect effects of NO. There is comprehensive evidence of an increased production of NO in IBD. Reynolds etal. [6] have found increased levels of NO in the lumen of the colon in patients with active UC. Similar data were obtained Ljung et al. [7] who found rectal NO increased in patients with active UC and CD. Although previous studies have only noticed the increased activity of NOS in the intestinal mucosa in patients with UC [8], subsequent work showed increased NO production in the mucosa in patients with CD [9]. The results of recent studies support the hypothesis that NO is an important etiological factor for the development of chronic inflammatory bowel disease and ulcerative colitis. Increased concentrations of NO and iNOS overexpression were found in altered inflammatory mucosa of patients with IBD, which may contribute to the process of deterioration and exacerbation of immune responses [10]. Active inflammation of the colon lining is associated with increased production of cytokines (TNF-α, IL-1 and IFN-γ) that are released by inflammatory cells (macrophages and neutrophils), endothelial cells and smooth muscles of the digestive tract. These cytokines induce iNOS, which causes an increased release of NO and the consequent creation of reactive products such as peroxynitrite, hydrogen peroxide and superoxide anions. Peroxynitrite exert cytotoxic effects and can cause membrane lipid peroxidation with an additional increase in permeability, causing breakdown ofDNA chains and activation of poly (ADP ribose) - synthase, which leads to energy consumption and impairment of intracellular ATP. Peroxynitrite once formed causes tissue damage and inflammation [1, 11]. Since NO can be measured with the use of non-invasive methods, the aim of this study was toexamine the possibility of its usage as potential serological marker in differential diagnosis of IBD, and in IBD activity assessment.
MATERIALS AND METHODS
Patients
The study included 30 patients with ulcerative colitis (UC) and 30 patients with Crohn’s disease (CD). Based on UC activity index (UCAI) defined by Seo et al. [12] and Activity index (AI) for CD patients defined by Van Hees et al. [13] studied groups were divided into subgroups of those displaying the active and inactive phases of the disease. Control group (n=30) represented age and gender-matched, apparently healthy individuals. All subjects involved in the study went through detailed anamnestic questionnaire, medical history, physical examination and standard laboratory analyses. Written informed consent was obtained from all of the study participants. The study was carried out at the Institute for Physiology and Biochemistry of Faculty of Medicine in Sarajevo, with an approval from the Local Ethic Committee. Investigations were carried out in accordance with the Declaration of Helsinki as revised in 2000.
NO measurement
The serum nitric oxide concentration was determined by measuring nitrite concentration, a stable metabolic product of NO with oxygen. Conversion of nitrates (NO3-) to nitrites (NO2-) was done with elementary zinc. The nitrite concentration was determined by classic colorimetrical Griess reaction [14]. Blood samples for the determination of serum NO levels were taken from the cubital vein and allowed to coagulate. After coagulation the samples were centrifuged at 2000 g for 5 min, and the serum samples were deproteinized by adding 750 μl of distilled water and 50 μl of 30% ZnSO4 to 250 μl of each serum sample. Samples were further centrifuged at 2000g for 10 min and frozen at -20°C until the analysis was performed. The procedure included suspending 4 mg of elemental zinc in 100 μl of distilled water and adding it to 500 μl of supernatants. Also, 16 μl of 5% acetic acid and 384 μl of distilled water were added to it, thus reaching the total volume of 1 ml. Samples prepared in this way were put on a vortex for 5 minutes at room temperature and centrifuged at 700 g for 2.5 minutes. Then, 500 μl of supernatant was mixed with an equal volume of the Griess reagent. The Griess reagent consists of equal volumes of 0.1% naphthylethylene diamine dihydrochloride in distilled water and 1% sulfanilamide in 5% H3PO4. These two components were mixed 12 hours prior to usage and stored in a cold place. The supernatants and the Griess reagent mixtures were put on a vortex for 10 minutes and the light absorbance (optical density) was measured with a spectrophotometer at 546 nm. The nitrate concentration was read from the standard curve with a known NaNO2 concentration (ranging from 1.56 – 100 μmol). Blind samples were composed of the Griess reagent and distilled water.
Statistical analysis
The Shapiro-Wilk test of normality was used to test the distribution of variables. Since all variables were skewed they are presented as median and interquartile ranges. The difference in values of tested parameters was assessed by Kruskal-Wallis test. Afterwards, Mann-Whithey U-test was used to compare differences between two groups. A p value of <0.05 was considered statistically significant. Sensitivity, specificity, positive and negative predictive value were calculated according to the following formula [15]:
Sensitivity = a/(a + c)
Specificity = d/(b + d)
Positive predictive value = a/(a + b)
Negative predictive value = d/(c + d)
Where: a = true-positive cases; b = false-positive cases; c = false-negative cases; d = true-negative cases. Receiver operating characteristic (ROC) curves were constructed by calculating the sensitivities and specificities of serum NO concentration at several cut-off points. The software used was SPSS for Windows (version 13.0; SPSS, Chicago, IL, USA).
RESULTS
Serum nitric oxide concentration
Serum NO concentration of the studied five groups are showed in Figure 1. Median serum NO concentration was statistically different (p=0.0005) between UC patients (median 15.25 μmol/L; range 13.47 – 19.88 μmol/L), CD patients (median 14.54 μmol/L; range 13.03 –16.32 μmol/L) and healthy controls (median 13.29 μmol/L; range 12.40 – 13.92 μmol/L). Comparison of the serum NO level of active UC patients (median 20.24 μmol/L; range 18.19 – 21.31 μmol/L) with inactive UC patients (median 13.65 μmol/L; range 13.03 – 14.36 μmol/L) showed a significant difference (p=0.0005). A significant difference in serum NO level was also found when active CD patients (median 15.61 μmol/L; range 14.54 – 16.68 μmol/L) were compared with the group of inactive CD patients (median 13.11 μmol/L; range 12.05 – 14.54 μmol/L) (p=0.0005). The ROC curves for serum NO level in the patients with IBD vs healthy controls, active UC patients vs inactive UC patients and active CD patients vs inactive CD are shown in Figure 2. Serum NO level was the most accurate for differentiating patients with active UC from patients with inactive UC (AUC 1.00). For differentiation between patients with active and inactive CD or differentiation between IBD patients and healthy controls serum NO level also had a good diagnostic accuracy (AUC 0.857 and 0.763). Based on the proposed cut-off values, the sensitivity and specificity of the serum NO concentration were calculated. Table 1. shows the predictive power of serum NO level in distinguishing patients with IBD and healthy controls, active UC patients from inactive UC patients and active CD patients from inactive CD patients.
FIGURE 1.
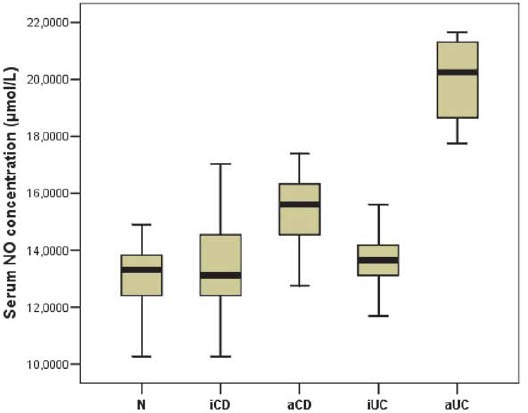
Box-and-whisker plots of serum NO levels (μmol/L) in the five studied groups. The solid horizontal lines denote the median value, the box represents the 25% and 75% interquartile ranges and the whiskers represent minimum and maximum values.
N = normal, healthy control; iCD = inactive Crohn’s disease; aCD = active Crohn’s disease; iUC = inactive ulcerative colitis; aUC = active ulcerative colitis.
FIGURE 2.
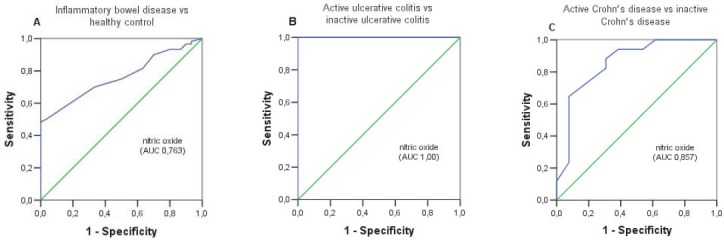
(A) Receiver operating characteristic (ROC) curve of serum NO level for differentiation between IBD patients and healthy controls. (B) ROC curve of serum NO level for differentiation between active and inactive UC patients. (C) ROC curve of serum NO level for differentiation between active and inactive CD patients.
TABLE 1.
Optimal Cut-off, Area under the curve with 95% confidence interval (AUC, 95% CI), sensitivity, specificity, positive and negative predictive value of serum NO concentration in differenting between IBD patients and healthy control, active and inactive UC patients, and active and inactive CD patients.

DISCUSSION
The immunology of IBD continues to be an intense area of investigations for clues to the pathogenesis of ulcerative colitis and Crohn’s disease. Typical for complex diseases, IBD research is continuously evolving. Without abandoning traditional areas of study, such as cellular and humoral immunity and cytokines, examination is broadening to explore new molecules and biologic phenomena [3]. Inflammatory mediators, cytokines, free radicals, as well as mucosal immune system provide a basis not only for the understanding of inflammatory processes but also for examining the mechanisms that cause the reduction of inflammation and provide adequate diagnostic and therapeutic approach. It is now widely held that oxidants, including free radicals, such as nitric oxide, play a key role in the pathophysiology of tissue injury in IBD, particularly in the initiation and perpetuation of inflammation and in the subsequent tissue damage [16, 17]. The aim of this study was to investigate changes in serum NO concentration and the possibility of its use as potential serum biomarker in disease activity assessment and in differential diagnosis of ulcerative colitis and Crohn’s disease. The reasons why we examined serum NO concentration as a potential laboratory marker in IBD was: firstly, to gain an objective measurement of disease activity as symptoms are often subjective; and secondly, to avoid invasive (endoscopic) procedures which are often a burden to the patient. The results of our study showed that the serum NO level was significantly higher in IBD patients compared to the control group (p=0.001). Although the serum NO concentration was higher in UC patients compared to CD patients, there was no statistically significant difference between the compared groups (p=0.12). Our results are in the accordance with the results of Oudkerk et al. [17] who also found no statistically significant difference in the median serum NO concentration between patients with UC and CD patients. However, when we compared active UC patients with inactive UC patients we found a statistically significant difference in serum NO level (p=0.0005). Comparing active CD patients with inactive CD patients we also found a significant difference in serum NO concentration (p=0.0005). Our results confirm findings from previous studies such as the study by Oudkerk et al. [17] who also observed significant higher serum NO level in UC and CD patients in active phase of the disease compared to inactive phase. Numerous experimental studies have shown raised NO concentrations in active phase of disease not only in serum but also in stool [18], urine [19] and in the lumen of the rectum [20] in patients with IBD. Levine et al. [18] conducted a study which demonstrated that IBD patients with active colitis have increased stool and plasma NO concentration compared to IBD patients with normal colonic histology and those with mild, nonspecific colonic inflammation. Goggins et al. [19] demonstrated that urinary nitrite concentration, a stable end product of NO in patients with active IBD were significanty different from levels in inactive IBD patients. These authors suggest that measurement of urinary nitrite may serve as a useful marker for monitoring disease activity in IBD. Based on the significant difference in serum NO level between active IBD and inactive IBD patients, an attempt was made to differentiate active and inactive IBD patients. For clinical decision making, the selected cut-off value of a laboratory test should provide the best diagnostic performance for either ruling out or ruling in the particular disease. The ROC curve analysis is a graphic method which can be used to determine this optimal cut-off level. In addition, it is a precise and valid measure of diagnostic accuracy [21]. In this study, based on the selected optimal cut-off value by ROC curve analysis, serum NO level showed a high specificity (100%) and high sensitivity (100%) in distinguishing patients with active UC from inactive UC patients; moderate specificity (69%) and a good sensitivity (88%) in distinguishing patients with active CD from inactive CD patients. We further tested whether serum NO level, as a serological marker, could help in differentiating patiens with ulcerative colitis and Cronh’s disease. Our study has not demonstrated a statistically significant difference in NO level between ulcerative colitis and Cronh’s disease (p=0.12), even though the patients with ulcerative colitis had higher serum NO levels compared to the patients with Crohn’s disease. The lack of such difference may be due to different distribution and activation of iNOS in these patients. Earlier study by Middelton et al. [22] has shown a marked increase in NO synthase activity only in the inflamed colonic mucosa of patients with ulcerative colitis. The cellular source of iNOS isoform in the inflamed mucosa may be product of resident or infiltrating leucocytes (neutrophils or macrophages) or mucosal vascular smooth muscle or endothelium. Induction of 1NOS in these cells may occur due to stimulatory cytokines (TNF and IL-1), the mucosal levels of which are increased in ulcerative colitis [23]. In contrast to ulcerative colitis, there was no increase in NO synthase activity detected in the inflamed colonic mucosa from patients with Crohn’s disease, suggesting that there is no induction of NO synthase in the colon in this inflammatory bowel disease [8]. The failure to detect induction of NO synthase in the inflamed tissue of Crohn’s disease patients may reflect a deficiency in the induction process or could be due to mucosal formation of inhibitory cytokines such as IL-4 or IL-10, which can prevent NO synthase induction. The balance of stimulatory versus inhibitory cytokines could determine the expression of NO synthase in both ulcerative colitis and Crohn’s disease.
CONCLUSION
Significant serum NO concentration increment during the active phase of disease and decrease during the inactive phase conveys possible use of serum NO level for monitoring disease activity in both types of IBD. Due to an absence of significant differences in serum NO concentration among patients with UC and CD, serum NO can not be recommended for IBD differential diagnosis use.
DECLARATION OF INTEREST
The authors state that there is no conflict of interest.
REFERENCES
- 1.Calcerrada P, Peluffo G, Radi R. Nitric oxide-derived oxidants with a focus on peroxynitrite molecular targets, cellular responses and therapeutic implications. Curr Pharm Des. 2011;17(35):3905–32. doi: 10.2174/138161211798357719. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 3.Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle, Immunology. 2004;113:427–437. doi: 10.1111/j.1365-2567.2004.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palatka K, Serfozo Z, Vereb Z, Hargitay Z, Lontay B, Erdodi F, Ban-falvi G, Nemes Z, Udvardy M, Altorjay I. Changes in the expression and distribution of the inducible and endothelial nitric oxide syn-thase in mucosal biopsy specimens of inflammatory bowel disease. Scand J Gastroenterol. 2005;40(6):670–80. doi: 10.1080/00365520510015539. [DOI] [PubMed] [Google Scholar]
- 5.Friedman S, Blumberg RS. Inflammatory bowel disease. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine. 18th ed. Chapter 295. New York, NY: McGraw-Hill; 2011. [Google Scholar]
- 6.Reynolds PD, Middleton SJ, Hansford GM, Hunter JO. Confirmation of nitric oxide synthesis in active ulcerative colitis by infra-red diode laser spectroscopy. Eur J Gastroenterol Hepatol. 1997;9:463–466. doi: 10.1097/00042737-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Ljung T, Lundberg S, Varsanyi M, Johansson C, Schmidt PT, Herulf M, Lundberg JO, Hellstrom PM. Rectal nitric oxide as biomarker in treatment of inflammatory bowel disease: Responders versus nonresponders. World J Gastroenterol. 2006;12(21):3386–3392. doi: 10.3748/wjg.v12.i21.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boughton-Smith NK, Evans SM, Hawkey CJ, Cole AT, Balsitis M, Whittle BJ, Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993;342:338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H, Miura S, Shigematsu T, Ohkubo N, Tsuzuki Y, Kurose I, Higuchi H, Akiba Y, Hokari R, Hirokawa M, Serizawa H, Ishii H. Increased nitric oxide production and inducible nitric oxide syn-thase activity in colonic mucosa patients with active ulcerative colitis and Crohn's disease. Dig Dis Sci. 1997;42(5):1047–1054. doi: 10.1023/a:1018849405922. [DOI] [PubMed] [Google Scholar]
- 10.Roediger WE, Lawson MJ, Nance SH, Radcliffe BC. Detectable colonic nitrite levels in inflammatory bowel disease mucosal or bacterial malfunction? Digestion. 1986;35:199–204. doi: 10.1159/000199368. [DOI] [PubMed] [Google Scholar]
- 11.Middleton SJ, Shorthouse M, Hunter JO. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993;341:465–466. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- 12.Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol. 1992;87(8):971–976. [PubMed] [Google Scholar]
- 13.van Hees PA, van Elteren PH, van Lier HJ, van Tongeren JH. An index of inflammatory activity in patients with Crohn's disease. Gut. 1980;21:279–286. doi: 10.1136/gut.21.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibragic S, Sofic E, Suljic E, Avdagic N, Bajraktarevic A, Tahirovic I. Serum nitric oxide concentrations in patients with multiple sclerosis and patients with epilepsy. J Neural Transm. 2012;119(1):7–11. doi: 10.1007/s00702-011-0686-6. [DOI] [PubMed] [Google Scholar]
- 15.Sox HC, Blatt MA, Higgins MC, Marton KI. London: Butterworth; 1989. Medical Decision Making; pp. 67–146. [Google Scholar]
- 16.Keshavarzian A, Banan A, Farhadi A, Komanduri S, Mutlu E, Zhang Y, Fields JZ. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. 2003;52:720–728. doi: 10.1136/gut.52.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oudkerk Pool M, Bouma G, Visser JJ, Kolkman JJ, Tran DD, Meu-wissen SG, Peña AS. Serum nitrate levels in ulcerative colitis and Crohn's disease. Scand J Gastroenterol. 1995;30(8):784–8. doi: 10.3109/00365529509096328. [DOI] [PubMed] [Google Scholar]
- 18.Levine JJ, Pettei MJ, Valderrama E, Gold DM, Kessler BH, Tracht-man H. Nitric oxide and inflammatory bowel disease: evidence for local intestinal production in children with active colonic disease. J Pediatric Gastroenterol Nutr. 1998;26(1):34–38. doi: 10.1097/00005176-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Goggins MG, Shah SA, Goh J, Cherukuri A, Weir DG, Kelleher D, Mahmud N. Increased urinary nitrite, a marker of nitric oxide in active inflamatory bowel disease. Mediators of inflamation. 2001;10:69–73. doi: 10.1080/09629350120054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herulf M, Ljung T, Hellström PM, Weitzberg E, Lundberg JO. Increased luminal nitric oxide in inflammatory bowel disease as shown with a novel minimally invasive method. Scan J Gastroen-terol. 1998;33(2):164–169. doi: 10.1080/00365529850166897. [DOI] [PubMed] [Google Scholar]
- 21.Swets JA. Measuring the accuracy of diagnostic system. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 22.Middleton SJ, Shorthouse M, Hunter JO. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993;341:465–6. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- 23.Boughton-Smith NK. Pathological and therapeutic implications for nitric oxide in inflammatory bowel disease. J R Soc Med. 1994;87(6):312–4. doi: 10.1177/014107689408700602. [DOI] [PMC free article] [PubMed] [Google Scholar]


