Abstract
The aim of this study was to determine surface roughness and topography of polished dental resin-based nanocomposites.
Four representative dental resin-based nanocomposites were tested in the study: two nanohybrids (Filtek Z550 and Tetric EvoCeram) and two nanofilled (Filtek Ultimate Body and Filtek Ultimate Translucent); and two reference materials: one microfilled (Gradia Direct) and one microhybrid (Filtek Z250). Polymerized cylindrical specimens (4 mm x 2 mm) were polished with multi-step polishing system-Super Snap. Immediately after the polishing, topography of each specimen was examined by Veeco di CP-II Atomic Force Microscope. Specimen’s surface has been scanned in 6 points in contact mode with CONT20A-CP tips. 1 Hz scan rate and 256 x 256 resolution were used to obtain topography on a 90 μm x 90 μm scanning area. Measured topography data were processed by Image Processing and Data Analysis V2.1.15 software. Following parameters were compared among specimens: average roughness and maximum peak-to-valley distance.
All of the tested materials had similar average surface roughness after finishing and polishing procedure. The lowest values occurred in the material Filtek Ultimate Body, and the highest in the Filtek Z550. When interpreting maximum peak-to-valley distance the larger differences in values (up to 100%) occurred in Filtek Z550, Filtek Z250 and Filtek Ultimate Body, which is a result of the deep polishing channels and tracks. Type, size, distribution of fillers and filler loading in tested materials, didn’t influence average roughness values, but had an impact on maximum peak-to-valley distance values.
KEY WORDS: dental polishing and finishing, resin-based composite, nanocomposite, nanoparticles, atomic force microscopy, surface roughness, topography
INTRODUCTION
Nanotechnology is the science and engineering involved in the design, synthesis, characterization and application of materials and devices whose smallest functional organization in, at least, one dimension is on the nanometer scale (one-billionth of a meter, 10-9 m) [1]. Something that we call nanomaterial or nanodevice has the smallest dimension about 100 nm or less, i.e. maximum 1 x 10-7 m. Nanotechnology is a multidisciplinary field of scientific research, which can be used in the numerous application areas [2]. Potential benefits of using nanomaterials and nanorobots in medical and dental applications are one of the main topics when talking about nanoadvances [3]. In the field of dentistry, nanotechnological improvements may lead to advanced preventive, diagnostic and therapy procedures [4, 5, 6]. Currently, nanoproducts that have the widest application in routine dental clinical practice are resin-based nanocomposites. Dental resin-based composites (RBC) are tooth-colored restorative materials that consist of organic resin matrix, inorganic filler particles, silane coupling agents and initiators and activators for the photo-polymerization [7]. Overall characteristics of the material may be improved by the research and work on improving the individual components of RBCs [8]. Fillers in composites have multiple roles: to reduce polymerization shrinkage, the coefficient of thermal expansion and water sorption and solubility; to mechanically reinforce the material; to improve optical and aesthetic characteristics of the material; to enable better initial polishing and polish retention, and to reduce wear during the masticatory forces [7, 9-13]. Formulation of filler particles, have been passed from macro-, micro-, down to the nanoparticles [14]. Microhybrid composites, so-called universal restorative composites, are composed of filler particles of different sizes (15-20 μm and 0.01-0.05 μm) and have good mechanical properties for use in the lateral occlusal region, but relatively poor aesthetic qualities, to be used in the esthetic zone [15]. Microfilled composites have been developed in order to obtain high-quality aesthetic materials that meet the needs of restorative dentistry in the esthetic zone. Microfilled composites have average particle size in range of 0.01-0.05 μm. Due to the relative poor mechanical strength, these materials are indicated for use in low-stress oral regions [15]. Trying to create a material that meets both of these properties, the mechanical resistance and the aesthetic and polishing qualities, nanofillers have been developed [12]. In order to produce particles smaller than 100 nm, the mode of production of particles for RBCs had to be changed from the “top-down” milling and grinding procedure to the “bottom-up” manufacturing approach (direct molecular assembly) [12, 16]. Current dental resin-based nanocomposites can be divided into two main groups: nanohybrids and nanofilled composites (nanofills) [16]. Nanohybrids consists of particles of various sizes, particles in micrometric and in nanometric range [7]. Nanofills consist of particles of nearly uniform size, all in nanometric dimensions, with the ability to create nanoclusters as secondarily formed fillers [7, 12]. Nanocomposites have excellent mechanical properties, similar as microhybrids, which make them able to withstand high occlusal forces, in lateral masticatory regions. On the other hand, they have great polish ability and polish retention, superior optical and aesthetic characteristics [12]. Surface quality of resin-based composite is a very important characteristic of the final restoration. Smooth surface of the restoration is necessary to obtain clinical durability and good aesthetic appearance, and to prevent discoloration and staining. Also, dental plaque, as a main cause of peri-odontal diseases and secondary caries, is less retained on the smooth surface [17]. Polishing treatments can improve the wear behavior of the material, as well [18, 19]. Finishing procedure is a necessary clinical step to establish a proper reconstruction of dental crowns and to restore anatomical and morphological form of the tooth. Another reason for this procedure is to remove the resin-rich surface layer, which remains after polymerization and removal of the matrix from the material [20]. Polishing procedure makes the surface smoother and removes surface damages like grooves, lines and furrows created during finishing phase [18]. Aim of this study was to determine surface roughness and topography of contemporary RBCs using atomic force microscopy analysis. The two main goals were: (a) analysis of the impact of particle size and loading on the surface roughness and topography after finishing and polishing procedure, and (b) the impact of the multi-step finishing and polishing procedure, itself, on the surface topography of RBCs.
MATERIALS AND METHODS
Materials
Four representative dental resin-based nanocomposites were tested in the study: two nanohybrids (Filtek Z550 and Tetric EvoCeram) and two nanofilled (Filtek Ultimate Body and Filtek Ultimate Translucent); and two reference materials: one microfilled (Gradia Direct) and one microhybrid (Filtek Z250). Detailed information about material used in the study are shown in the Table 1.
TABLE 1.
Details of the materials tested in the study*
Procedure for preparing the specimens
One specimen of each material was made by using cylindrical plastic molds (4 mm diameter x 2 mm depth). Plastic molds were placed on the glass microscope slide, filled with material and covered with a polyester strip and a glass slide, taking care to obtain a flat surface without any defects and entrapped air. Material was then polymerized for 40 seconds with a SmartLite® IQTM 2 LED unit (Dentsply Caulk). After removing glass plate and polyester strip from the top of the samples, they were polished with multi-step polishing system- Super Snap (Shofu, Inc. Kyoto, Japan). During the polishing procedure, each abrasive disk was used only once for each material, in the dry condition, for 1 minute, using handpiece rotating 10 000 revolutions per minute (recommended speed by manufacturer). One single operator did all of the polishing treatments, trying to simulate clinical finishing and polishing procedure. Two mutually perpendicular grinding directions were used during polishing procedure (Figure 1). Detailed information about the polishing system used in the study are shown in Table 2. Immediately after the polishing treatment, topography of each specimen was examined by Veeco di CP-II Atomic Force Microscope. Specimen’s surface has been scanned in 6 points (two points at specimen’s center, two points at specimen’s perimeter and two points at half distance between specimen’s center and perimeter (Figure 2) in contact mode with CONT20A-CP tips. 1 Hz scan rate and 256 x 256 resolution were used to obtain topography on a 90 μm x 90 μm scanning area. Before the scanning, specimen’s surface has been blown through with cold air by hairdryer. Cleaning specimen’s surface with alcohol created damaged surface. Measured topography data were processed by Image Processing and Data Analysis v2.1.15 software. Following parameters were compared among specimens: average roughness (Ra) and maximum peak-to-valley distance (Rp-v).
FIGURE 1.
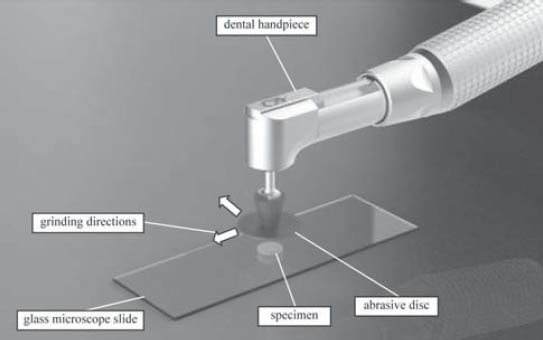
Grinding setup and grinding directions
TABLE 2.
Details about polishing system used in the study
FIGURE 2.

AFM measurement points on specimen’s surface. (1 - specimen’s center, 2 - half-distance between specimen’s center and circumference, 3 - specimen’s circumference)
RESULTS
The results obtained by this study are presented in Table 3 displaying measured values of (Ra) and (Rp-v) roughness parameters of specimens used, and in Figure 3 and 4 where these values are graphically presented. Figures 5 to 10 present 3D images of surface topography on each specimen obtained by AFM. Each figure contains six 3D AFM images that are obtained on each measuring point on specimen (two points on specimen’s center, two points on specimen’s perimeter and two points at half distance between specimen’s center and perimeter). From the analysis of the results showed in Figure 3, it can be seen that although there are different materials tested in the study (nanohybrids, nanofills, microfills and microhybrids- with different filler composition, size and volumetric loading of the material), all the materials have similar average surface roughness (Ra) after finishing and polishing procedure. The lowest (Ra) values occurred in the material Filtek Ultimate Body, and the highest in GC Gradia Direct and Filtek Z550. Filtek Ultimate Translucent and Tetric EvoCeram showed the most consistent results of all the measurements on the sample (Figure 3), which can be confirmed by the uniform 3D AFM images, as well (Figure 8, and Figure 6). When interpreting (Rp-v), the larger differences in values (up to 100%) occurred in Filtek Z550, Filtek Z250 and Filtek Ultimate Body, which is the result of the deep polishing channels and tracks. During qualitative analysis of 3D AFM images it can be seen that material surfaces contain various channels and polishing tracks of different size, flat areas or local damages. By analyzing images it can be noticed that Tetric EvoCeram exhibits highly-uniform surface quality on all of the measuring points (Figure 6). Visible polishing track and local damages feature GC Gradia Direct’s surface (Figure 9) and similar is on Filtek Zsso’s surface (Figure 5). Moreover, by analyzing topography of Gradia Direct, regions with flat surfaces can be distinguished, which don’t appear on any other material in this study. Surface of Filtek Z250 is featured by individual deep channels and less visible polishing tracks and local damages (Figure 10) which are not channel-like type. Filtek Ultimate Body has small polishing tracks without any defects on measuring points on the specimen’s center (Figure 7a and b) and with deep channels and defects on measuring points close to specimen’s circumference (Figure 7c, d, e, f). Filtek Ultimate Translucent demonstrated a uniform surface quality regardless of the measuring point (in the center, half-distance between center and circumference and at circumference of the specimen), which is confirmed by very close values of (Ra) and (Rp-v) on all of the measuring points. Measured roughness of all materials used in this study was lower than 100 nm.
TABLE 3.
Values of (Ra) and (R) surface parameters on specimens used in this study
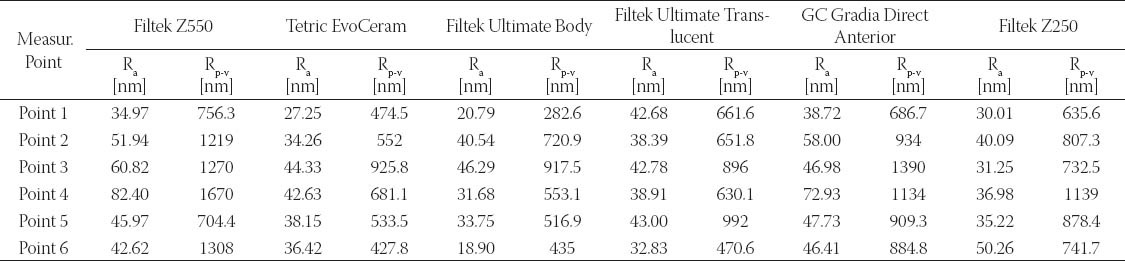
FIGURE 3.
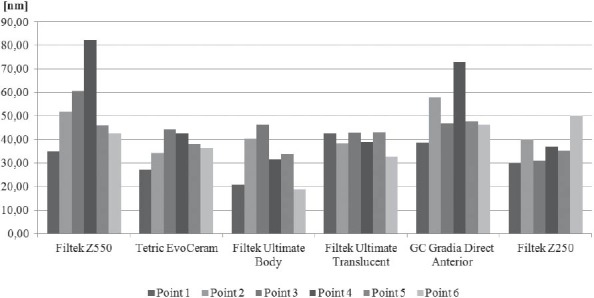
Comparison of average roughness (Ra) among specimens after polishing
FIGURE 4.
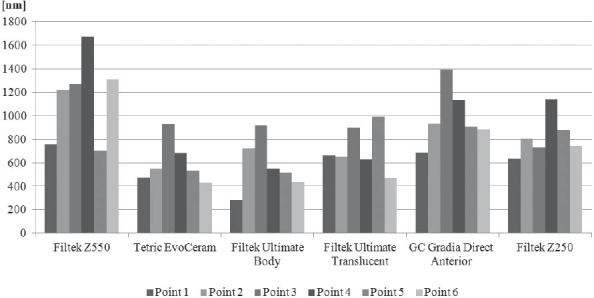
Comparison of peak-to-valley (R) distance among specimens after polishing
FIGURE 5.
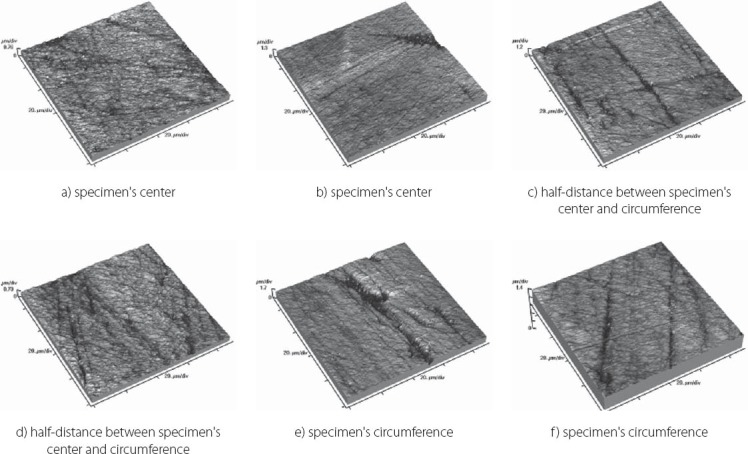
Topography of Filtek Z550 sample
FIGURE 10.
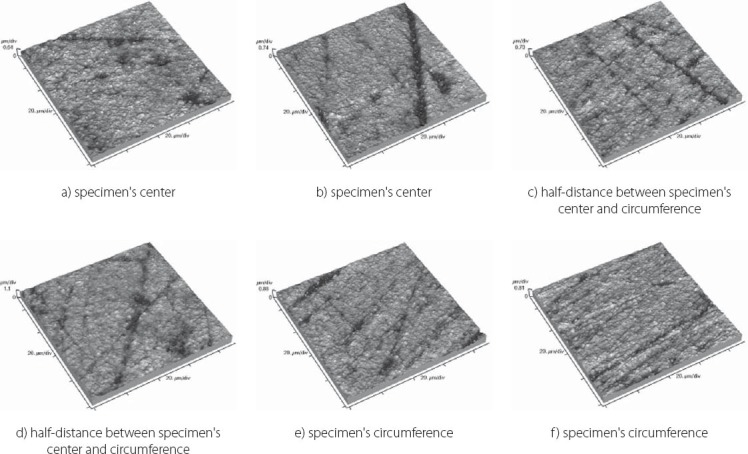
Topography of Filtek Z250 sample
FIGURE 8.
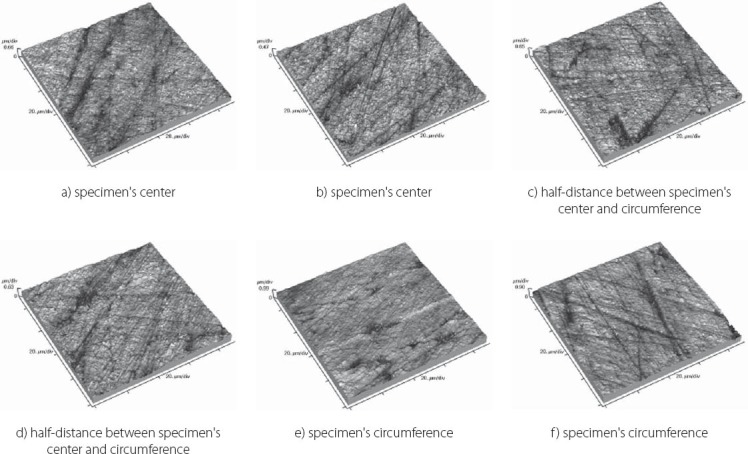
Topography of Filtek Ultimate Translucent sample
FIGURE 6.
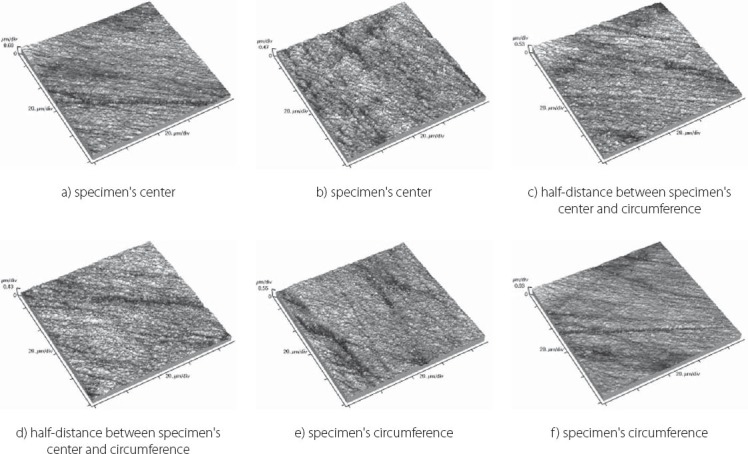
Topography of Tetric EvoCeram sample
FIGURE 9.
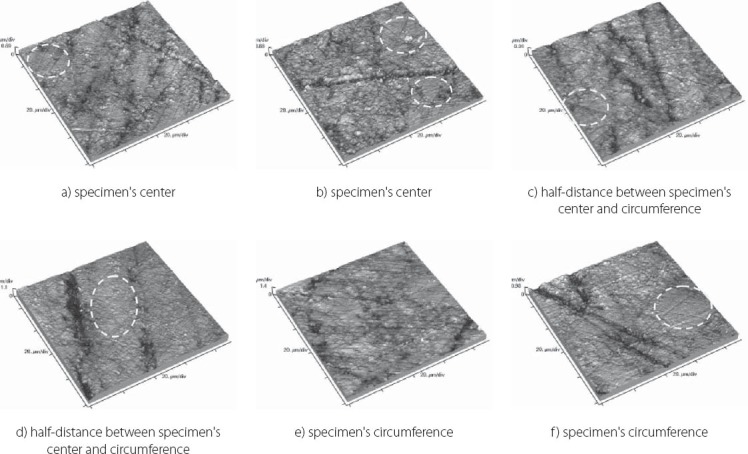
Topography of GC Gradia Direct Anterior sample with indicated flat surface regions
FIGURE 7.
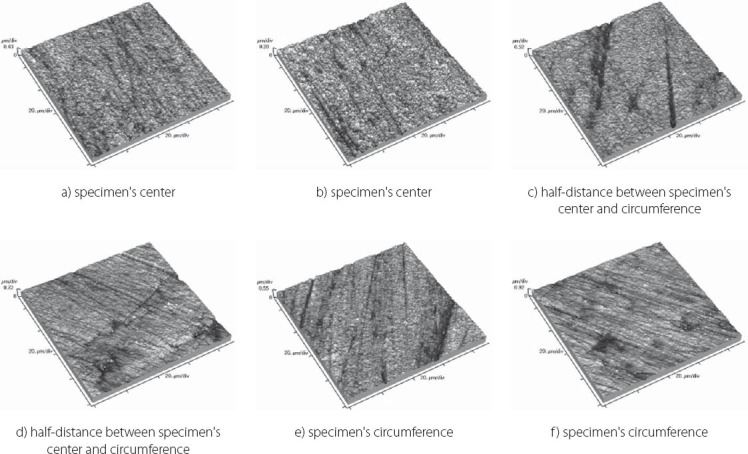
Topography of Filtek Ultimate Body sample
DISCUSSION
After finishing and polishing procedure, all the materials had similar average surface roughness (Ra) which indicates that the used abrasive disks successfully reduced the average roughness of the materials on the relatively equal value. The lowest (Ra) values of Filtek Ultimate Body can be explained by material filler composition. This material is a nanofilled composite, filled only with nanometer size particles, from which some are dispersed and others create nanoclusters, as secondary formed fillers [12]. The size of these nanoclusters can range from about 0.6 to 10 μm, from which the polishing procedure usually plucks out the individual primary nanoparticles, and not a whole nanocluster [12, 21, 22]. Although rare, there is a possibility of the whole nanocluster dislodgement from the surface layer of the material, which can be seen as a nearly rounded surface defect that is not channel or track-shaped [19]. The highest (Ra) value of Filtek Z550 can be explained by the fact that this material is a nanohybrid composite, composed of micrometer particles, whose size range up to 3 μm, and of very small nanometer particles of 20 nm. The existence of larger micrometer particles in this material causes either protruding or dislodge-ment of fillers from the surface layer during polishing, remaining residual surface irregularities and defects [21, 23]. Consistency of the measured results of Tetric EvoCeram can be explained by very high percentage of inorganic filler (82.5 vol%) in this material, and it’s optimal composition of heterogeneous filler particles, which provide this uniformly behavior of the material. Although Tetric EvoCeram belongs to the same material type as Filtek Z550, these materials differ in the volumetric filler proportion. In terms of uniformity of results, nanofilled composites also stood out, noting that Filtek Ultimate Translucent showed more uniform (Ra) values than Filtek Ultimate Body. The only differences, which could be indicated between these two materials, are: the size of the clusters, which are about 20 μm in Translucent, and about 10 μm in Body material; and a slightly lower volume percentage of filler in Translucent (63.3 vol % in Body, 55.6 vol % in Translucent). It is possible that these double-sized clusters in Translucent favor more homogeneous distribution of particles in the composite, so that it implies such uniform (Ra) results of this material. Higher (Rp-v) values in Filtek Z550, Filtek Z250 and Filtek Ultimate Body and deep channels and polishing tracks are expected, because these are hybrid materials, whose larger microparticles protrude from the polished surface or are pulled out by abrasive disk leaving surface voids [24]. Large differences in the profile (Rp-v) of Filtek Ultimate Body are showing a certain heterogeneity of distribution of particles in the material and the existence of segments that are, more or less, susceptible to cleavage. It is important to emphasize that the (Rp-v) is a relative indicator of surface roughness and that it does not show absolute values of height or depth of channels in the material. Qualitative analysis of 3D AFM images confirmed that Tetric EvoCeram has highly-uniform surface quality on all of the measuring points (Figure 6), such as the roughness analysis found. Polishing damages on GC Gradia Direct’s surface can be explained by a wear process of this microfilled material, whose surface resistance to abrasive disc treatment is lower. This is directly related to the size of filler particles and it’s relatively poor capability for volumetric loading, which leaves areas with weak and soft, low-filled resin [25]. On the other hand, although nanofills are similarly volumetrically filled like microfills, they contain nanoparticles which have the unique physicochemical properties [12, 26]. Those properties are not only the matter of particle size, but also of the qualities that this small size particles cause [12, 26]. Thus, they have reinforcing influence on material that comprises them, making high surface resistance to wear caused by abrasive disc [12]. Unique flat surfaces on Gradia Direct can be explained by the existence of pre-polymerized fillers, taking as much as 42% by volume in the material. The “flat fields” on the AFM images can be explained by the fact that the pre-polymerized fillers have improved bonding properties and that almost any of the particles in this field do not pluck out during polishing procedure [19]. The disadvantage of this material is relatively low wear resistance, compared to nanofilled materials, and therefore reliability of polished surface could be short [25]. The local damages on the surface of Filtek Z250 and on Filtek Z550, which are not channel-like type, are probably caused by plucking out of the entire microparticles during polishing process and by remaining the residual surface defects, which were verified by (Ra) and (Rp-v) values [12]. Filtek Ultimate Body and Filtek Ultimate Translucent although two very similar nanofilled materials, represented differences in surface appearance on measuring points, depending on the location of the measurement. Through repetitive movements during polishing process, abrasive disc was falling off from the specimen’s circumference which could cause an engraving of the edge of abrasive disc into the material sample. The only difference between Filtek Ultimate Body and Filtek Ultimate Translucent is in terms of nanocluster size, which is 0.6-10 μm for Body and 0.6-20 μm for Translucent material. Remaining particles are totally the same. It could be possible that larger clusters favor Filtek Ultimate Translucent in terms of polishing properties and wear resistance caused by abrasive disc whether it is in the specimen’s center or circumference. It is important to emphasize that rough surfaces favor bacterial adhesion and biofilm formation on the teeth and restorations, which can further cause secondary caries, gingival and periodontal diseases [17, 27]. There are no agreed reference data on the limit roughness below which the bacteria would not adhere [28]. The most commonly mentioned limit is below 0.2 μm for adherence of dental biofilm [17, 27]. Maybe it is most accurate to say, that it depends on the bacteria species. Roughness in this study, lower than 100 nm for all material types, is far from the mentioned limit.
CONCLUSION
Within the limitation of this study, it can be concluded that the type, size, distribution of fillers and filler loading of all of the tested materials, didn’t affect average surface roughness of the samples after finishing and polishing procedure. On the other hand, different material compositions affected the topography of the polished surfaces of the materials. We should be careful when using the prefix nano-, when it comes to nanohybrids, because the overall material properties depend on all kinds of filler particles, their volume fraction and distribution. Only materials that have the optimal composition of heterogeneous filler particles, which provide their consistent behavior, or materials that consist of all particles in nanometer dimensions, allow more uniform surface topography after polishing treatment. Multi-step polishing abrasive discs, left channels on all materials regardless of composition. It is recommended for future studies to examine whether other polishing systems would leave fewer traces on polished samples.
ACKNOWLEDGEMENTS
This paper represents a part of the research realized in the framework of the project “Research and development of modeling methods and approaches in manufacturing of dental recoveries with the application of modern technologies and computer aided systems” – TR 035020, financed by the Ministry of Science and Technological Development of the Republic of Serbia (Lainović, Blažić, Marković, Ivanišević). This paper is a part of research included into the project “Project TESLA: science with accelerators and accelerator technologies”, financed by Serbian Ministry of Science and Technological Development. The authors are grateful for the financial support (Vilotić, Kakaš). The authors would like to thank 3M (East) AG company branch in Serbia, Ivoclar Vivadent products distributers for Serbia, and Mikodental, Šabac-general dealers of Shofu, Japan for Serbia, for the material support of this study.
DECLARATION OF INTEREST
There are no scientific or financial conflicts of interest.
REFERENCES
- 1.Sahoo SK, Parveen S, Panda JJ. The present and future of nanotech-nology in human health care. Nanomedicine. 2007;3 (1):20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Editorial. The future of nanotechnologies. Technovation. 2012;32:157–160. [Google Scholar]
- 3.Boisseau P, Loubaton B. Nanomedicine, Nanotechnology in medicine. C.R. Physique. 2011;12:620–636. [Google Scholar]
- 4.Gupta J. Nanotechnology applications in medicine and dentistry. J Investig Clin Dent. 2011;2:81–88. doi: 10.1111/j.2041-1626.2011.00046.x. [DOI] [PubMed] [Google Scholar]
- 5.Emerich DF. Nanomedicine- prospective therapeutic and diagnostic applications. Expert Opin Biol Ther. 2005;5:1–5. doi: 10.1517/14712598.5.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Subramani K, Ahmed W. Waltham, MA: Elsevier Inc; 2012. Emerging Nanotechnologies in Dentistry: Processes, Materials and Applications. [Google Scholar]
- 7.Chen MH. Update of dental nanocomposites. J Dent Res. 2010;89(6):549–560. doi: 10.1177/0022034510363765. [DOI] [PubMed] [Google Scholar]
- 8.Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90(4):402–416. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaled SMZ, Miron RJ, Hamilton DW, Charpentier PA, Rizkalla AS. Reinforcent of resin based cement with titania nanotubes. Dent Mater. 2010;26:169–178. doi: 10.1016/j.dental.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Curtis AR, Palin WM, Fleming GJP, Shortall ACC, Marquis PM. The mechanical properties of nanofilled resin-based composites: The impact of dry and wet cycling pre-loading on bi-axial flexure strength. Dent Mater. 2009;25:188–197. doi: 10.1016/j.dental.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Yu B, Lim H, Lee Y. Influence of nano- and micro-filler proportions on the optical property stability of experimental dental resin composites. Mater Design. 2010;31:4719–4724. [Google Scholar]
- 12.Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. JADA. 2003;134:1382–1390. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 13.Heintze SD, Zellweger G, Zappini G. The relationship between physical parameters and wear of dental composites. Wear. 2007;263:1138–1146. [Google Scholar]
- 14.Ferracane J. Resin composite- state of the art. Dent Mater. 2011;27(1):29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Sideridou ID, Karabela MM, Vouvoudi ECh. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dent Mater. 2011;27:598–607. doi: 10.1016/j.dental.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Saunders SA. Current practicality of nanotechnology in dentistry. Part I: Focus on nanocomposite restoratives and biomimetics. Clin Cosmet Investig Dent. 2009;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- 17.Antonson SA, Yazici AR, Kilinc E, Antonson DE, Hardigan PC. Comparison of different finishing/polishing systems on surface roughness and gloss of resin composites. J Dent. 2011;39 (Suppl 1):e9–e17. doi: 10.1016/j.jdent.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Erdemir U, Yildiz E, Eren MM, Ozsoy A, Topcu FT. Effects of polishing systems on the surface roughness of tooth-colored materials. J Dent Sci. 2012 Article in press. [Google Scholar]
- 19.Janus J, Fauxpoint G, Arntz Y, Pelletier H, Etienne O. Surface roughness and morphology of three nanocomposites after two dif-ferent polishing treatments by a multitechnique approach. Dent Mater. 2010;26:416–425. doi: 10.1016/j.dental.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Morgan M. Finishing and polishing of direct posterior restoration. Pract Proced Aesthet Dent. 2004;16:211–217. [PubMed] [Google Scholar]
- 21.Turssi CP, Ferracane JL, Serra MC. Abrasive wear of resin composites as related to finishing and polishing procedures. Dent Mater. 2005;21(7):641–648. doi: 10.1016/j.dental.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Nagem FH, D’Azevedo MT, Nagem HD, Marsola FP. Surface roughness of composite resins after finishing and polishing. Braz Dent J. 2003;14(1):37–41. doi: 10.1590/s0103-64402003000100007. [DOI] [PubMed] [Google Scholar]
- 23.Jafferies SR. Abrasive finishing and polishing in restorative dentistry: a state-of-the-art review. Dent Clin North Am. 2007;51(2):379–397. doi: 10.1016/j.cden.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 24.ErgÜcÜ Z, TÜrkÜn LS. Surface roughness of novel resin composites polished with one-step system. Oper Dent. 2007;32(2):185–192. doi: 10.2341/06-56. [DOI] [PubMed] [Google Scholar]
- 25.Lim BS, Ferracane JL, Condon JR, Adey JD. Effect of filler fraction and filler surface treatment on wear of microfilled composites. Dent Mater. 2002;18:1–11. doi: 10.1016/s0109-5641(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 26.Trajković V, Marković Z. Nanomedicina: stanje i perspektive. In: Raković D, Uskoković D, editors. Biomaterijali. Beograd: Institut tehnickih nauka Srpske akademije nauka i umetnosti: Društvo za istraživanje materijala; 2010. pp. 762–777. [Google Scholar]
- 27.Bashetty H, Joshi S. The effect of one-step and multi-step polishing systems on surface texture of two different resin composites. J Conserv Dent. 2010;13(1):34–38. doi: 10.4103/0972-0707.62637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei L, Busscher HJ, Mei HC, Ren Y. Influence on surface roughness on streptococcal adhesion forces to composite. Dent Mater. 2011;27:770–778. doi: 10.1016/j.dental.2011.03.017. [DOI] [PubMed] [Google Scholar]


