Abstract
As a source of biologically active compounds, buckwheat has beneficial effects in nutrition due to its high content of flavonoids, particularly rutin.
Aim of our study was to examine effects of buckwheat on plasma lipid status and phospholipids fatty acids composition, histological and parameters of oxidative stress in Wistar rats fed a high-fat diet. This study showed that buckwheat leaf and flower (BLF) mixture supplementation significantly reduce weight gain, plasma lipid concentrations and atherogenic index in rats fed a high-fat diet. Treatment of the high-fat group of animals with buckwheat significantly increased percentage of n-6 fatty acids as well as eicosapentaenoic acid (EPA) and decreased percentage of saturated fatty acids (SFA) and oleic acid. Buckwheat antioxidant effects diminished negative influence of high-fat diet in hyperlipidemic rats, while pathohistological analysis of liver confirmed changes after high-fat consumption. Our results showed hypolipidemic, antiatherogenic and antioxidative features of buckwheat leaf and flower mixture, and these parts of the plant with the highest rutin content could be beneficial in prevention and curing of hyperlipidemia.
KEY WORDS: buckwheat, plasma lipids, plasma phospholipids fatty acids, oxidative stress, hypolipidemic effect
INTRODUCTION
Buckwheat (Fagopyrum esculentum) is a herbaceous plant, which belongs to the Polygonaceae family and is important food source which contains proteins with high biological value and balanced amino acid composition, fibres, vitamins B1 and B2, zinc, copper, manganese and selenium [1]. Buckwheat grains and hulls contain components with healing properties and biological activity, such as flavonoids and flavon, phenolic acid, condensed tannins, phytosterols and fagopyrins. Flavonoids are phytonutrients with chelating properties, acting as antioxidants inhibiting lipid peroxidation, chelate redox-active metals, and attenuate reactive oxygen species (ROS) damages [2]. Buckwheat’s flavonoid compounds decrease blood cholesterol, helping the prevention of a high blood pressure. In addition, buckwheat reduces cellular proliferation and therefore it protects the colon against carcinogenesis [3]. The antioxidant activity in buckwheat exhibited a statistically significant relationship with its total phenolics, as well as rutin content [4]. Rutin, a flavonoid composed of flavonol quercetin and disaccharide rutinose, has anti-inflammatory, hypotensive effect. Rutin/quercetin inhibits oxidation of lipoproteins, which suggests that rutin could reduce the risk for arteriosclerosis [5]. Buckwheat seed contains more rutin than do most plants. The rutin content of the flower part is higher than that of other parts of the buckwheat (flower > leaves > seed > stem > root). About 2–10% of rutin per dry weight can be found in buckwheat flowers and leaves, and their total phenolics content is higher than that of seeds [4]. Dietary fats can modulate the plasma phospholipids fatty acid composition (FAs) [6]. Alterations in these lipid classes are of special interest because plasma phospholipids (PL) mirror the tissue PL status and these functional and pathological consequences can be correlated [7]. Since dietary fat is one of the most important environmental factors associated with the cardiovascular diseases incidence, this study aimed to investigate hypolipidemic and antioxidant effects of buckwheat in rats fed a high-fat diet. Because, fatty acids (FAs) composition of PL highly correlates with dietary intake [8], one of our aims was to examine effects of buckwheat supplementation in rats on FAs composition in PL.
MATERIALS AND METHODS
Plant material
Buckwheat (Fagopyrum esculentum – Moench) leaf and flower (BLF) parts in dried powder form were obtained from Institute for Medicinal Plants Research “Dr Josif Pančić”, Belgrade, Serbia, where the voucher specimen (No. 31210911) was deposited. Polyphenolic content of BLF mixture was: rutin 4.99%, quercetin 0.04%, hyperoside 0.39%, gallic acid 0.09%, protocatechuic acid 0.04%, caffeic acid 0.11%, catechin 0.01% and chlorogenic acid 0.16% (wt/ wt), determined using high performance liquid chromatography with diode-array detection (HPLC/DAD) [9].
Experimental animals and diets
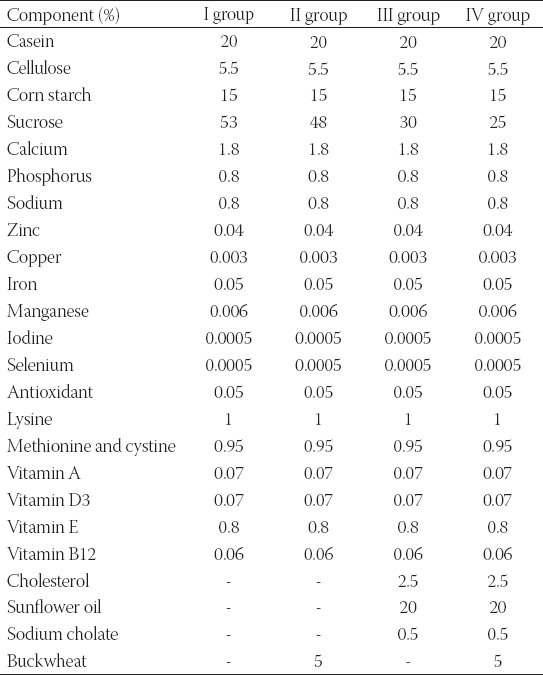
Experiments were carried out on forty male Wistar rats, (four months aged, b.w. 310-440 g), obtained from the vivarium (Galenika a.d., Belgrade, Serbia). All the experiments and protocols employed in the study were reviewed and approved by the Institutional Animal Care and Use Committee [No. III-2011-01]. Experimental animals were housed in groups of two or three per standard cage, in a room with a 12 h light-dark cycle and an ambient temperature of 24°C. All rats were fed a pellitized commercial chow diet for 2 weeks after arrival. They were then randomly divided into five groups. The animals of the group I (control) were fed normal chow (n=10). The rats of the group II (buckwheat) were fed normal chow with 5% BLF mixture (n=s). High-fat (HF) groups III and IV were fed a lipogenic diet [10] consisting of 2.5% cholesterol, 20% sunflower oil and 0.5% sodium cholate added to normal chow without (group III, n=13) or with 5% BLF mixture (group IV, n=5). This regime was maintained 13 weeks. The animals of group V were maintained in the same food regime as the animals in group III (n=7). After 7 weeks, the animals doubled plasma cholesterol concentration and they were maintained in the same food regime as the animals in group IV for 6 weeks. The composition of the experimental diet is shown in Table 1. The animals were given food and tap water ad libitum during the experimental period. Food consumption and weight gain were measured daily and weekly, respectively. After 2, 7 and 13 weeks of experiment, the animals were fasted overnight and then sacrificed under ether anesthesia. Blood samples (5-6 ml) were collected from the inferior vena cava in tubes containing heparin, and plasma was separated for biochemical analysis. The livers were removed, rinsed with saline and stored at -80 °C. The abdominal aorta, pieces of heart, liver, kidney and brain tissues were removed from the rats, rinsed with saline and fixed in a buffer solution of 4% formalin for the pathohistological analysis.
TABLE 1.
Composition of the experimental diets.
Biochemical analysis
The levels of plasma total cholesterol (TC) and triglycerides (TG), alanine transaminase (ALT) and aspartate transaminase (AST) were measured using the automated enzymatic methods (EliTech Diagnostic, Sees, France). Plasma high-density lipoprotein cholesterol (HDL) was measured after precipitation with phosphotungstic acid and magnesium chloride [11]. Low-density lipoprotein cholesterol (LDL) was calculated using formula by Friedewald et al. [12]. The PL fraction was isolated from the extracted lipids by one-dimensional thin layer chromatography (TLC) with neutral lipid solvent system of hexane-diethyl ether-μ acetic acid (87:2:1, v/v) using Silica Gel GF plates (C. Merck, Darmstadt, Germany). The extraction, preparation of FAs methyl esters and gas chromatography (GC) analysis were performed as previously described [13]. Comparing sample peak retention times with authentic standards (Sigma Chemical Co, St. Louis, MO, USA) and/or the PUFA-2 standard mixture (Supelco Inc., Belleforte, PA, USA) individual FAs methyl esters were identified. Hepatic lipid peroxidation, antioxidant enzyme activities and free radical scavenging assays
After partly defrosting, liver was homogenized on ice-cold in the 50mM phosphate buffer, pH 7.0 (buffer: tissue 1:10(w/v). Homogenization was performed in homogenizer Ultra Turex (Janke Kinkel) [14]. Homogenized tissue was centrifuged at 10000g for 10minutes at +4°C and the activity of xanthine oxidase (XOD), catalase (CAT), glutathione S-transferase (GST) and other parameters of oxidative stress were measured in supernatant. The protein content was determined by the method of Bradford [15]. Activities of all determined enzymes were expressed in units per mg proteins (U/mg). XOD activity was assayed according to the Bergmayer method [16] CAT activity was determined according to Clairborne [17] GST activity was measured according to the Habig et al. [18] method. The amount of the reduced glutathione (GSH) was measured after the homogenate precipitation with 4% sulfosalicylic acid (30% w/v) and its centrifugation at 12000 g for 5 min and, then, measured by the Ellman method [19]. The data are expressed as nmol of GSH per mg protein. The superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities were determined using commercially available kits (purchased from RANDOX Laboratories, Antrim, United Kingdom). The malondialdehyde levels (MDA) were estimated by TBARS Assay Kit (Cayman Chemicals, USA). The antioxidant activity of the liver supernatants was measured on the basis of the radical scavenging ability using the stable radical, 1,1-diphenyl-2-picrylhydrazyl (DPPH) [20-22]. The DPPH radical scavenging activity (%) was calculated as [1- (sample absorbance /control absorbance)] X 100. The IC50 values (mg per liver tissue) denoted the amount of the sample, which was required to scavenge 50% of the DPPH free radicals. The spectrophotometric assays were performed with a Nicolet Evolution 100 UV/VIS scanning spectrophotometer equipped with a cell positioned or with a multi plate reader Thermo LabSystem Multiskan EX.
Pathohistological analysis
Fixed tissues (aorta, heart, liver, kidney and brain) were processed routinely for paraffin embedding, and 4μm sections were prepared and stained with hematoxylin-eosin, stained areas were viewed using an optical microscope with magnifying power of x 100 and x 400.
Statistical analysis
The results were expressed as mean values ± standard deviation (SD). The statistical significance was determined using one-way analysis of variance (ANOVA). The differences between control and experimental diets were determined by the Turkey’s test. Values of p<0.05 were considered significant.
RESULTS
Food intake, weight gain and organ weights
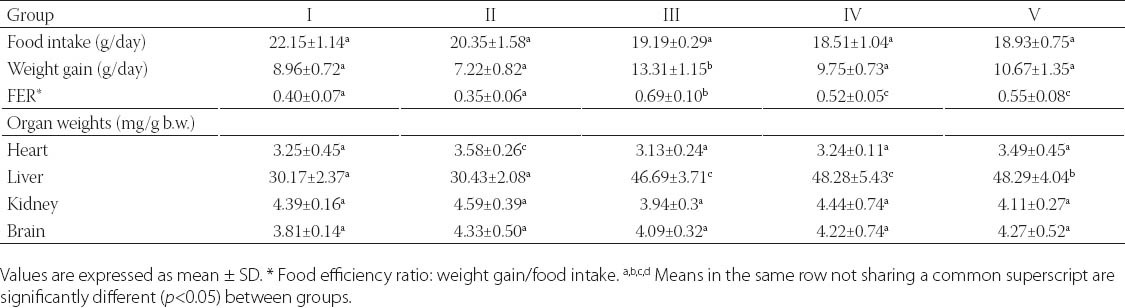
Food intake was equivalent between all groups that allows us to compare effects of BLF mixture (Table 2). After 13 weeks of the experiment, the body weight gain was significantly higher (p<0.05) in the group III compared to other groups of animals. The average weight gains of the IV and V group were not significantly different from that of the I and II group (p>0.05). However, the food efficiency ratio (FER) of the IV and V group exhibited similar values that appeared to be significantly higher than of the I and II group (p<0.05). Compared with I, II, IV and V group, there was significantly higher FER in the III group of animals (p<0.05). Organ weights were expressed as their relative weight per body weight (Table 2). The relative weight of heart was significantly higher in the II group than in other groups (p<0.05). The relative weights of liver were significantly higher in HF groups than in the I and II group (p<0.05).
TABLE 2.
Food intake, weight gain, and organ weights in control and experimental groups of rats.
Plasma lipid levels and transaminase activities
Concentrations of plasma lipids and transaminase activities after 2, 7 and 13 weeks of the experiment are shown in Table 3. After 2 weeks, a significant difference was observed in HDL levels, atherogenic index (AI), ALT and AST activities, while after 7 weeks a significant difference was observed in all plasma lipid levels, AI and AST/ALT activities between I and III group. An increase in TC and AI by 106.8% and 351.9% respectively was observed in III group of animals. After that period, feeding regime was changed to the V group from a lipogenic diet to a lipogenic diet with BLF. In the next 6 weeks the supplementation of BLF to the V group significantly lowered TG by 40.2%, TC by 25.9%, LDL by 68.4% and AI by 60.6% compared to the III group (p<0.05). Also, after 13 weeks the supplementation of BLF to the IV group significantly lowered TG by 12.0%, TC by 27.8%, LDL by 61.1% and AI by 40.4% compared to the III group (p<0.05), which indicated that the BLF mixture had anti-hyperlipidemic effect. HDL concentration and transaminase activities were not significantly different (p>0.05) between III, IV and V groups of animals. After 13 weeks of the experiment there were no significant differences in plasma lipid levels, AI and transaminase activities between I, II, IV and V group (p>0.05), except significant differences observed in HDL and LDL levels comparing I and II with IV and V group (p<0.05). Compared with I and II group, there were significant differences in all analyzed parameters in the III group of animals with induced hyperlipidemia (p<0.05).
TABLE 3.
Biochemical parameters in plasma of control and experimental groups of rats.

Fatty acid analysis
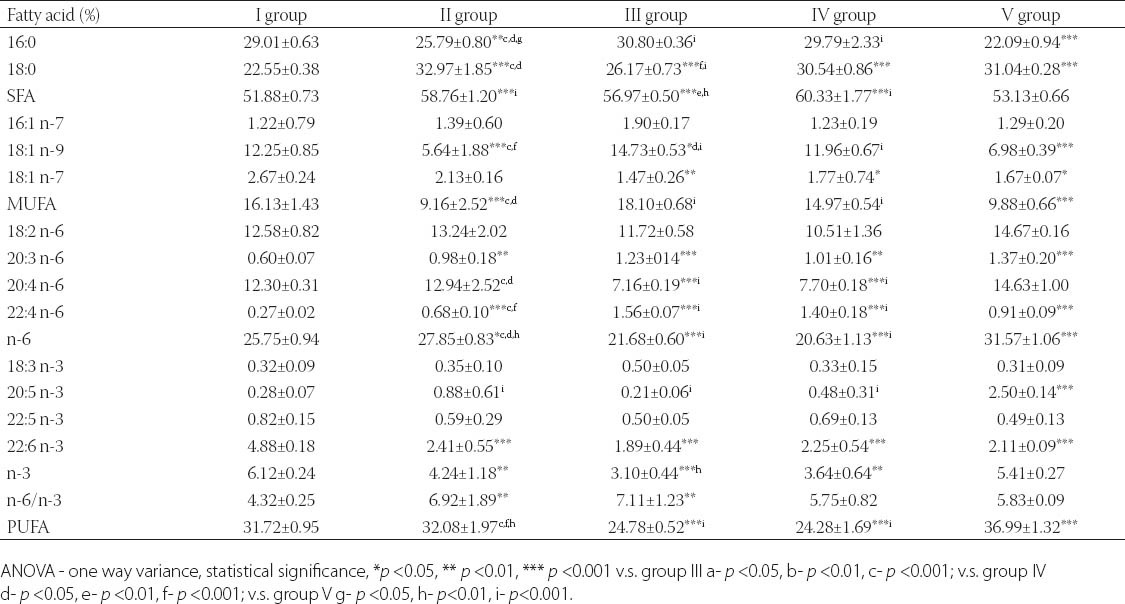
Results of FA analysis of PL (Table 4) showed that the lowest percentage of palmitic acid (16:0) was in V group, significantly lower compared to all other examined groups. Percentage of stearic acid (18:0) significantly increased in group V compared to I and group III. Therefore, percentage of saturated fatty acids (SFA) decreased in group V compared to II, III and IV group. Our results showed that percentage of oleic acid (18:1 n-9) decreased in group V as well as in group II, compared to other groups. Percentage of monounsaturated fatty acids (MUFA) statistically decreased in groups II and V compared to groups III and IV (p<0.001). Analysis of polyunsaturated fatty acids (PUFA) profiles did not show differences in levels of precursors of n-6 and n-3 families, i.e. linoleic acid (LA, 18:2 n-6) and alpha-linolenic acid (ALA, 18:3 n-3) between examined groups, but there were statistical significant differences in concentration of their metabolites in plasma phospholipids. Our results showed that lipogenic diet decreases percentage of arachidonic acid (AA, 20:4 n-6) in III and IV group, while between other groups there were no differences. On the other hand, level of n-6 PUFA increased in groups II and V compared to I, III and IV group. Percentage of eicosapentaenoic acid (EPA, 20:5 n-3) increased in group V compared to other examined groups. Percentage of docosahexaenoic acid (DHA, 22:6 n-3) statistically increased in control, compared to other groups, and did not differ between groups II, III, IV and V. In this study ratio of n-6/n-3 did not differ between groups I, IV and V, while in groups II and III was statistically increased compared to control, and n-6 FA increased percentage could have negative consequences on health.
TABLE 4.
Plasma phospholipids fatty acids profiles in control and experimental groups of rats.
Hepatic antioxidant activity
It could be observed that group III induced a significant increase (p<0.05) in the liver MDA levels compared to groups I and II (Figure 1), resulting in the oxidative damage to the lipids. The activities of SOD (significantly p<0.05) and GSH-Px (slightly) were decreased in the group III. Particularly, the high XOD activity (p<0.05) was recorded in given group compared with all other experimental groups (Figure 2). Statistically the significant increase (p<0.05) in the CAT activity was observed in the group III (Figure 2) which indicated that the CAT activity was one of the most important enzyme defenses against hydrogen peroxide formation, especially when there was no high activity of GSH-Px as it was detected in this group. As shown in Figure 2, the levels of GSH were significantly decreased (p<0.05) in the group III. Furthermore, the significantly high (p<0.05) IC50 radical scavenger activity (the DPPH assay) was observed in this group (Figure 1). The GST activity increased significantly (p<0.05) in the liver of the group III (Figure 2) considering liver as the metabolic centre for the detoxification [23]. The data presented in the Figure 1 show that there was no significant difference in the MDA levels of the groups I and II in the comparison to the group IV. In other enzymatic and non enzymatic parameters of the antioxidative status no significant differences were detected between the groups IV and V, except for the GSH-Px levels which were significantly higher in the group V and nearly the same as in the groups I and II. It indicated a beneficial effect of the buckwheat enriched diet. Although the GSH level in the groups IV and V didnt show an increment in its level, IC50 (the DPPH assay) significantly decreased (p<0.05) in both IV and V groups (Figure 1).
FIGURE 1.

DPPH (IC50) (A) and MDA (B) levels in control and experimental groups of rats. Values are expressed as mean ± SD. Means with the same letters are not significantly different at p<0.05.
FIGURE 2.
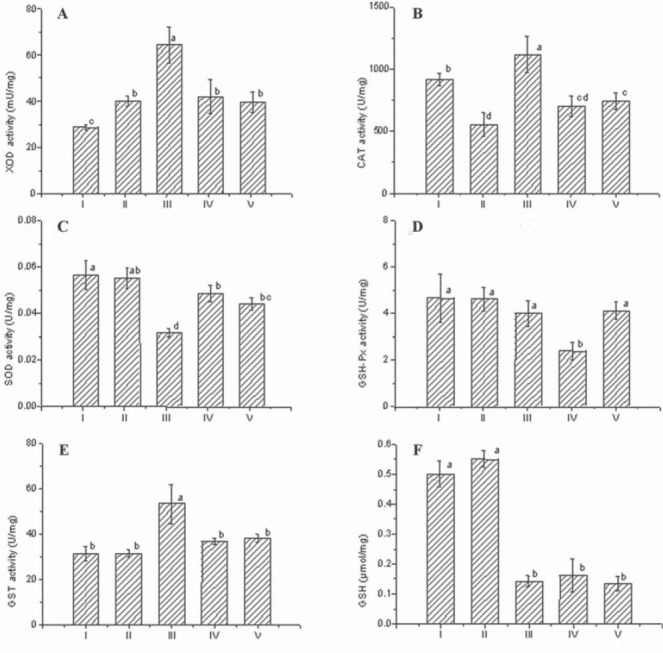
XOD (A), CAT (B), SOD (C), GSH-Px (D), GST (E) and GSH (F) activity in control and experimental groups of rats. Values are expressed as mean ± SD. Means with the same letters are not significantly different at p<0.05.
Morphological results
After 7 weeks of fat rich diet there was no change in aortic intima specific for early atherosclerosis, although adipocytes of periaortic adipose tissue were somewhat enlarged and containing more fat in group III (Figure 3), compared to animals with regular diet (I group, Figure 4). Histology of other organs remained normal. Histological findings of organs were normal in animals fed a regular and food enriched with BLF mixture (I and II group), after 13 week of the experiment (Figures 5 and 6). Significant changes were observed in liver of animals in III, IV and V group. Grossly, liver was enlarged, softer, yellowish and greasy. Other examined organs had no gross changes. Pathohistological findings in liver specimens obtained from HF groups of animals correlates with steatohepatitis: fatty degeneration of hepatocyte cytoplasm (approximately 70% of hepatocytes), mostly of microvesicular type, mild to moderate mononuclear inflammatory infiltrate in portal tracts, groups of lymphocytes inside hepatic lobular parenchyme (lobular inflammatory activity) and rupture of endothelium in few central veins (Figures 7-10).
FIGURE 3.
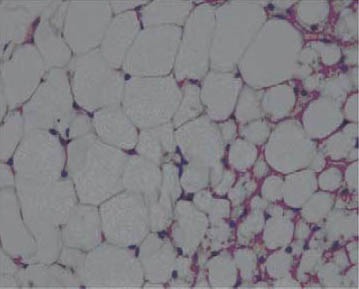
Periaortic adipose tissue (III group), HE x 400.
FIGURE 4.
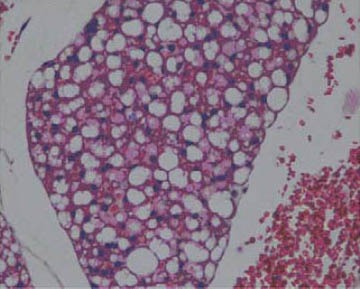
Periaortic adipose tissue (I group), HE x 400.
FIGURE 5.
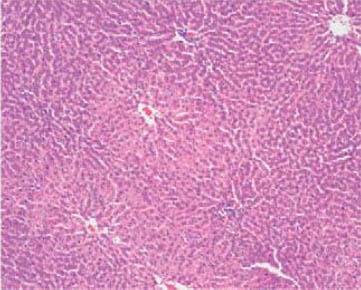
Normal architecture of hepatic parenchyme (I group), HE x 100.
FIGURE 6.
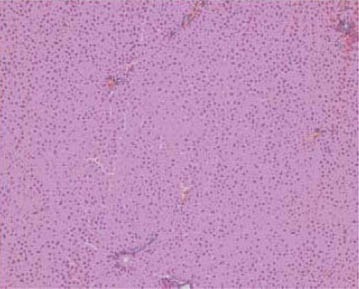
Normal architecture of hepatic parenchyme (II group), HE x 100.
FIGURE 7.
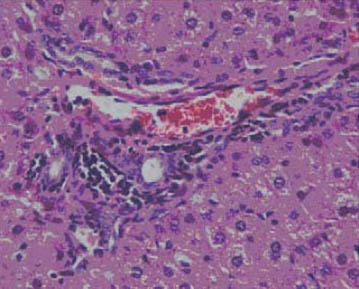
Moderate mononuclear inflammatory infiltrate in portal tract (III group), HE x 400.
FIGURE 10.
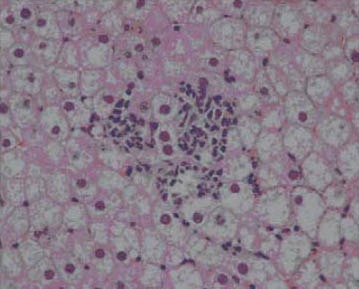
Fatty change in hepatocyte cytoplasm with focus of lobular inflammatory activity (IV group), HE x 400.
FIGURE 8.
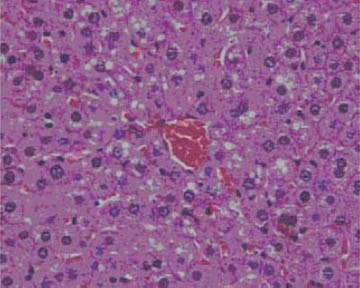
Fatty change of centrolobular hepatocytes, mostly microvesicular type (III group), HE x 400
FIGURE 9.

Endothelial rupture of central vein (III group), HE x 400.
DISCUSSION
BLF mixture supplementation contributed to the decreased body weight gain when compared high-fat group, and such results get along with the studies reporting that polyphenols exert an inhibitory effect on growth by decreasing protein digestibility [24]. It was supposed that the weights of the liver in the HF groups were increased by the high-fat diet. However, the kidney and brain weights were not significantly different between the groups (p<0.05). In our study, as well as in previously feeding experiments with rats [24] was observed that cholesterol, fat component and cholic acid addition to normal diet increase plasma cholesterol concentration, i.e. enhancing hyperlipidemic effect. TG, TC, LDL concentrations and AI were significantly lower in BLF mixture supplemented rats fed a high-fat diet, but this supplement had no effect in cholesterol-free diet groups. It has been reported that the hypocholesterolemic effect of dietary fiber and polyphenolic compounds were more effective in hypercholesterolemic animals and humans than in normocholesterolemic ones [25]. FAs profiles in PL were determined after 13 weeks of the experiment. Palmitic acid raise plasma cholesterol, induce apoptosis in adult rat cardiomyocytes and has a dramatic destructive effect on the myofibrillar apparatus [26]. In contrast, stearic acid may have neutral or even positive effects on health because it has been shown to exert cardioprotective [27] and cancer-protective effects [28]. Therefore, BLF mixture intake has beneficial effects on SFA/ PL and health. Although, oleic acid is considered beneficial for health [29], our results showed that hypocholesterolemic effect in group V is consequence of some other factors rather than oleic acid. AA is a precursor for the synthesis of leukotriene B4, thromboxane A2, and prostaglandin E2, as mediators of inflammation [30]. Low concentrations of AA is consequence of decreased level of n-6 fatty acids, and may cause infertility in both males and females [31, 32]. Also, decreased level of total PUFA, mainly of n-6 family, was observed in patients with insulin resistance [33]. BLF mixture intake increased percentage of n-6 PUFA in plasma PL of examined animals. These findings are very important, considering that n-6 PUFA are necessary for liver and kidney function, heart contractility, and skin permeability [34]. EPA is precursor of prostaglandin PGE-3 and leukotriene LTB-4. It has the ability to partially block the conversion of n-6 FA to harmful eicosanoids, thereby reducing the cardiovascular risk and inhibiting tumor genesis [35, 36]. Percentage of ALA did not differ between examined groups, supposing BLF mixture intake increase enzyme activity in EPA biosinthesys. DHA plays the important role in neural and retinal development, may protect against Alzheimer disease [37] while deficiency in n-3 PUFA may be associated with metabolic syndrome in adults [38]. For optimal human nutrition, the ratio of n-6/n-3 must not be higher than 10 [39]. The results presented in this study demonstrated that supplementation of BLF mixture improved FAs profiles in rats with induced hypercholesterolemia, decreasing percentage of palmitic acid and SFA, and by increasing percentage of stearic acid and total PUFA, compared to control group. Low percentage of oleic acid in animals which consumed BLF mixture could be improve by addition of olive oil in usual diet. The activity of enzymes, such as CAT, SOD, XOD, GSH-Px, GST, were measured to determine the antioxidant activity in vivo. The MDA, GSH and DPPH levels were also measured in this study, after 13 weeks of the experiment. Feeding a high-fat diet resulted in a large amount of fat intake and a further increment of PUFA in the liver susceptible to the lipid peroxidation. The resent study [40, 41] stated that the increased levels of MDA in the hyperlipidemic rats could be attributed to the increased ROS production and/or the deficiency of the antioxidant defence system. High amount of the XOD activity in the group III resulted in the high amount of the hydrogen peroxide content. BLF enriched diet could be a good choice for lowering the MDA levels in the hyperlipidemic animals. We assume that this results indicates that the antioxidant effect of the BLF enriched food may depend on a different phenolic content (high level of rutin and quercetin), which may provide a mechanism for the buckwheat pharmacological activity. The consumption of the BLF enriched food both by the groups IV and V resulted in the return of the antioxidant protection to the level of the normal state (to the levels of the control and the buckwheat groups). Accordingly, the significant decrease (p<0.05) of GST, CAT and XOD activities were recorded in the cited groups in comparison to the group III in this study. The significant increased (p<0.05) of the SOD activity in the liver homogenates of the hyperlipidemic rats fed a BLF enriched food was detected, confirming the good antioxidant status in the groups IV and V. There were no differences in histological findings between HF groups of animals. Our morphological findings correlate with findings of other authors [10].
CONCLUSIONS
This study showed that the supplementation of the BLF mixture significantly reduced body weight gain, plasma lipid concentrations and atherogenic index in rats fed a high-fat diet. An efficacy of lipid-lowering action of the BLF mixture indicates that these parts of the plant with the highest rutin content have anti-hyperlipidemic effect and could be beneficial for regulation of lipid metabolism or prevention of hyper-lipidemia. Treatment with BLF in fat-fed groups increased percentage of n-6 FA and EPA, decreased percentage of SFA, MUFA (oleic acid) and partly AA. Considering our results buckwheat should be consume with olive oil in usual diet habits, but future studies should addressed it. The obtained results of the hepatic antioxidant status of hyperlipidemic rats are consistent with the assumption that the buckwheat antioxidant effects affect the body’s antioxidant status and the liver, as the central metabolic organ, in a favourable way to diminish the negative influence of a high-fat diet consumption. Fat rich diet induced gross and histological changes of liver typical of steatosis and steatohepatitis, without significant resolution following supplementation of the BLF mixture.
ACKNOWLEDGMENTS
This work is a part of the Project (TR–31029) supported by the Ministry of Education, Science and Technological Development, Republic of Serbia. We are grateful to Dr Slavica Ristić and senior veterinary technician Branka Veselinović (Center for Biomedical Investigation, Galenika a.d., Belgrade, Serbia) who donated experimental animals. We are thankful to Professor Dr Dušan Lalošević (Pasteur Institute, Novi Sad, Serbia) who has enabled our experiments with rats in Pasteur Institute’s vivarium.
DECLARATION OF INTEREST
Authors designed, organized, performed the experimental work and wrote the manuscript, and declare no conflict of interest in this research.
REFERENCES
- 1.Jeong-Sun L, Song-Hae B, Seon-Min J, Hye-Jin K, Kyung-Min D, Yong-Bok P, et al. Antihyperlipidemic effects of buckwheat leaf and flower in rats fed a high-fat diet. Food Chem. 2010;119(1):235–240. [Google Scholar]
- 2.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidant: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Ishikawa W, Huang X, Tomotake H, Kayashita J, Watanabe H, Kato NA. Buckwheat protein product suppresses 1,2-dimethylhydrazine-induced colon carcinogenesis in rats by reducing cell proliferation. J Nutr. 2001;131:1850–1853. doi: 10.1093/jn/131.6.1850. [DOI] [PubMed] [Google Scholar]
- 4.Holasova M, Fiedlerova V, Smrcinova H, Orsak M, Lachman J, Vavreinova S. Buckwheat – the source of antioxidant activity in functional foods. Food Res Int. 2002;35(2-3):207–211. [Google Scholar]
- 5.Valenzuela A, Sanhueza J, Alonso P, Corbari A, Nieto S. Inhibitary action of onventional food-grade natural antioxidants and of natural antioxidants of new development on the thermal-induced oxidation of cholesterol. Int J Food Sci Nutr. 2004;55:155–162. doi: 10.1080/09637480410001666496. [DOI] [PubMed] [Google Scholar]
- 6.Willett WC. Dietary fat and obesity: an unconvincing relation. Am J Clin Nutr. 1998;68:1149–1150. doi: 10.1093/ajcn/68.6.1149. [DOI] [PubMed] [Google Scholar]
- 7.Pan DA, Storlien LH. Dietary lipid profile is a determinant of tissue phospholipid fatty acid composition and rate of weight gain in rats J Nutr. 1993;123:512–519. doi: 10.1093/jn/123.3.512. [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The atherosclerosis risk in communities (ARIC) study investigators. Am J Clin Nutr. 1995;62:564–571. doi: 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- 9.Mišan A, Beljkaš B, Milovanovic I, Mandic A, Sakac M, Sedej I. Belgrade, Serbia: 2011. Sep, Herbal drug Fagopyri Herba as a source of bioactive phenolic compounds. In Proceedings of the 16 th European Conference on Analytical Chemistry (Euroanalysis 16) p. CH21. [Google Scholar]
- 10.Bolkent S, Yanardag R, Karabulut-Bulan O, Yesilyaprak B. Protective role of Melissa officinalis L. extract on liver of hyperlipidemic rats: a morphological and biochemical study. J Ethnopharmacol. 2005;99(3):391–398. doi: 10.1016/j.jep.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 11.Lopes-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23:882–884. [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 13.Popovic T, Ranic M, Bulajic P, Milicevic M, Arsic A, Vucic V, et al. Effects of n-3 fatty acids supplementation on plasma phospholipids fatty acid composition in patients with obstructive jaundicea pilot study. J Clin Biochem Nutr. 2009;45(3):370–375. doi: 10.3164/jcbn.09-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krummer S, Thiermann H, Worek F, Eyer P. Equipotent cholinesterase reactivation in vitro by the nerve agent antidotes HI 6 dichloride and HI 6 dimethanesulfonate. Arch Toxicol. 2002;76:589–595. doi: 10.1007/s00204-002-0382-2. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Bergmeyer HU. Weinheim: Verlag Chenue/New York: Academic Press Inc; 1974. Methoden der enzymatischen analyse; pp. 644–649. [Google Scholar]
- 17.Clairborne A. Catalase activity. In: Greenwald RA, editor. CRC Handbook of methods for oxygen radical research. Boca Raton, FL: C.R.C. Press Inc; 1984. pp. 283–284. [Google Scholar]
- 18.Habig WH, Pabst MJ, Jacoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 19.Ellman GC. Tissue sulflydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Ebrahimzadeh MA, Bahramian F. Antioxidant activity of Crataegus pentaegyna subsp. elburensis fruits extracts used in traditional medicine in Iran. Pak J Biol Sci. 2009;12(5):413–419. doi: 10.3923/pjbs.2009.413.419. [DOI] [PubMed] [Google Scholar]
- 21.Ebrahimzadeh M A, Ehsanifar S, Eslami B. Sambucus ebulus elburensis fruits: A good source for antioxidants. Pharmacogn Mag. 2009;5(19):213–218. [Google Scholar]
- 22.Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 2009;22(3):277–281. [PubMed] [Google Scholar]
- 23.Torres MCL, Soares NFF, Maia JF. Pârametros cinéticos da glutationa S-transferase e sua ativçãao por extratos de vegetais. Cienc Tecnol Aliment. 2004;24(2):243–248. [Google Scholar]
- 24.Tebib R, Bitri L, Besançon P, Rouanet JM. Polymeric grape seed tannins prevent plasma cholesterol changes in high-cholesterol-fed rats. Food Chem. 1994;49(4):403–406. [Google Scholar]
- 25.Lairon D. Dietary fibres: effects on lipid metabolism and mechanisms of action. Eur J Clin Nutr. 1996;50(3):125–133. [PubMed] [Google Scholar]
- 26.Dyntar D, Eppenberger-Eberhardt M, Maedler K, Pruschy M, Ep-penberger HM, Spinas GA, et al. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 2001;50:2105–2113. doi: 10.2337/diabetes.50.9.2105. [DOI] [PubMed] [Google Scholar]
- 27.Kelly FD, Sinclair AJ, Mann NJ, Turner AH, Abedin L, Li D. A stearic acid-rich improves thrombogenic and atherogenic risk factor profiles in healthy males. Eur J Clin Nutr. 2001;55(2):88–96. doi: 10.1038/sj.ejcn.1601122. [DOI] [PubMed] [Google Scholar]
- 28.Evans LM, Cowey SL, Siegal GP, Hardy RW. Stearate preferentially induces apoptosis in human breast cancer cells. Nutr Cancer. 2009;61:746–753. doi: 10.1080/01635580902825597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haug A, Hostmark AT, Harstad OM. Bovine milk of human nutrition - a review. Lipids Health Dis. 2007;6:25–41. doi: 10.1186/1476-511X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawakami Y, Murakami Y, Kawakami T, Muroyama N, Takiue T, Moritani Y, et al. Abnormal fatty acid profile of blood cell phospholipids and dietary fatty acid intake in patients with ulcerative colitis. J Clin Biochem Nutr. 2005;37(3):95–102. [Google Scholar]
- 31.Kinsella JE, Broughton KS, Whelan JW. Dietary unsaturated fatty acids: interactions and possible needs in relation to eicosanoid synthesis. J Nutr Biochem. 1990;1:123–141. doi: 10.1016/0955-2863(90)90011-9. [DOI] [PubMed] [Google Scholar]
- 32.Seo T, Blaner WS, Deckelbaum RJ. Omega-3 fatty acids: molecular approaches to optimal biological outcomes. Curr Opin Lipidol. 2005;16:11–18. doi: 10.1097/00041433-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. New Engl J Med. 1993;328(4):238–244. doi: 10.1056/NEJM199301283280404. [DOI] [PubMed] [Google Scholar]
- 34.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Wilson WM. Plasma phospholipid arachidonic acid and growth and development of preterm infants. In: Koletzko B, Okken A, Rey J, Salle B, Van Biervliet JP, editors. Recent advances in infants feeding. Stuttgart-New York: Georg Thieme Verlag; 1992. pp. 22–27. [Google Scholar]
- 35.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arsic A, Prekajski N, Vucic V, Tepsic J, Popovic T, Vrvic M, et al. Milk in human nutrition: comparison of fatty acid profiles. Acta Veterinaria (Beograd) 2009;59(5-6):569–578. [Google Scholar]
- 37.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 38.Kabagambe EK, Tsai MY, Hopkins PN, Ordovas JM, Peacock JM, Borecki IB, et al. Erythrocyte fatty acid composition and the metabolic syndrome: a national heart, lung, and blood Institute GOLDN study. Clin Chem. 2008;54:154–162. doi: 10.1373/clinchem.2007.095059. [DOI] [PubMed] [Google Scholar]
- 39.Gerster H. Can adults adequately convert alfa-linolenic acid to eicosapentaenoic acid and docosahexaenioc acid. Int J Vitam Nutr Res. 1998;68:159–173. [PubMed] [Google Scholar]
- 40.Feng L, Yu C, Ying K, Hua J, Dai X. Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res Int. 2011;44(1):404–409. [Google Scholar]
- 41.Yang R, Shi Y, Hao G, Li W, Le G. Increasing oxidative stress with progressive hyperlipidemia in human: relation between malondialdehyde and atherogenic index. J Clin Biochem Nutr. 2008;43(3):154–158. doi: 10.3164/jcbn.2008044. [DOI] [PMC free article] [PubMed] [Google Scholar]


