Abstract
The objective of our work was to evaluate the effect of interferon-γ (IFN-γ) on cytokine expression in rat acute pancreatitis (AP).
AP was introduced to rats which were divided into Control, AP and IFN-γ group. Rats in the AP and IFN-γ group were sacrificed as 6, 12 and 24 h after IFN-γ treatment. The serum amylase (AMA), endotoxin and cytokines were detected. The pathological examination and immunofluorescence staining of pancreas for TNF-α, NF-κB and IL-18 were performed.
The serum AMA increased significantly at 6 h and reduced at 48 h after AP. The increase in IFN-γ was higher than that in AMA. IL-18 increased in the AP and IFN group, and IFN increased markedly at 48 h after AP. IL-27 reduced at 24 h after AP compared with AP group. In the AP group, the immunostaining of cytokines increased. In the IFN group, the edema in the pancreas was more severe, and NF-κB and IL-18 expression was higher than that in the other two groups. IFN-γ can increase serum IL-18 and reduce IL-27 in AP.
IFN-γ can increase serum IL-18 and reduce serum IL-27 in AP. The increase in NF-κB and IL-18 may exert influence on pro-inflammatory cytokines to deteriorate inflammation in the pancreas. Thus, to control the IFN-γ might has promise to attenuate pancreatitis.
KEY WORDS: pancreatitis, interferon-γ, nuclear factor-κB, tumor necrosis factor-α, interleukin-27
INTRODUCTION
Acute pancreatitis is a clinical entity that is believed to have intracellular activation of digestive enzymes and autodigestion of the pancreas as its central pathophysiologic cause [1, 2]. As early as 1960s and 1970s, investigators felt that some mediators released during AP entered the systemic circulation, thereby causing dysfunction of distant organs [3]. Efforts to unravel the events in the evolution of tissue damage in AP have shown a number of inflammatory mediators to be involved [4, 5]. The proinflammatory response is countered by an anti-inflammatory response, and an imbalance between these two systems leads to localized tissue destruction and distant organ damage. Cytokines lie at the heart of the problem and are involved in all aspects of the cascade leading to systemic inflammatory response syndrome and multiple organ dysfunction syndrome [6]. Acute pancreatitis (AP) is a common disease in clinical practice, and its pathogenesis is closely related to the abnormal activation of pancreatin, microcirculatory disturbance, reperfusion induced injury and release of inflammatory mediators and cytokines. Recently, increasing attention has been paid to the role of cytokines in the pathogenesis of AP. In the present study, AP was introduced to rats which were then treated with interferon-γ (IFN-γ) and the cytokines were measured before and after treatment. This study aimed to investigate the potential effect of IFN-γ on the production of cytokines in AP.
MATERIALS AND METHODS
Preparation of AP animal model
The study was conducted in the Animal Center of Shanghai Tenth People’s Hospital. Male SD rats (n=90) weighing 180-220 g were randomly assigned into control group, AP group and IFN-γ group. Rats in the AP group and IFN-γ were sacrificed at 6 h, 12 h, 24 h and 48 h after IFN-γ treatment (n=10 per subgroup). In the AP group, rats were fasted for 24h. Following anesthesia with diethyl ether and routine sterilization, ligation of duodenum was done to induce AP during which a catheter was inserted in the intestine aiming to avoid intestinal obstruction [7]. In the IFN-γ group, IFN-γ was injected via the tail vein. In the control group, duodenum was not ligated. After surgery, animals were fasted but given ad libitum access to water. At different time points, animals were sacrificed and the heart, mesenteric lymph nodes, pancreas, iliac lymph nodes and ilium were collected for use.
Detections
Automatic biochemical analyzer, limulus synthetic matrix azo-dye method and ELISA (Wuhan Boster Biotech Co., Ltd) were employed to detect the serum amylase (AMA), endotoxin and cytokines (TNF-α, IL-18 and IL-27), respectively. Under an aseptic condition, the mesenteric lymph nodes were collected and added to LB medium. One day later, these lymph nodes were seeded into culture plate for bacterial culture. The number of bacterial colonies was counted and the type of bacteria also determined.
Pathological examination and immunofluorescence staining
The pancreas was collected and processed for pathological examination with HE staining. Immunofluorescence staining was done to detect the expression of cytokines in the pancreas. The paraffin embedded sections were deparaffinized and dehydrated. After antigen retrieval, sections were treated with 0.1% trypsin at 37°C for 40 min. Following treatment with 5% BSA for blocking, sections were incubated with primary antibody (1:50) at 4°C overnight and then with FITC conjugated secondary antibody. CD68 was used to label macrophages during which the secondary antibody was labeled with Rhodamine. Incubation was done at 37°C for 1 h. Mounting was performed with glycerophosphate and sections were observed under a fluorescence microscope. The expression of TNF-α, IL-18 and NF-κB was detected in these sections.
Statistical analysis
Data were expressed as mean ± standard deviation ( ). Statistical analysis was performed with SPSS version 12.0 for Windows and one way analysis of variance employed for comparisons. A value of p<0.05 was considered statistically significant.
RESULTS
Changes in serum AMA, endotoxin andIL-27
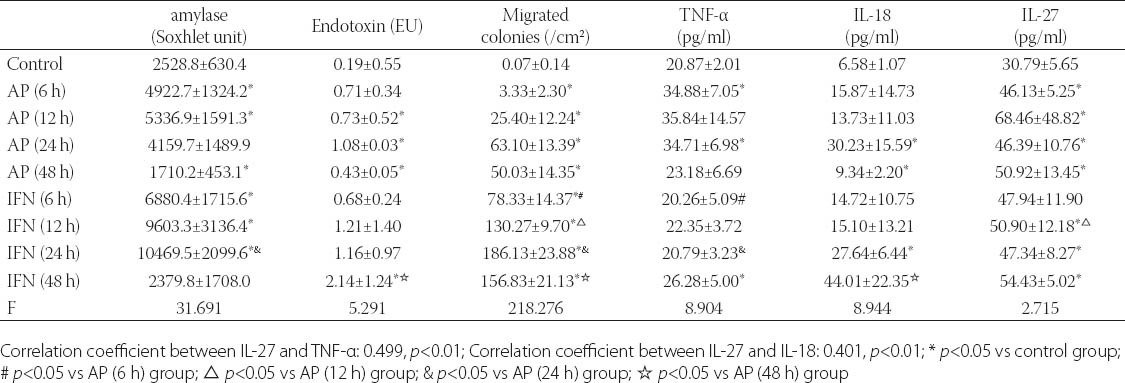
As shown in Table 1, the serum AMA increased at 6 h after AP and reduced at 48 h, and the increase in AMA was the most obvious in the IFN group. The endotoxin increased in different groups to different extents, and the increase was the most evident in the IFN group at 48 h after AP (p<0.05). The amount of bacteria increased in the AP group and IFN group, and was the highest in the IFN group (p<0.05). TNF-α increased in the AP group, but remained unchanged in the IFN group. IL-18 increased in both AP group and IFN group, and was the highest in the IFN group at 48 h after AP (p<0.05). IL-27 increased in both AP group and IFN group, and reached a maximal level at 24 h after AP. At 24 h after AP, the IL-27 was lower in the IFN group than in the AP group (p <0.05).
TABLE 1.
Biochemical parameters in rats of different groups (n=10 per group)
Pathological examination of pancreas
As shown in Figure 1, the pancreatic cells were intact and swelling of interstitial space was absent (Figure 1-A). At 12 h after AP, the pancreatic cells were swelling, vascular congestion was noted, and swelling of interstitial space was present accompanied by infiltration of inflammatory cells (Figure 1-B) in the AP group. At 12 h after AP, the above pathological changes were also found but more severe (Figure 1-C).
FIGURE 1.
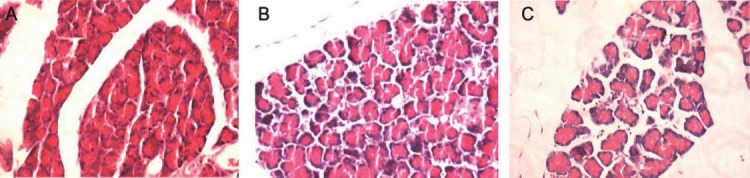
Pathological examination of pancreas (HE staining, x20). A: control group; B: AP group (12 h); C: IFN group (12 h).
Immunofluorescence staining of TNF-α, NF-κB and IL-18 in pancreas
The expression of TNF-α, NF-κB and IL-18 was detected in the pancreas (Figure 2). As shown in the Figure 2, the expression of TNF-α, NF-κB and IL-18 at a very low level in the control group. The expression of TNF-α and NF-κB at 12 h after AP and IL-18 expression at 48 h after AP were markedly increased in the AP group. The TNF-α expression in the IFN group at 12 h was higher than that in the control group but lower than that in the AP at corresponding time point. The NF-κB expression in the IFN group at 12 h increased significantly. The IL-18 in the IFN group at 48 h was dramatically increased when compared with control group and AP group at 48 after AP.
FIGURE 2.
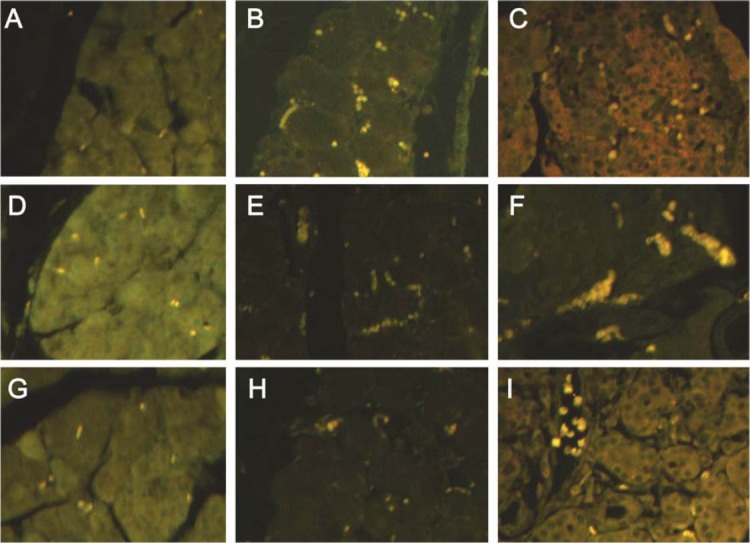
Expression of TNF-α, NF-κB and IL-18 (immunofluorescence staining; TNF-α: x20; NF-κB and IL-18: x40). A, D, G: control group; B, E: AP group (12 h); H: AP group (48 h); C, F: IFN group (12 h); I: IFN group (48 h).
DISCUSSION
The pathogenesis of AP is complex. Studies have shown that some cytokines play important roles in the pathogenesis of AP. As an initiator in the occurrence of AP, TNF-α is one of important cytokines affecting AP and plays a pro-inflammatory role in AP at early stage. TNF is the primary member of the inflammatory cytokine family, primary inducers of IL-6 and IL-8 production, and known to initiate and propagate nearly all of the detrimental consequences of severe sepsis [8] nearly all experimental models of pancreatitis have implicated TNF as a major pathologic cytokine associated with local and systemic tissue destruction. TNF production was closely associated with induction of other genes within their same gene-family [9]. Likewise, as TNF is produced during AP, so too are its receptors [10]. IL-18 is a cytokine that belongs to the IL-1 superfamily [11]. IL-18 levels reflect the severity of acute pancreatitis and display a significant negative correlation with the concentrations of antioxidative damage factors, serum selenium and glutathione peroxidases (GPx). IL-18 is one of the key mediators of inflammation in the pathogenesis of acute pancreatitis [12]. Elevation of serum IL-18 levels may mediate acute pancreatitis associated liver injury [13, 14]. IL-18 may serve as an additional marker to monitor the severity of inflammation during pancreatitis since its tissue production is delayed and appears after that of more commonly investigated cytokines [15]. IL-18 appears to protect the pancreas during early induced-induced AP in mice, probably through induction of NO release from an iNOS source. IL-18 may be a target for new AP therapeutics[16]. IL-18 is mainly produced by monocytes / macrophages and intestinal epithelial cells and endothelial cells also have the ability to secret it. Generally, IL-8 binds to its receptor exerting biological effect. In vitro studies reveal that IL-18 can promote the proliferation of T cells and increase the activity of T cells and NK cells; may elevate the production of some cytokines including TNF-α, IFN-γ, IL-1 and IL -8 but reduce the IL-10 production. In AP patients, the IL-18 increases significantly and is closely related to the severity of AP. NF-κB is a multifunctional transcriptional factor. Under physiological conditions, NF-κB binds to IKB leading to the inactivation of NF-κB. Once activated, NF-κB separates from IKB and enter the nucleus initiating the transcription of target genes. There is evidence showing that the NF-κB is significantly activated in AP patients and closely associated with the severity of AP. TNF-α is an activator of NF-κB. After binding to receptors on cells, TNF-α may phosphorylate IKB leading to the degradation of IKB which results in activation of NF-κB. The activated NF-κB may further increase the synthesis and release of TNF-α. In this manner, a large amount of cytokines is released, which further complicates the AP. Interleukin-27 (IL-27) is a heterodimeric cytokine belonging to the IL-12 family and composed of EBI3 and p28 protein [17]. IL-27 and its receptor WSX-1 have originally been reported as critical factors for initial commitment of Th1 differentiation and The Th1-promoting effect of IL-27 has been reported both in in vivo and in vitro experiments [18, 19]. Yoshimura et al found that IL-27 via IL-27R complex (WSX-1 plus gp130) had a suppressive function on cytokine production by fully activated CD4+ T cells and significant contribution of STAT3 to the IL-27-mediated cytokine suppression in fully activated CD4+ T cells [20]. Thus, IL-27/WSX-1 have two-sided roles; one is for Th1 differentiation and the other for cytokine suppression [21]. Our results showed IL-27 increased in AP. The changes in IL-27 might exert effect on AP. Lucas et al. [22] reported that IL-27 could stimulate the generation of IFN-γ by NK cells, promote the proliferation of CD4+ T and coordinate the IL-12 induced differentiation of naïve CD4+ T cells into Th1 cells. IL-27 can up-regulate the ICAM-1 expression in naïve CD4+ T cells, and the interaction between ICAM-1 and LFA-1 may promote the differentiation of naïve CD4+ T cells into Th1 cells [23]. In addition, studies also revealed that IL-27 may exert immunosuppressive effect to regulate the immune system, avoid the over-expression of inflammatory cytokines and inhibit the autoimmune response [24]. In experiments on mice, IL-27 was found to attenuate the collagen induced arthritis and to inhibit the delayed hypersensitivity. Moreover, U-27 could reduce the production of pro-inflammatory cytokines including IFN-γ [25]. IFN-γ is mainly produced by T lymphocytes, B lymphocytes, NK cells and monocytes/macrophages, and also known as an immune interferon and plays an important role in the regulation of immune response. In addition, IFN-γ can also promote the proliferation of Th1 cells and strengthen the immune response via Th1 cells. IFN-γ can also inhibit the activity of Th2 cells and further suppress the humoral immunity. Thus, IFN-γ is a key factor in the immune response of humans and possesses multiple bioeffects. IFN-γ can regulate the expression of CD80/86, two costimulatory molecules of MHC-I and MHC-II, promote the production of inducible nitric oxide synthesase (iNOS) and activate the oxygen dependent and independent bactericidal system of macrophages leading to the macrophage activation. IFN-γ is a potent activator of macrophages, has the ability to activate macrophages and NK cells and regulate the functions of dendritic cells, T cells and B cells and involves in innate immunity and acquired immunity. IFN-γ can exert synergistic effect with LPS to induce the production of pro-inflammatory cytokines including IL-6, TNF-α and IL-12 [26]. In normal intestinal epithelial cells, the TLR4 expression is at a low level, and LPS fails to induce the NF-κB activation. After incubation with IFN-γ, the TLR4 expression in the intestinal epithelial cells increases markedly, and subsequent stimulation with LPS may induce the potent activation of NF-κB. This suggests that IFN-γ can up-regulate the TLR4 expression in intestinal epithelial cells, facilitate the response of these cells to LPS and indirectly affects NF-κB [27].
CONCLUSIONS
Taken together, our findings indicate that IFN-γ treatment may induce the changes in some cytokines in AP which are characterized by reduction in IL27 and increase in IL-18. This suggests that IFN-γ may inhibit the IL-27 production to a certain extent. Currently, the pathway involving in the IFN-γ induced suppression of IL-27 is still unclear, and might be associated with JAK-STAT mediated transcription activation [28, 29]. Thus, we speculate that to control the IFN-γ may attenuate AP to a certain extent, which provides evidence for the clinical treatment of AP.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Fund (30940034)
DECLARATION OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Leach SD, Gorelick FS, Modlin IM. New perspectives on acute pancreatitis. Scand J Gastroenterol Suppl. 1992:19229–38. doi: 10.3109/00365529209095976. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330(17):1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- 3.Carey LC. Extra-abdominal manifestations of acute pancreatitis. Surgery. 1979;86(2):337–342. [PubMed] [Google Scholar]
- 4.Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47(4):546–552. doi: 10.1136/gut.47.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175(1):76–83. doi: 10.1016/s0002-9610(97)00240-7. [DOI] [PubMed] [Google Scholar]
- 6.Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9(4):401–410. doi: 10.1007/s005340200049. [DOI] [PubMed] [Google Scholar]
- 7.Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford) 2006;(4):264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowry SF. Cytokine mediators of immunity and inflammation. Arch Surg. 1993;128(11):1235–1241. doi: 10.1001/archsurg.1993.01420230063010. [DOI] [PubMed] [Google Scholar]
- 9.Fink G, Norman J. Pancreatitis induces specific changes in the intrapancreatic expression of the TNF family of genes. Pancreas. 1995:11428–430. [Google Scholar]
- 10.de Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg. 1996;83(3):349–353. doi: 10.1002/bjs.1800830317. [DOI] [PubMed] [Google Scholar]
- 11.Lebel-Binay S, Berger A, Zinzindohoue F, Cugnenc P, Thiounn N, Fridman WH, et al. Interleukin-18:biological properties and clinical implications. Eur Cytokine Netw. 2000;11(1):15–26. [PubMed] [Google Scholar]
- 12.Perejaslov A, Chooklin S, Bihalskyy I. Implication of interleukin 18 and intercellular adhesion molecule (ICAM)-1 in acute pancreatitis. Hepatogastroenterology. 2008;55(86-87):1806–1813. [PubMed] [Google Scholar]
- 13.Yuan BS, Zhu RM, Braddock M, Zhang XH, Shi W, Zheng MH. Interleukin-18:a pro-inflammatory cytokine that plays an important role in acute pancreatitis. Expert Opin Ther Targets. 2007;11(10):1261–1271. doi: 10.1517/14728222.11.10.1261. [DOI] [PubMed] [Google Scholar]
- 14.Ueda T, Takeyama Y, Yasuda T, Matsumura N, Sawa H, Nakajima T, et al. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol. 2006;41(2):158–165. doi: 10.1007/s00535-005-1735-4. [DOI] [PubMed] [Google Scholar]
- 15.Pastor CM, Morel DR, Vonlaufen A, Schiffer E, Lescuyer P, Frossard JL. Delayed production of IL-18 in lungs and pancreas of rats with acute pancreatitis. Pancreatology. 2010;10(6):752–757. doi: 10.1159/000317283. [DOI] [PubMed] [Google Scholar]
- 16.Ueno N, Kashiwamura S, Ueda H, Okamura H, Tsuji NM, Hosohara K, et al. Role of interleukin 18 in nitric oxide production and pancreatic damage during acute pancreatitis. Shock. 2005;24(6):564–570. doi: 10.1097/01.shk.0000184285.57375.bc. [DOI] [PubMed] [Google Scholar]
- 17.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15(4):569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407(6806):916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, et al. Two-sided roles of IL-27:induction of Th1 differentiation on naive CD4+T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177(8):5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida H, Hamano S, Miyazaki Y. The double identity of WSX-1 (IL-27R) as an initiator and attenuator of immune responses. Curr Immunol Rev. 2005:55–60. [Google Scholar]
- 22.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100(25):15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, et al. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175(4):2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida H, Miyazaki Y. Interleukin 27 signaling pathways in regulation of immune and autoimmune responses. Int J Biochem Cell Biol. 2008;40(11):2379–2383. doi: 10.1016/j.biocel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki Y, Shimanoe Y, Wang S, Yoshida H. Amelioration of delayed-type hypersensitivity responses by IL-27 administration. Biochem Biophys Res Commun. 2008;373(3):397–402. doi: 10.1016/j.bbrc.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Kong HJ, Li H, Huang B, Yang M, Zhu C, et al. IRF-8/in- terferon (IFN) consensus sequence-binding protein is involved in Toll-like receptor (TLR) signaling and contributes to the cross-talk between TLR and IFN-gamma signaling pathways. J Biol Chem. 2006;281(15):10073–10080. doi: 10.1074/jbc.M507788200. [DOI] [PubMed] [Google Scholar]
- 27.Tamai R, Sugawara S, Takeuchi O, Akira S, Takada H. Synergistic effects of lipopolysaccharide and interferon-gamma in inducing interleukin-8 production in human monocytic THP-i cells is accompanied by up-regulation of CD14, Toll-like receptor 4, MD-2 and MyD88 expression. J Endotoxin Res. 2003;9(3):145–153. doi: 10.1179/096805103125001540. [DOI] [PubMed] [Google Scholar]
- 28.Lighthouse J, Naito Y, Helmy A, Hotten P, Fuji H, Min CH, et al. Endotoxinemia and benzodiazepine-like substances in compensated cirrhotic patients: a randomized study comparing the effect of rifaximine alone and in association with a symbiotic preparation. Hepatol Res. 2004;28(3):155–160. doi: 10.1016/j.hepres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Rayes N, Seehofer D, Hansen S, Boucsein K, Muller AR, Serke S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74(1):123–127. doi: 10.1097/00007890-200207150-00021. [DOI] [PubMed] [Google Scholar]


