Abstract
We investigated the effects of urantide, a receptor antagonist of urotensin II (U-II), on the expression of U-II and its receptor GPR14 in rat vascular smooth muscle cells. Vascular smooth muscle cells from rat thoracic aorta were cultured by explant method. Subjects in this experiment were divided into eight groups: normal control group (group C), U-II group (group M), positive control group (Flu group) and urantide-treated groups (10-10, 10-9, 10-8, 10-7 and 10-6 mol/L). Cultured vascular smooth muscle cells in vitro were studied by immunocytochemistry, biochemistry, and flow cytometry. U-II (10-8 mol/L) promoted the proliferation of vascular smooth muscle cells at each time point, influenced cell cycle, increased proliferation index and S-phase cell fraction, and dramatically promoted the expression of U-II and GPR14. In the concentration range from 10-10 to 10-6 mol/L, urantide dramatically inhibited the proliferation of vascular smooth muscle cells and the protein expression of U-II and GPR14, especially at a concentration of 10-6 mol/L. U-II, binding with its receptor GPR14, promotes vascular smooth muscle cells proliferation and migration, which can be inhibited by urantide. This study provides an evidence for understanding the effects of U-II and its receptor GPR14 on vascular smooth muscle cells.
KEY WORDS: atherosclerosis, urantide, urotensin II, vascular smooth muscle cell (VSMC)
INTRODUCTION
The development of atherosclerotic plaques is a slow and gradual process, pathologically characterized by the proliferation and migration of vascular smooth muscle cells [1-10]. Urotensin II (U-II) is an autocrine/paracrine growth factor with mitogenic activity, which has various non-hemodynamic effects such as stimulating cellular proliferation and promoting extracellular matrix expression [11, 12]. The mitogenic effects of U-II were firstly demonstrated in vascular smooth muscle cells of rat thoracic aorta. Tsai et al. demonstrated that both reactive oxygen species generation and epidermal growth factor receptor transactivation play critical roles in U-II signal transduction [13]. It was found that U-II increases ERK phosphorylation level in the MAPK pathway, promotes vascular smooth muscle cells proliferation via activation of the Rho A/Rho kinase pathway, thus contributes to the pathogenesis and development of atherosclerosis [14, 15]. Therefore, how to effectively suppress the mitogenic effects of U-II and inhibit the proliferation and migration of vascular smooth muscle cells has attracted great attention in the field of anti-arteriosclerosis. The mitogenic effect of U-II is mediated by its receptor GPR14 (G-protein coupled receptor 14) and hence it can be inhibited by GPR14 antagonist [16]. Urantide is a receptor antagonist peptide derived from human U-II, which is the most powerful known antagonist of GPR14 receptor with an antagonistic effect 50 to 100 times higher than other antagonists [17], although it may act as partial agonist in some animals and human cell line [18]. Up to now, research on urantide mainly focused on myocardial ischemic injury and vascular remodeling. Effects of urantide on atherosclerosis have been scarcely reported. Herein, under the intervention of the receptor antagonist urantide of U-II, we studied the expression of U-II and its receptor GPR14 in vascular smooth muscle cells cultured in vitro. The possible mechanism of effects of urantide was also explored. This study provides an evidence for understanding the effects of U-II and its receptor GPR14 on vascular smooth muscle cells.
MATERIALS AND METHODS
The study protocol was approved by the Local Ethics Committee of Medical Research Center.
Materials
Male Wistar rats weighing 180 to 200 g were obtained from the Animal Department of Norman Bethune Division of Medical Sciences, Jilin University. Urantide was synthesized by HD Biosciences Ltd (Shanghai, China). DMEM powder culture medium and U-II were obtained from Gibco (Carlsbad, USA), fetal bovine serums (FBS) from Hao Yang Biological Manufacture Corporation (Tianjin, China), a-smooth muscle actin (a-SMA) antibody from Beijing Biosynthesis Biotechnology Co. Ltd, biotin-labeled secondary antibody IgG from Boster Biological Technology Co. Ltd (Wuhan, China), and strept avidin-biotin complex (SABC) Immunohistochemistry Kit and diaminobenzidine (DAB) Staining Kit from Fuzhou Maxim Biotech Inc (Fujian, China). The reverse transcription systems were purchased from Promega (Madison, WI, USA). Sequences of oligonucleotide primers for RT-PCR analysis were synthesized by Sai Bai Sheng (Beijing). The western blot kits were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Isolation and primary culture of vascular smooth muscle cells
Under etherization, Wistar rats were killed by dislocation of the neck, which was approved by the Local Ethics Committee of Medical Research Center. The skin over the thorax and abdomen was sterilized, and thoracic aorta was quickly taken out and immersed in 75% alcohol for about 1 min. Under aseptic conditions, the thoracic aorta was cut into 2–3 cm strips and rinsed in phosphate buffer saline (PBS, pH 7.4) repeatedly. The adventitia containing fibroblasts was carefully removed by microforceps. The adventitia-removed aorta was placed in culture dishes, cut longitudinally and washed in culture solution after the endomembrane was scraped with a blade. Then the tunica media was cut into 1 mm3 pieces, inoculated evenly in culture flasks by bent sippers, and incubated at 37 °C for 2 to 4 h. When tightly adherent to the wall, the tissue pieces were incubated with DMEM containing 10% FBS in a CO2-incubator for 3 days. When confluence was reached, vascular smooth muscle cells were subcultured.
Morphological observation and identification of vascular smooth muscle cells
Morphology of vascular smooth muscle cells was observed under an optical microscope with hematoxylin and eosin staining. Vascular smooth muscle cells identification was performed by SABC immunohistochemical staining according to kit instruction. The primary antibody a-SMA was diluted 1:100. DAB developer was freshly prepared as needed. Positive cells were observed under an optical microscope at 100× magnification and the percentage of positive cells was calculated.
Effects of urantide on vascular smooth muscle cells proliferation and cell cycle Grouping
Vascular smooth muscle cells in logarithmic growth phase were used and divided into eight groups: (1) control group (group C) where cells were continuously cultured in 10% FBS DMEM; (2) U-II group (group M) where 10-8 mol/L U-II was added; (3) positive control group (Flu group) where in the presence of 10-8 mol/L U-II and 10-7 mmol/L fluoxetine (Flu); (4) urantide-treated groups where in the presence of 10-8 mol/L U-II, 10-10, 10-9, 10-8, 10-7 and 10-6 mol/L of urantide were added, respectively. Four parallel experiments were performed for each group.
Detection of vascular smooth muscle cell viability
Vascular smooth muscle cells viability was detected at 8, 12, 24, 48 and 72 h by methyl thiazolyl tetrazolium (MTT) assay. Viability curves were made in each group.
Cell cycle analysis by flow cytometry
Cell cycle analysis was performed using a flow cytometer (FACS Aria, BD Corporation, Franklin Lakes, USA) according to manufacturer’s instructions [19, 20]. After 48-hour culture, vascular smooth muscle cells were digested with 0.25% trypsin. Cells were collected, rinsed with PBS and fixed in 70% cold ethanol overnight. After centrifugation, cells were resuspended with propidium iodide working solution and underwent a chemical reaction in dark at 5 °C for 30 min. Cell cycle distribution was detected and flow cytometric data were analyzed with Modfit LT 3.0 software. Proliferation index (PI) and S-phase cell fraction (SPF) of vascular smooth muscle cells were calculated. PI = (G2M+S)/(G0G1+S+G2M); SPF = S/(G0G1+S+G2M) [21].
Effects of urantide on the gene and protein expression of U-II and GPR14 in vascular smooth muscle cells
Vascular smooth muscle cells in logarithmic growth phase were cultured in conditioned medium for 48 h and then collected for detection. qRT-PCR and Western blot analysis were used to detect the gene and protein expression of U-II and GPR14 in vascular smooth muscle cells. For the RT-PCR analysis, two micrograms of total RNA was reversetranscribed into single-strand cDNA using of M-MLV reverse transcriptase and oligo (dT)18 primers. RT-PCR was carried out according to a modified procedure [22]. The primers 5’-GCA TCT TCA CCC TGA CCA TAA-3’ (sense) and 5’-CCC AGAAGA GAA GGA CGA TAC C-3’ (antisense) were used to amplify a 270-bp fragment of the rat UT cDNA. The primer pair for GAPDH used as an internal control was 5’-ATCTGGCACCACACCTTC-3’ (sense) and 5’-AGC-CAGGTCCAGACGCA-3’ (antisense) for a 260-bp fragment. Reverse transcription was carried out at 42 °C for 15 min and then terminated at 95 °C for 5 min. Target cDNA was amplified by quantitative PCR with 0.2 mM dNTPs, 2 mM MgCl2, 2.5 U Taq DNA polymerase, 1 mM each primer, 1× PCR buffer and ddH2O to a final volume of 25 μL. Thermal cycling involved an initial denaturation of 5min at 94 °C, then 40 cycles of 30 s at 94 °C, 30 s at 57 °C and 30 s at 72°C, followed by an extension step of 5 min at 72 °C. The PCR products were resolved on 2% agarose gel; the gel was stained with ethidium bromide. Peak fluorescent area of the target gene and control transcripts was measured in GeneScan under UV light by use of ScionImage software (GEL/Chemi Doc, Bio-Rad), and a standard curve of the ratio was drawn. For the Western blot analysis, total protein (100 μg) was separated by SDS-PAGE and blotted onto nitrocellulose membrane (Hybond ECL, Amersham). The membranes were then blocked overnight at 4–8 °C with 1% BSA in TBST solution, and then incubated with primary antibodies rabbit polyclonal against rat UT (1:500), mouse polyclonal against rat ERK1/2 or p-ERK1/2 (1:1000), rabbit polyclonal antibody against rat PKC or p-PKC (1:1000), and rabbit polyclonal against rat p-P38 MAPK and GADPH (1:5000). After 3 washes with TBST, the membranes were incubated with HRP-labeled secondary antibody in TBST. After extensive washing in TBST, membranes were developed using of an enhanced chemiluminescence kit (NEN Life Science Products, Boston, MA, USA).
Statistical analysis
Statistical analysis was executed by SPSS 13.0 software. The results are presented as mean ± SD. The differences between each group were analyzed with analysis of variance or rank sum test. Probability values less than 0.05 were considered to indicate statistically significant differences.
RESULTS
Morphological observation and identification of vascular smooth muscle cells
Second-passage cultured vascular smooth muscle cells were stained with hematoxylin and eosin and observed under an optical microscope. Vascular smooth muscle cells are rhombus, cestiform, triangle or stellate in shape and cell nuclei are mostly oval. The cytoplasm appears pinky, and nucleus is blue-purple (Figure 1). Over 98% cultured cells were positive with SABC immunohistochemical staining for a-SMA. The cytoplasm appears claybank and granular, and the nucleus is unstained (Figure 2).
FIGURE 1.
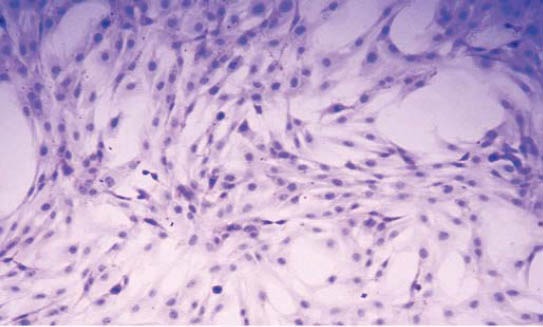
Morphologies of vascular smooth muscle cells stained with hematoxylin and eosin (×100).
FIGURE 2.
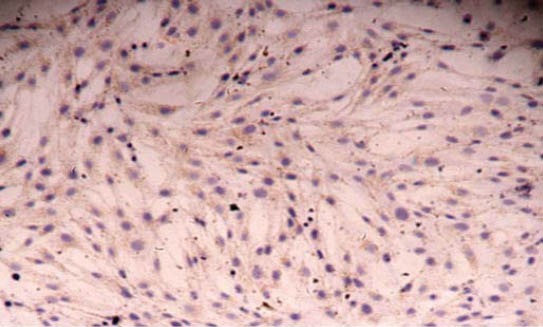
Immunohistochemical staining of vascular smooth muscle cells with strept avidin-biotin complex (×100).
Effects of urantide on vascular smooth muscle cell proliferation
In group M, U-II dramatically promoted vascular smooth muscle cells proliferation at each time point when compared with group C (p<0.01). In urantide-treated group, urantide inhibited vascular smooth muscle cells proliferation with time and concentration dependence in the concentration range from 10-10 to 10-6, mol/L, and the differences were found to be significant in comparison with group M. The inhibitory effect of 10-6 mol/L urantide was most evident, very close to that of the positive control group (Figure 3). It should be mentioned that before treatment all the groups have comparable cell numbers as indicated by MTT assay.
FIGURE 3.
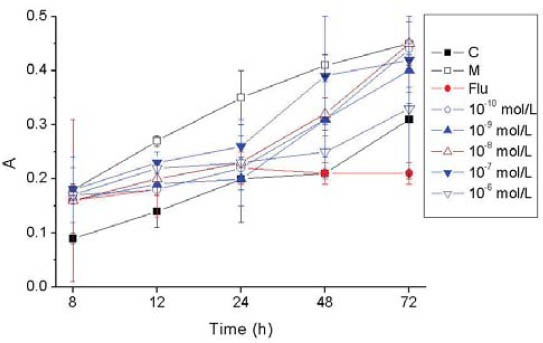
Effects of concentrations of Urantide on the proliferation of vascular smooth muscle cells. p<0.01: group M vs. group C; p<0.05 urantide groups vs group M. Label A of y-axis is an arbitrary unit of absorbance.
Effects of urantide on cell cycle of vascular smooth muscle cells
Cell cycle is evaluated with PI and SPF. PI indicates the percentage of the total number of cells in proliferative phases of the cell cycle, and SPF indicates the percentage of cells in the S-phase (DNA Synthesis Phase). In group M, both PI and SPF are dramatically higher than those in group C (p<0.01). After 48-hour culture with urantide of different concentrations, PI and SPF are both dramatically lower than those in group M, especially in 10-6 mol/L urantide group (Figures 4 and 5).
FIGURE 4.
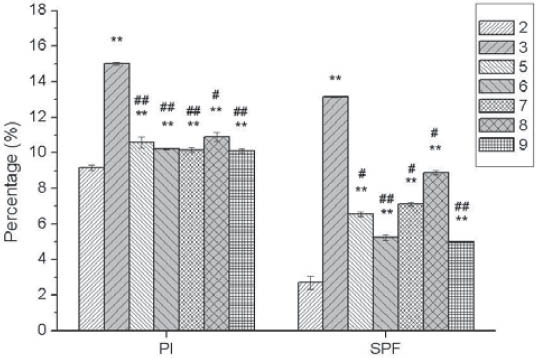
Effects of concentrations of Urantide on cell cycle of vascular smooth muscle cells. 2: Control group (group C); 3: U-II group (group M); 5-9: 10-10, 10-9, 10-8, 10-7, and 10-6 mol/L Urantide group. *p<0.05, **p<0.01 vs group C; #p<0.05, ##p<0.01 vs group M.
FIGURE 5.
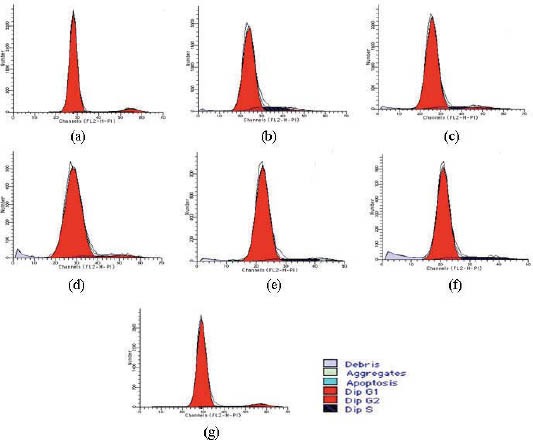
Effects of Urantide concentrations on cell cylcle of vascular smooth muscle cells. (a): Control group (group C); (b): U-II group (group M); (c-g): 10-10, 10-9, 10-8, 10-7 and 10-6 mol/L Urantide groups.
Effects of urantide on U-II and GPR14 mRNA expression in vascular smooth muscle cells
In group M, the expression of U-II and GPR14 mRNA dramatically increased compared with group C (p<0.01). In urantide-treated group, the expression of U-II and GPR14 mRNA were dramatically down-regulated by urantide of different concentrations ranging from 10-10 to 10-6 mol/L, especially by 10-6 mol/L urantide (Figure 6).
FIGURE 6.
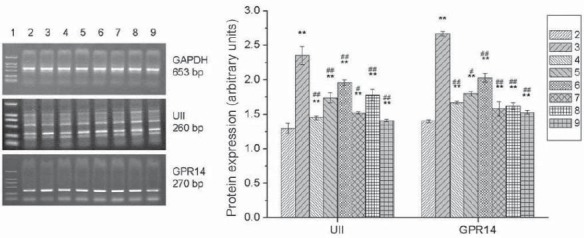
Effects of Urantide on U-II and GPR14 mRNA expression in vascular smooth muscle cells. 1: Marker; 2: Control group (group C); 3: U-II group (group M); 4: Positive control group (Flu group); 5-9: 10-10, 10-9, 10-8, 10-7, and 10-6 mol/L Urantide group. *p<0.05, **p<0.01 vs group C; #p<0.05, ##p<0.01 vs group M.
Effects of urantide on protein expression of U-II and GPR14 in vascular smooth muscle cells
In group M, the protein expression of U-II and GPR14 in vascular smooth muscle cells were dramatically up-regulated by U-II, compared with group C (p<0.01). In urantide-treated group, the protein expression of U-II and GPR14 were dramatically inhibited by urantide at different concentrations (p<0.01) (Figure 7). It should be noted that the inhibition of U-II protein expression by urantide seems to follow a dose-response curve, the reason of which needs further investigation.
FIGURE 7.
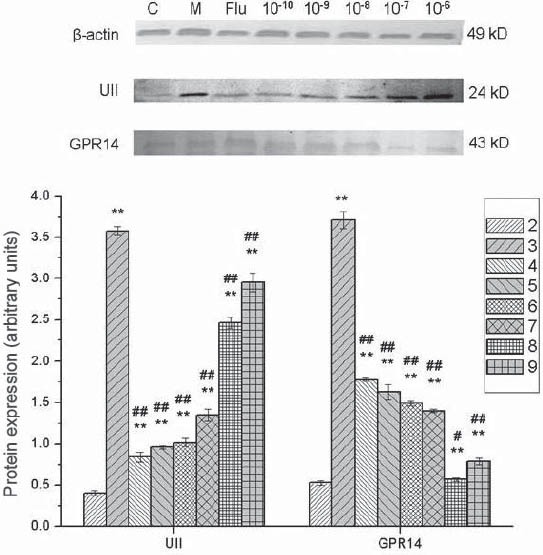
Effects of urantide on protein expression of U-II and GPR14 in vascular smooth muscle cells. 1: Marker; 2: Control group (group C); 3: U-II group (group M); 4: Positive control group (Flu group); 5-9: 10-10, 10-9, 10-8, 10-7, and 10-6 mol/L Urantide group. **p<0.01 vs group C; #p<0.05, ##p<0.01 vs group M.
DISCUSSION
U-II is a somatostatin-like cyclic peptide initially isolated from the caudal neurosecretory system of teleost fish, existing widely in different species from mollusks to mammals [23]. The receptor GPR14 is a specific U-II receptor in human body, mainly expressed in vascular and cardiac tissue [24]. U-II and its receptor GPR14 form U-II/GPR14 system and induce series of biological effects, such as vascular constriction and mitogenic effects. Numerous studies have shown that U-II plays an important role in the regulation of cardiovascular homeostasis and in the development of cardiovascular diseases. The systemic hemodynamics of U-II and the effects on vessel walls and cells could be among main mechanisms in the occurrence and development of atherosclerosis [25]. Hence, the application of U-II receptor antagonist may have potential and great clinical value for the prevention and treatment of atherosclerosis. Urantide is a GPR14 receptor antagonist peptide derived from human U-II, as the most powerful known antagonist of GPR14 receptor with an antagonistic effect 50 to 100 times higher than other antagonists. The antagonistic effect of urantide is highly selective, only acting against the vasoconstrictive effect of U-II by ignoring the responses produced by different spasmogenic compounds, such as noradrenaline and ET-1 [26, 27]. Zhang et al. [28] found that exogenous U-II may upregulate mRNA levels of the GPR14 receptor and increase collagen and cell proliferation, all of which would contribute to intimal hyperplasia after angioplasty. In studies on isolated rat aorta, urantide can competitively antagonized the vasoconstriction induced by U-II, obviously inhibited the up-regulation of cholesterol acyltransferase-1 in human monocyte-derived macrophages and prevented the development of foam cells [29]. In this work, we investigated the expression of U-II and its receptor GPR14 in vascular smooth muscle cells in vitro under the intervention of U-II and its receptor antagonist urantide. We found that U-II (10-8 mol/L) promoted vascular smooth muscle cells proliferation at each time point, influenced cell cycle and increased PI and SPF, and dramatically promoted protein expression of U-II and GPR14. In the concentration range of 10-10 to 10-6 mol/L, urantide dramatically inhibited vascular smooth muscle cells proliferation and protein expression of U-II and GPR14, especially at a concentration of 10-6 mol/L. The present data suggest that U-II promoted the mitogenic effect of U-II and increased the expression of U-II and its receptor GPR14 in rat vascular smooth muscle cells, which can be antagonized by urantide. It has been reported that U-II protein and mRNA levels were significantly elevated in the arteries of patients with coronary atherosclerosis in comparison to healthy arteries and U-II acts in synergy with mildly oxidized LDL inducing vascular smooth muscle cells proliferation [30, 31]. It can be concluded that U-II binding with its receptor GPR14 promotes the proliferation and migration of vascular smooth muscle cells, being able to be inhibited by urantide, providing a theoretical basis for the clinical application of urantide. It should be noted that the UII effect is not only direct to vascular smooth muscle cells but also to the endothelium. In fact, it has been demonstrate that UII pathway is present in endothelial cells too and that its effect on vascular tissues is trough NO release where a mechanism of action it has been proposed [32]. This evidence should be taken in account since the effect of urantide in vivo could be different depending on physiological or pathological conditions also due to the fact that urantide may act as partial agonist in some animals and human cell line; and it is unknown what it does in human. To date, urantide is still in the stage of fundamental research and show great potential as an anti-atherosclerosis drug. Moreover, investigations on urantide will benefit the elucidation of pathophysiological role of U-II and its receptor GPR14 in cardiovascular diseases, especially in atherosclerosis.
CONCLUSIONS
We have investigated the effects and mechanisms and the receptor antagonist urantide of urotensin II (U-II) on the expression of U-II and its receptor GPR14 in rat vascular smooth muscle cells. U-II promoted the proliferation of vascular smooth muscle cells, influenced cell cycle, increased proliferation index and S-phase cell fraction, and dramatically promoted the expression of U-II and GPR14. Urantide inhibited the proliferation of vascular smooth muscle cells and the expression of U-II and GPR14. Furthermore, this inhibition shows dependence on the concentration.
DECLARATION OF INTEREST
All authors have read and approved this manuscript. Neither the submitted paper nor any similar paper, in whole or in part has been or will be published in any other primary scientific journal. No conflict of interest exists in the submission of this manuscript.
REFERENCES
- 1.Stoneman VE, Bennett MR. Role of Fas/Fas-L in vascular cell apoptosis. J Cardiovasc Pharmacol. 2009;53(2):100–108. doi: 10.1097/FJC.0b013e318198fe60. [DOI] [PubMed] [Google Scholar]
- 2.Ljuca F, Drevenšek G, Zerem E. Contribution of Ras farnesyl transferase, MAP kinase and cytochrome P-450 metabolites to endothelin-i induced hypertension. Bosn J Basic Med Sci. 2011;II(2):84–86. doi: 10.17305/bjbms.2011.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anacak GY, Ustünes L, Dilsiz OY, Ergul A. Lacidipine has antiatherosclerotic effects independent of its actions on lipid metabolism and blood pressure. Vascul Pharmacol. 2010;53(5-6):193–199. doi: 10.1016/j.vph.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ragino YI, Chernjavski AM, Polonskaja YV, Tchimbal SY, Redjkin DA, Semaeva EV, et al. Blood levels of inflammatory and destructive biomarkers in coronary atherosclerosis of different severity. Bull Exp Biol Med. 2010;149(5):587–590. doi: 10.1007/s10517-010-0999-8. [DOI] [PubMed] [Google Scholar]
- 5.Sobolev VV, Starodubtseva NL, Piruzyan AL, Minnibaev MT, Sautin ME, Tumanov VP, et al. Comparative study of the expression of ATF-3 and ATF-4 genes in vessels involved into atherosclerosis process and in psoriatic skin. Bull Exp Biol Med. 2011;151(6):713–716. doi: 10.1007/s10517-011-1423-8. [DOI] [PubMed] [Google Scholar]
- 6.Mesihovic-Dinarevic S, Kulic M, Kreso A. Cardiovascular screening in young athletes in sarajevo canton. Bosn J Basic Med Sci. 2010;10(3):227–233. doi: 10.17305/bjbms.2010.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tikhaze AK, Viigimaa M, Konovalova GG, Kumskova EM, Abina EA, Zemtsovskaya G, et al. Interrelation between malonyl dialdehyde-dependent modification and cholesterol content in low-density lipoproteins. Bull Exp Biol Med. 2010;149(2):184–186. doi: 10.1007/s10517-010-0903-6. [DOI] [PubMed] [Google Scholar]
- 8.Girotra S, Keelan M, Weinstein AR, Mittleman MA, Mukamal KJ. Relation of heart rate response to exercise with prognosis and atherosclerotic progression after coronary artery bypass grafting. Am J Cardiol. 2009;103(10):1386–1390. doi: 10.1016/j.amjcard.2009.01.346. [DOI] [PubMed] [Google Scholar]
- 9.Moheimani F, Moore L, Ferrante JV, Trout N, Hii CS, Ferrante A. Inhibition of atherosclerotic lesion development in the ApoE- /- mouse by a novel β-oxa polyunsaturated fatty acid. J Cardiovasc Pharmacol. 2010;56(4):431–439. doi: 10.1097/FJC.0b013e3181f1d420. [DOI] [PubMed] [Google Scholar]
- 10.Wan LS, Li JW, Ke BB, Xu ZK. Ordered microporous membranes templated by breath figures for. size-selective separation. J Am Chem Soc. 2012;134(1):95–98. doi: 10.1021/ja2092745. [DOI] [PubMed] [Google Scholar]
- 11.Li K, Ding W-H, Shi L-B, Zhang L-F, Han X-N, Tang C-S. Role of vascular remodeling for urotensin ii in rat thoracic aorta after balloon injury. Chin J Pathophys. 2008;24(4):674–679. [Google Scholar]
- 12.Esposito G, Sanzari E, Serafino LD, Cipolletta E, Concilio AD, Iaccarino G, Rapacciuolo A, Chiariello M. Word Cong Card 2006. Spain: 2006. Urotensin II inhibition prevents smooth muscle cell proliferation and neointima formation following balloon angioplasty through apoptosis modulation and g-betagamma signaling; p. 5486. [Google Scholar]
- 13.Tsai CS, Loh SH, Liu JC, Lin JW, Chen YL, Chen CH, Cheng TH. Urotensin ii-induced endothelin-i expression and cell proliferation via epidermal growth factor receptor transactivation in rat aortic smooth muscle cells. Atherosclerosis. 2009;206(1):86–94. doi: 10.1016/j.atherosclerosis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Matsusaka S, Wakabayashi I. Enhancement of vascular smooth muscle cell migration by urotensin II. Naunyn Schmiedebergs Arch Pharmacol. 2006;373(5):381–386. doi: 10.1007/s00210-006-0086-x. [DOI] [PubMed] [Google Scholar]
- 15.Jordao AA, Junior, Domenici FA, Lataro RC, Portari GV, Vannuc-chi H. Effect of methionine load on homocysteine levels, lipid peroxidation and DNA damage in rats receiving ethanol. Braz J Pharm Sci. 2009;45:709–714. [Google Scholar]
- 16.Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans. 2003;31(Pt 1):20–24. doi: 10.1042/bst0310020. [DOI] [PubMed] [Google Scholar]
- 17.Conlon JM, Yano K, Waugh D, Hazon N. Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J Exp Zool. 1996;275(2-3):226–238. [PubMed] [Google Scholar]
- 18.Behm DJ, Stankus G, Doe CP, Willette RN, Sarau HM, Foley JJ, et al. The peptidic urotensin-II receptor ligand GSK248451 possesses less intrinsic activity than the low-efficacy partial agonists SB- 710411 and urantide in native mammalian tissues and recombinant cell systems. Br J Pharmacol. 2006;148(2):173–190. doi: 10.1038/sj.bjp.0706716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge Y, Zhang J, Cao J, Wu Q, Sun L, Guo L, et al. TFF1 inhibits proliferation and induces apoptosis of gastric cancer cells in vitro. Bosn J Basic Med Sci. 2012;12(2):74–81. doi: 10.17305/bjbms.2012.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang D, Guo Y, Zhu Z, Chen W. Silence of p15 expression by RNAi enhances cisplatin resistance in hepatocellular carcinoma cells. Bosn J Basic Med Sci. 2012;12(1):4–9. doi: 10.17305/bjbms.2012.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song D, Wang H-l, Wang S, Zhang X-h. 5-hydroxytryptamine- induced proliferation of pulmonary artery smooth muscle cells are extracellular signal-regulated kinase pathway dependenti. Acta Pharmacologica Sinica. 2005;26(5):563–567. doi: 10.1111/j.1745-7254.2005.00071.x. [DOI] [PubMed] [Google Scholar]
- 22.Zachar V, Thomas R, Goustin A. Absolute quantification of target DNA: A simple competitive pcr for efficient analysis of multiple samples. Nucleic Acids Res. 1993;21:2017–2018. doi: 10.1093/nar/21.8.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulouarn Y, Lihrmann I, Jegou S, Anouar Y, Tostivint H, Beauvil-lain JC, Conlon JM, Bern HA, Vaudry H. Cloning of the cdna encoding the urotensin ii precursor in frog and human reveals intense expression of the urotensin 11 gene in motoneurons of the spinal cord. Proc Nat Acad Sci. 1998;95(26):15803–15808. doi: 10.1073/pnas.95.26.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401(6750):282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 25.Camarda V, Song W, Marzola E, Spagnol M, Guerrini R, Salvadori S, et al. Urantide mimics urotensin-ii induced calcium release in cells expressing recombinant ut receptors. Eur J Pharmacology. 2004;498(1-3):83–86. doi: 10.1016/j.ejphar.2004.07.089. [DOI] [PubMed] [Google Scholar]
- 26.Camarda V, Spagnol M, Song W, Vergura R, Roth AL, Thompson JP, et al. In vitro and in vivo pharmacological characterization of the novel ut receptor ligand [pen(5), dtrp(7), dab(8)] urotensin ii(4- 11) (ufp-803) Brit J Pharm. 2006;147(1):92–100. doi: 10.1038/sj.bjp.0706438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns DG, Ao Z, Naselsky D, Herold CL, Maniscalco K, Sarov-Blat L, et al. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol. 2004;370(4):238–250. doi: 10.1007/s00210-004-0980-z. [DOI] [PubMed] [Google Scholar]
- 28.Zhang LF, Ding WH, Shi LB, Li K, Haom YJ, Ke YN, et al. Effects of exogenous urotensin II on vascular remodelling after balloon injury. Clin Exp Pharmacol Physiol. 2010;37(4):477–481. doi: 10.1111/j.1440-1681.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- 29.Sauzeau V, Le Mellionnec E, Bertoglio J, Scalbert E, Pacaud P, Loirand G. Human urotensin ii-induced contraction and arterial smooth muscle cell proliferation are mediated by rhoa and rhokinase. Circulation Res. 2001;88(11):1102–1104. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulos P, Bousette N, Giaid A. Urotensin-ii and cardiovascular remodeling. Peptides. 2008;29(5):764–769. doi: 10.1016/j.peptides.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Guidolin D, Albertin G, Ribatti D. Urotensin-ii as an angiogenic factor. Peptides. 2010;31(6):1219–1224. doi: 10.1016/j.peptides.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 32.d’Emmanuele di Villa Bianca R, Cirino G, Mitidieri E, Coletta C, Grassia G, Roviezzo F, et al. Urotensin II: a novel target in human corpus cavernosum. J Sex Med. 2010;7(5):1778–1. doi: 10.1111/j.1743-6109.2009.01450.x. [DOI] [PubMed] [Google Scholar]


