Abstract
We investigated the etiopathogenetic role of manganese superoxide dismutase (MnSOD) (Ala-çVal) and glutathione peroxidase (GSH-Px) (Pro 197 Leu) gene polymorphisms in patients diagnosed with major depressive disorder (MDD) and bipolar I disorder (BD).
Eighty patients with MDD, 82 patients with BD (total 162 patients) and 96 healthy controls were enrolled in this study and genotyped using a Real Time-Quantitative Polymer Chain Reaction (RT-qPCR)-based method.
The patients with BD and MDD and the controls had a similar distribution of the genotypes and alleles in the Ala-9Val MnSOD gene polymorphism. Comparison of the MDD group and control group regarding the Pro 197 Leu GSH-Px gene polymorphism revealed similar genotype distribution but different allele distribution. The BD group and control group were similar both for genotypes and for alleles when compared regarding the Pro 197 Leu GSH-Px gene polymorphism. The combined analysis (MDD plus BD) also failed to find any association between the Ala-9Val MnSOD and Pro 197 Leu GSH-Px gene polymorphism.
Although small statistical power of the current study the significant difference between patients with depression and the control group for the Pro 197 Leu GSH-Px polymorphism indicates that the distribution of these alleles may have a contribution in the physiopathogenesis of depression. One of the limitation of the current study is that the sample size is too small. Understanding of the exact role of Pro 197 Leu GSH-Px polymorphism in the development of depression needs to further studies with more sample size and high statistical power.
KEY WORDS: bipolar I disorder, major depressive disorder, manganese superoxide dismutase, glutathione peroxidase, polymorphism
INTRODUCTION
Free radicals and/or disorders of the antioxidant defence system have frequently been blamed for the pathology of many disorders through the tissue destruction they cause in recent years in the medical literature. The creation and destruction of free radicals are normally balanced in the organism and this is called oxidative balance. The organism is not affected by free radicals as long as the oxidative balance is preserved. This balance is disturbed by an increase in the rate these radicals are created or a decrease in the rate they are eliminated, this is called oxidative stress and causes tissue damage as a result of the severe imbalance between free radical creation and the antioxidant defence mechanism [1]. Superoxide dismutases (SOD) and glutathione peroxidase (GSH-Px) are antioxidant defence enzymes and play an important preventive role against the damage caused by free radicals in cells [2]. GSH-Px is a selenium-dependent enzyme that is expressed in the human brain, and plays an important role in the detoxification of reactive oxygen and reactive nitrogen species-related oxidative stress [3]. SOD is a manganese-dependent enzyme found in the mitochondria and is encoded by the nuclear DNA. Manganese superoxide dismutase (MnSOD) scavenges the greatest number of superoxide anions produced in the mitochondria among all known isoforms of superoxide dismutases [4]. Mood disorders are generally named affective disorders. Depression defines patients who are in the depressed mood and feeling low while the term bipolar defines patients in both the high (mania) mood and the low (depressed) mood [5]. Some studies report increased SOD levels in mood disorders (BD and MDD) with a decrease or no change in GSH-Px levels [6-8]. Other studies have reported a change in SOD and GSH-Px levels with treatment [6, 9-11]. As regards studies on gene polymorphism, the Pae et al. [12] have compared major depressive disorder and bipolar I disorder patients for the Ala-9Val MnSOD gene polymorphism while the Galecki et al. [13] investigated the relationship between the Ile-58Thr and Ala-9Val MnSOD gene polymorphisms and recurrent depressive disorders. We did not come across any study on the relationship between mood disorders (BD and MDD) and the GSH-Px gene polymorphism. We aimed to compare patients with a diagnosis of BD and MDD with a healthy group regarding the Ala-9 Val MnSOD and Pro 197 Leu GSH-Px gene polymorphisms using the real-time quantitative polymerase chain reaction (RT-qPCR) method.
MATERIALS AND METHODS
Patients
We included a total of 82 bipolar I patients (BD) and 80 major depressive disorder (MDD) patients diagnosed according to the DSM-IV (APA, 1994) [14] diagnostic criteria and 96 age- and sex-matched health control subjects. The Structured Clinical Interview, DSM-IV Axis I Disorders-Clinical Version (SCID-I-CV), [15] was administered to all patients. The family history, prior history of suicide attempts and psychotic episodes (the subjects had a precise history of psychiatric evaluations and treatments from psychiatrists) were also obtained. The study inclusion criteria for the patient and control groups were the lack of an axis I and II disorder according to DSM-IV, no additional neurological disorder, no mental retardation, no substance addiction, and no chronic systemic disease (diabetes mellitus, hypertension, etc.). The study was started after the necessary consent forms from the patient and control groups and local ethic committee decisions were obtained.
Genotyping
A venous blood sample (2 mL) was drawn for routine analysis and placed in tubes containing EDTA. DNA isolation was performed from whole blood using the Roche High Pure PCR Template Preparation Kit. GSH-Px and MnSOD polymorphism screening was performed based on the real-time PCR method using a Light Cycler 480 II Real-Time PCR thermal cycler. The increase in the fluorescence of the product obtained by the amplification of DNA was followed in real time using the real-time polymerase chain reaction (RT-PCR) method. Gene polymorphism was determined by analyzing the detailed melting curve of the PCR product obtained. Both GSH-Px and SOD2 primers and probes sequences are given below.
GSH-Px:
Forward Primer: ACTTTGAGAAGTTCCTGGTG
Reverse Primer: TTCCTCCCTCGTAGGTTTAG
Probe 1: CAGACCATTGACATCGAGCCTGACATCGAA-Fluorescein
Probe 2: LC Red 640-TGCTGTCTCAAGGGCCCAG-Phosphate
SOD2:
Forward Primer: GCTGTGCTTTCTCGTCT
Reverse Primer: GGTGACGTTCAGGTTGTT
Probe 1: CGGCTTTGGGGTATCTGGG-Fluorescein
Probe 2: LC Red 640-CCAGGCAGAAGCACAGCCTC-Phosphate
Statistical analysis
The data obtained from the study were analyzed with the Statistical Package for the Social Sciences (SPSS) 15.0 package software. Categorical variables reported as number and percent. We used Pearson Chi-square and Fisher exact test as appropriate for comparison categorical variables. A value of p<0.05 was considered significant.
RESULTS
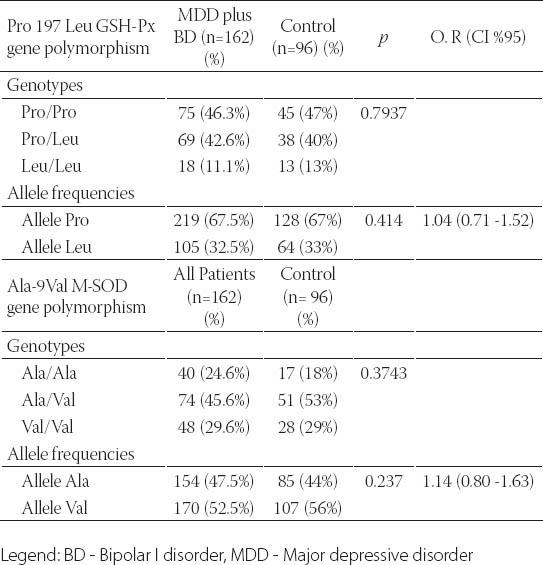
A total of 258 subjects, consisting of 82 BD patients, 80 MDD patients and 96 healthy subjects were included in the study. There was no significant difference between the BD, MDD and healthy control groups for age and gender (Table 1). The BD and MDD patients and the control subjects had a similar distribution of the genotypes and alleles in the Ala-9Val MnSOD gene polymorphism. The combined analysis (MDD plus BD) also failed to find any association between the Ala-9Val MnSOD gene polymorphism and any mood disorder (Tables 2, 3 and 4). Comparison of the MDD group and control group for the Pro 197 Leu GSH-Px gene polymorphism showed similar genotype distribution while there was a statistically significant difference between the distribution of the alleles. The rate of GSH-Px Pro allele carriers was higher and that of GSH-Px Leu carriers lower in the depression patients compared to the control group. Comparison of the BD group and control group for the Pro 197 Leu GSH-Px gene polymorphism revealed a similar distribution regarding the genotypes and alleles. The combined analysis (MDD plus BD) failed to find any association with the Pro 197 Leu GSH-Px gene polymorphism (Tables 2, 3 and 4). Subgroup analyses showed that the distribution of the genotypes in the Ala-9Val MnSOD polymorphism was similar according to the subgroup classifications such as the presence or absence of psychotic episodes (p=0.134, BD; p =0.419, MDD) and the family history (p =0.968, BD; p =0.625, MDD) and suicide history (p=0.330, BD; p=1.000, MDD). Subgroup analyses also revealed that the distribution of the genotypes in the Pro 197 Leu GSH-Px gene polymorphism was similar according to the subgroup classifications such as the presence or absence of psychotic episodes (p=0.478, BD; p=0.946, MDD) and family history (p=0.630, BD; p=0.625, MDD) and suicide history (p=1.000, BD; p=1.000, MDD) (data available on request).
TABLE 1.
Sociodemographic features of the BD, MDD and healthy control groups
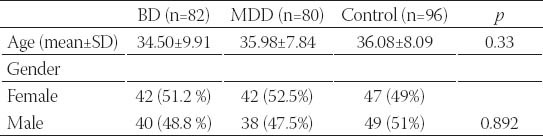
TABLE 2.
The genotype and allele distribution in the Pro 197 Leu GSH-Px and Ala-9Val MnSOD genes polymorphism in BD and controls
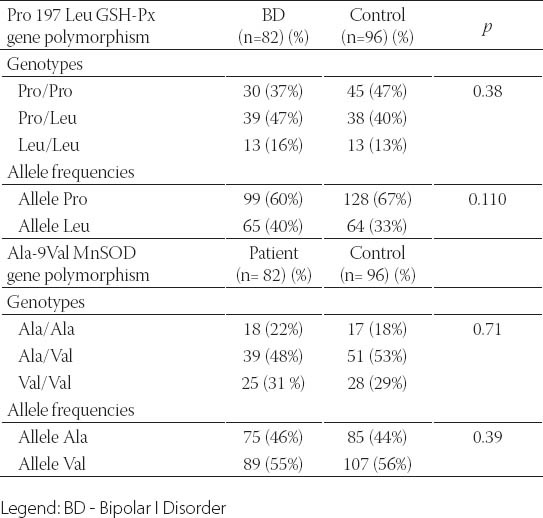
TABLE 3.
The genotype and allele distribution in the Pro 197 Leu GSH-Px and Ala-9Val MnSOD genes polymorphism in MDD and controls
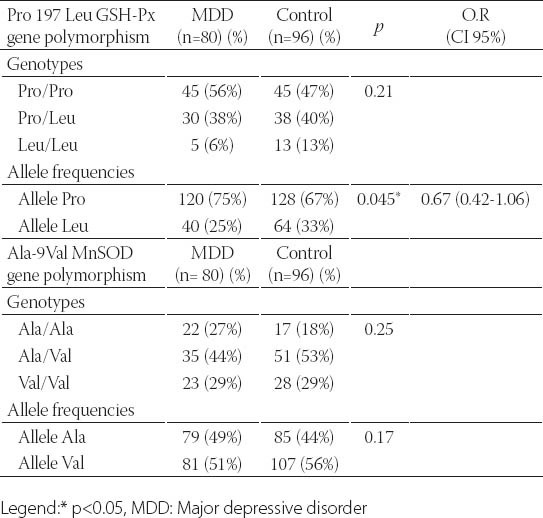
TABLE 4.
The genotype and allele distribution in the Pro 197 Leu GSH-Px and Ala-9Val MnSOD genes polymorphism in all patients (MDD plus BD) and controls
DISCUSSION
This study is the first to investigate whether a relationship is present between the Pro 197 Leu GSH-Px gene polymorphism and the development of mood disorders and their clinical variables. Although small statistical power of the current study it is found that there is a significant relationship between Pro 197 Leu GSH-Px polymorphism and MDD whereas it is not possible to say this concordance for BD. We did not find any relationship between the Pro 197 Leu GSH-Px polymorphism and the clinical variables of these mood disorders that we were able to detect (such as the presence or absence of psychotic episodes and the family history). We also investigated the relationship between these patients and the Ala-9 Val MnSOD gen polymorphism in addition to the Pro 197 Leu GSH-Px polymorphism in our patient groups. We did not find a significant relationship between the Ala-9 Val MnSOD polymorphism and mood disorders (BD, MDD) and their clinical variables (such as the presence or absence of psychotic episodes or the family history). We only found a relationship between the Pro 197 Leu GSH-Px gene polymorphism and MDD in our study. We did not find a relationship between the Pro 197 Leu GSH-Px gene polymorphism and BD. We also did not find a relationship between the Ala-9 Val MnSOD gene polymorphism and MDD or BD. We know that free radicals and antioxidant defense systems play an important role in the pathophysiology of mood disorders and both depression and BD, and the role of genes such as GSH-Px and SOD in oxidative stress in these disorders is definitely worth investigating. Genetic studies with BD and MDD patients include the Pae et al. [12] study comparing Korean patients with mood disorders (n=80 BP, n=61 MDD) and a healthy control group (n=106). They found that the Ala-9 Val MnSOD gene polymorphism showed a similar distribution both for genotypes and alleles between the BD and MDD patient groups and the healthy control group. Comparison by merging the BD and MDD groups and comparing this group with the healthy control group also showed a similar distribution for the Ala-9 Val MnSOD gene polymorphism genotypes and alleles. We similarly found similar Ala-9 Val MnSOD gene polymorphism genotype and al- lele distribution between the BD and MDD patient groups and the healthy control group and similarly between the merged group (BD plus MDD) and the health control group. In contrast to the Pae et al. [12] study, we also compared the BD and MDD patients and the healthy control group for the Pro 197 Leu GSH-Px gene polymorphism. We did not find a significant difference between the MDD group and the control group for the Pro 197 Leu GSH-Px gene polymorphism genotypes while such a difference was present for the alleles. Comparison of the BD and control groups for the Pro 197 Leu GSH-Px gene polymorphism showed no difference regarding genotypes or alleles. Comparison of the merged group (BD plus MDD) with the healthy control groups also showed similar genotype and allele distribution. Our study revealed that the GSH-Px Pro and Leu allele carrier state was different in depressed patients compared to the healthy control group. The Pro allele carrier rate was higher than in the healthy control group in the MDD patients while the Leu allele carrier rate was lower than in the healthy control group. A literature survey reveals that the Pro 197 Leu GSH-Px gene polymorphism has been studied in schizophrenics and patients who developed tardive dyskinesia due to drugs while it has not been studied in patients with mood disorders. Shinkai et al. [16] studied the Pro 197 Leu GSH-Px gen polymorphism in schizophrenic patients and found no difference between the patients and the healthy control group for the genotype or alleles. Other studies on the Pro 197 Leu GSH-Px gene polymorphism genotype and alleles in tardive dyskinesia patients have also reported no statistically significant difference between the tardive dyskinesia patients and the healthy control group [17, 18]. Our study results indicate that the increased Pro alleles in depression may cause high GSH-Px activity and lead to abnormal GSH-Px activity by disturbing the intracellular communication in mood disorders. The definite demonstration of the relationship between depression and antioxidant enzymes requires enzyme expression studies with a much larger scope so that the transcription and translation steps from DNA to enzyme synthesis can be elucidated. Demonstration of the enzyme structure at the molecular level is needed to show whether abnormal enzyme structure is present in this disorder. The main limitation of this study is the relatively small sample size. Although there is a significant relationship between Pro 197 Leu GSH-Px polymorphism and MDD; low statistical power of the current study is our another limitation.
CONCLUSIONS
Although small statistical power of the current study there is a significant relationship between Pro 197 Leu GSH-Px polymorphism and MDD. On the other hand, there is no significant relationship between Ala-9 Val MnSOD polymorphism and mood disorders. Understanding of the exact role of Pro 197 Leu GSH-Px polymorphism in the development of depression needs to further investigations with more sample size and high statistical power. None of the authors has any conflict of interest to disclose.
ACKNOWLEDGMENT
This study was supported by a grant the Gaziosmanpasa University Research Foundation.
DECLARATION OF INTEREST
None of the authors has any conflict of interest to disclose.
REFERENCES
- 1.Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the total antioxidant capacity the right tool? Redox Rep. 2004;9(3):145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- 2.Herken H, Uz E, Ozyurt H, Sogut S, Virit O, Akyol O. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol Psychiatry. 2001;6(1):66–73. doi: 10.1038/sj.mp.4000789. [DOI] [PubMed] [Google Scholar]
- 3.Lei XG, Cheng WH, McClung JP. Metabolic regulation and function of glutathione peroxidase-i. Annu Rev Nutr. 2007;27:41–61. doi: 10.1146/annurev.nutr.27.061406.093716. [DOI] [PubMed] [Google Scholar]
- 4.Hori H, Ohmori O, Shinkai T, Kojima H, Okano C, Suzuki T, et al. Manganese superoxide dismutase gene polymorphism and schizophrenia: relation to tardive dyskinesia. Neuropsychopharmacology. 2000;23(2):170–177. doi: 10.1016/S0893-133X(99)00156-6. [DOI] [PubMed] [Google Scholar]
- 5.Fagiolini A. Medical monitoring in patients bipolar disorders: a review of data. J Clin Psychiatry. 2008;69(6):16–18. doi: 10.4088/jcp.0608e16. [DOI] [PubMed] [Google Scholar]
- 6.Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20(2):171–175. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- 7.Kunz M, Gama CS, Andreazza AC, Salvador M, Ceresér KM, Gomes FA, et al. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1677–1681. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V, Jr, da Silva Vargas R, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett. 2007;421(1):33–36. doi: 10.1016/j.neulet.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol. 2004;19(2):89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kotan VO, Sarandol E, Kirhan E, Ozkaya G, Kirli S. Effects of longterm antidepressant treatment on oxidative status in major depressive disorder: a 24-week follow-up study. Prog Neuropsychophar macol Biol Psychiatry. 2011;35(5):1284–1290. doi: 10.1016/j.pnpbp.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Cumurcu BE, Ozyurt H, Etikan I, Demir S, Karlidag R. Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci. 2009;63(5):639–645. doi: 10.1111/j.1440-1819.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 12.Pae CU, Yoon SJ, Patkar A, Kim JJ, Jun TY, Lee C, Paik IH. Manganese superoxide dismutase (MnSOD: Ala-9Val) gene polymorphism and mood disorders: A preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):326–329. doi: 10.1016/j.pnpbp.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Galecki P, Smigielski J, Florkowski A, Bobinska K, Pietras T, Szem-raj J. Analysis of two polymorphisms of the manganese superoxide dismutase gene (Ile-58Thr and Ala-9Val) in patients with recurrent depressive disorder. Psychiatry Res. 2010;179(1):43–46. doi: 10.1016/j.psychres.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. 4th edition. Washington DC: APA; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW, Gibbon M. Washington, DC: APA; 1997. Structured clinical interview of DSM-IV disorders-clinician version (SCID-CV) [Google Scholar]
- 16.Shinkai T, De Luca V, Zai G, Shaikh S, Matsumoto C, Arnold PD, et al. No association between the Pro197Leu polymorphism in the glutathione peroxidase (GPX1) gene and schizophrenia. Psychiatr Genet. 2004;14(3):177–180. doi: 10.1097/00041444-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Al Hadithy AF, Ivanova SA, Pechlivanoglou P, Wilffert B, Semke A, Fedorenko O, et al. Missense polymorphisms in three oxidativestress enzymes (GSTP1, SOD2, and GPX1) and dyskinesias in Russian psychiatric inpatients from Siberia. Hum Psychopharmacol. 2010;25(1):84–91. doi: 10.1002/hup.1087. [DOI] [PubMed] [Google Scholar]
- 18.Shinkai T, Müller DJ, De Luca V, Shaikh S, Matsumoto C, Hwang R, et al. Genetic association analysis of the glutathione peroxidase (GPX1) gene polymorphism (Pro197Leu) with tardive dyskinesia. Psychiatry Res. 2006;141(2):123–128. doi: 10.1016/j.psychres.2004.06.023. [DOI] [PubMed] [Google Scholar]


