Abstract
The aim of our study was to report a series of 15 consecutive patients with aneurysms of the proximal segment (A1) of the anterior cerebral artery. In 15 patients with diagnosed A1 aneurysms, representing 2.1% of 720 aneurysm patients treated at a University Clinical Center between October 1999 and August 2012, clinical presentation, neuroradiological findings, surgical treatment methods and outcome were retrospectively analyzed. Mean patient age was 53.06 (range 32 to 75) years. Ten saccular aneurysms were treated with micro neurosurgical approach via standard pterional craniotomy, four fusiform aneurysms with coiling, and one fusiform aneurysm with stent. No patients died during the operation. The mean follow-up period was 32 months (range 5 months to 7 years). Clinical outcomes revealed good recovery in all patients. Despite the general opinion that A1 aneurysms are benign lesions, an increasing number of reports have demonstrated their potential complications. To date, due to the rarity of A1 aneurysms, only a few consecutive series have been reported. Conduct of multicenter studies are required in order to understand clinical features of A1 aneurysms and devise a proper treatment plan.
KEY WORDS: anterior cerebral artery aneurysm, A1 segment, digital subtraction angiography, endovascular treatment, subarachnoid hemorrhage
INTRODUCTION
The course of the proximal segment (A1) of the anterior cerebral artery (ACA) varies greatly according to its length and dominance, sometimes looping under the frontal lobe [1, 2]. A1 segment aneurysms constitute less than 1% of all intracranial aneurysms, but they are challenging to treat because of their small size and close relationship to the perforating arteries [3, 4, 5]. Generally, A1 segment aneurysms are smaller than other intracranial aneurysms [6-8]. The incidence of A1 aneurysms is low even in high-volume neurovascular centers, only a few cases every year are encountered. Suzuki et al., Handa et al., Yasargil et al., and Wakabayashi et al. are among the few describing a series of patients with A1 aneurysms; most other reports have been studied of individual case [3, 5, 9, 10]. The largest collection of A1 aneurysms published to date has been by Suzuki et al. in 1992 with 38 patients [5]. In this study, we present 15 patients who were surgically treated for A1 segment aneurysms and describe clinical status, radiologic findings, treatment, and outcome.
MATERIALS AND METHODS
Patients
This study was approved by the University Clinical Center Review Board. Seven hundred and twenty patients admitted with a diagnosis of aneurysm to the Department of Neurosurgery of our University Clinical Center between October 1999 and August 2012 were retrospectively analyzed. A diagnosis of “A1 aneurysm” was made on the basis of angiographic findings in fifteen patients who constituted the study group. On admission, some patients already had contrast-enhanced magnetic resonance (MR) angiography or computed tomography (CT) angiography was performed; however all patients underwent digital subtraction angiography (DSA) to visually identify aneurysms arising from the proximal A1 segment.
RESULTS
Fifteen patients met the eligibility criteria for the study. Of the 15 patients (five males, 10 females) whose records were reviewed, the mean age was 53.06 (range 32 to 75) years. The demographic and clinical data of the patients and the anatomic locations of the lesions are summarized in Table 1. CT showed subarachnoid hemorrhage in all patients. The combined imaging modalities confirmed a diagnosis of A1 segment of ACA aneurysms, eight (53.3%) on the left and seven (46.6%) on the right. Of the 15 A1 aneurysms, 13 (86.6%) were found on the proximal portion and two (13.3%) on the distal portion. Three patients (20%) were found to have intracerebral hematoma in the basis of frontal lobe, four (26.6%) had intraventricular hemorrhage. The aneurysms were saccular in 10 patients, and fusiform in five patients. Multiple aneurysms were seen in five patients (33.3%). Multiple aneurysms accompanying the A1 aneurysms arose from the middle cerebral artery (MCA), the internal carotid artery (ICA), and the anterior communicating artery (AComA). Contralateral A1 hypoplasia was seen in five patients (33.3%). The preoperative condition of the patients was assessed with World Federation of Neurological Surgeons (WFNS) SAH Grading System. Five patients were Grade I, eight were Grade II, and two were Grade III. The interval between the diagnosis and surgery was less than 72 hours in 10 patients who underwent micro neurosurgical approach. Ten saccular aneurysms were treated with micro neuro-surgical approach via standard pterional craniotomy (Figure 1), four fusiform aneurysms were treated with coiling of the aneurysm together with the parent artery, and one fusiform aneurysm was treated with stent-assisted coiling (Figure 2 and Figure 3). No patients died during the operation. The mean follow-up period was 32 months (range 5 months to 7 years). Good recovery was noted in all patients.
TABLE 1.
Summary of 15 patients with subarachnoid hemorrhage, aneurysms arising from the proximal (A1) segment of the anterior cerebral artery.
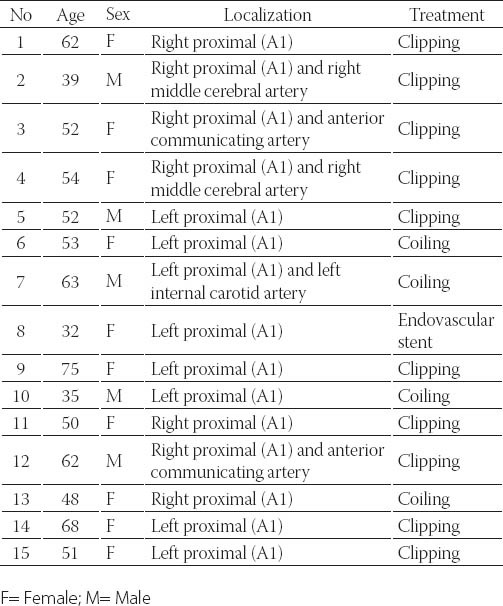
FIGURE 1.
Left internal carotid angiogram (A) demonstrating a saccular aneurysm in the left proximal (A1) anterior cerebral artery (arrow). Preoperative magneticresonance angiography imaging (B) showing the aneurysm (arrow). Postoperative left internal carotid angiogram (C) confirms clipping of the aneurysm and no residual filling (arrows) (Case 9).
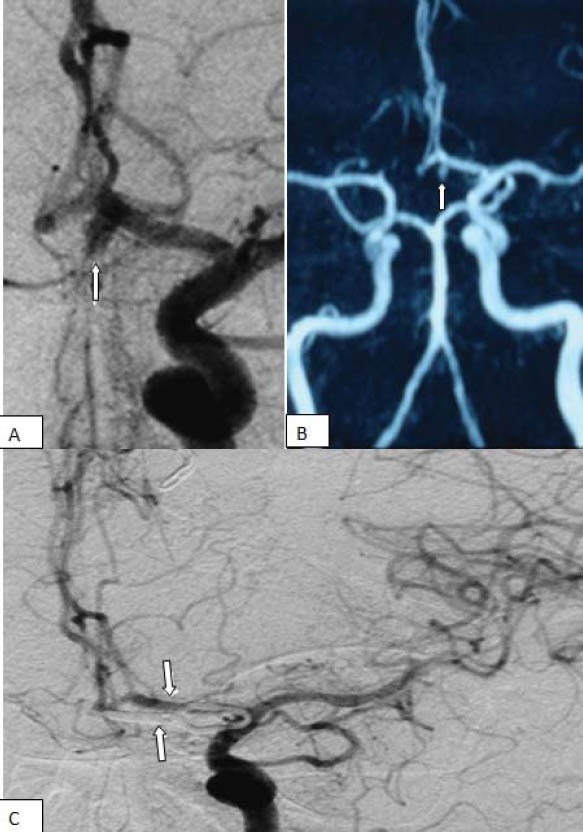
FIGURE 2.
Left internal carotid angiogram (A), demonstrating a wide necked saccular aneurysm of the left proximal (A1) anterior cerebral artery (arrows) and a saccular aneurysm in the ophthalmic segment. Post embolization angiography (B) shows total obliteration of the aneurysm (arrows) (Case 7).
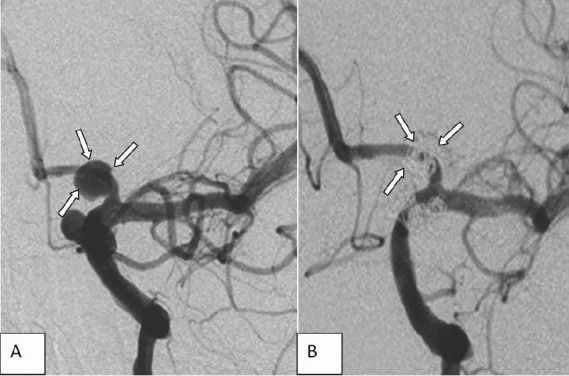
FIGURE 3.
Left internal carotid angiogram (A), demonstrating a fusiform an-eurysm in the left proximal (A1) anterior cerebral artery. Left internal carotid angiogram (B) shows complete obliteration of the aneurysm. Note occlusion of the parent artery (A1) which was well tolerated by the patient owing to patency of the anterior communicating artery (Case 6).
DISCUSSION
Our institution experience of A1 segment aneurysms is presented to expose our strategies related to surgical and endovascular treatment of 15 patients with ruptured aneurysms who were treated in our clinic over last 14 years. Aneurysms situated on the A1 segment of the ACA are known for their rarity. They have a low incidence (1% to 2%) and they are rarely described in literature [2, 3, 5, 6]. Similar to the other reports, the incidence in present study was 2.1%. In this series, similar as in those of Wakabayashi et al. [3] and Handa et al. [10], there are more female than male patients [3,10]. Most A1 aneurysm reported in the literature were located on the right side [3,7,10]. In the present study, eight (53.3%) aneurysms were found on the left and seven (46.6%) on the right side. Previous reports have particularly taken notice on numerous associated vascular anomalies at the locations of A1 segment aneurysms [3,5]. A high incidence of multiplicity (25%-70%) is also an important feature of A1 segment aneurysms [3, 5, 9, 11] and should prompt four-vessel angiography aimed to detection both A1 segment aneurysm and possible accompanying pathology at another location. If multiple aneurysms are present, determining which ones have bled and which one is most likely to bleed is essential. We noticed intracerebral hematoma in the basis of frontal lobe in three patients, and intraventricular hemorrhage in four. The most reliable diagnostic tools are DSA and three-dimensional reconstruction image. In our series the incidence of multiple aneurysms was lower (33.3%) than that in other reports [5, 9, 11]. The optimum timing of surgery is controversial and there is no good evidence that it has any significant effect on overall management outcome, especially in patients who are initially in poor neurological condition. At present, there is a tendency towards early surgery within the first three days after bleeding, before the possible onset of vasospasm. Such strategy reduces risk of aneurysmal rebleeding, allows for effective treatment of vasospasm, and do not increase surgical morbidity and mortality [12, 13]. Endovascular treatment is effective in preventing aneurysmal bleeding and provides an alternative to surgery in the treatment of ruptured intracranial aneurysms. Lubicz et al. [2] treated proximal ACA aneurysms with endovascular coiling and reported good results. The physician should propose selective endovascular treatment as an alternative therapeutic option to surgery. In the present study, four fusiform aneurysms were treated with coiling, and one fusiform aneurysm with stenting. The important characteristics of proximal A1 ACA aneurysms are perforating artery injuries and occlusions. Because of these specific features, A1 segment aneurysms are technically challenging to treat. Sparing the perforating arteries around the aneurysms is crucial for the satisfying neurological outcome after A1 segment aneurysm surgery. These arteries are delicate and frequently poorly visualized on preoperative angiography and brain CT angiography. They arise from the superior or posterior aspect of the A1 segment of the ACA [2]. From a microsurgical view, such an aneurysm is almost posterior to the A1 segment of the ACA, and the overlying parent artery interferes with the exposure and dissection of the aneurysm and perforating vessels. Limitations of our study include the retrospective design and relatively small number of our series. In addition, some details of history and factors that may influence the outcome may not be completely documented.
CONCLUSION
Despite the general opinion that these aneurysms are benign lesions, an increasing number of reports have stressed their potential complexity. To date, due to the rarity of A1 aneurysms, only a few collected cases have been reported. Conduct of multicenter studies is required in order to better understanding of characteristics of A1 aneurysms possibly leading to improvements in the treatment plan.
ACKNOWLEDGEMENT
The authors state that no conflict of interest exists for this manuscript.
REFERENCES
- [1].Kedia S, Daisy S, Mukherjee KK, Salunke P, Srinivasa R, Narain MS. Microsurgical anatomy of the anterior cerebral artery in Indian cadavers. Neurol India. 2013;61(2):117–121. doi: 10.4103/0028-3886.111113. [DOI] [PubMed] [Google Scholar]
- [2].Park HS, Choi JH, Kang M, Huh JT. Management of aneurysms of the proximal (A1) segment of the anterior cerebral artery. J Cere-brovasc Endovasc Neurosurg. 2013;15(1):13–19. doi: 10.7461/jcen.2013.15.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Handa J, Nakasu Y, Matsuda M, Kyoshima K. Aneurysms of the proximal anterior cerebral artery. Surg Neurol. 1984;22(5):486–490. doi: 10.1016/0090-3019(84)90308-2. [DOI] [PubMed] [Google Scholar]
- [4].Lee JM, Joo SP, Kim TS, Go EJ, Choi HY, Seo BR. Surgical management of anterior cerebral artery aneurysms of the proximal (A1) segment. World Neurosurg. 2010;74(4-5):478–482. doi: 10.1016/j.wneu.2010.06.040. [DOI] [PubMed] [Google Scholar]
- [5].Suzuki M, Onuma T, Sakurai Y, Mizoi K, Ogawa A, Yoshimoto T. Aneurysms arising from the proximal (Ai) segment of the anterior cerebral artery: a study of 38 cases. J Neurosurg. 1992;76:455–8. doi: 10.3171/jns.1992.76.3.0455. [DOI] [PubMed] [Google Scholar]
- [6].Wanibuchi M, Kurokawa Y, Ishiguro M, Fujishige M, Inaba K. Characteristics of aneurysms arising from the horizontal portion of the anterior cerebral artery. Surg Neurol. 2001;55(3):148–154. doi: 10.1016/s0090-3019(01)00396-2. [DOI] [PubMed] [Google Scholar]
- [7].Lehecka M, Niemelä M, Hernesniemi J. Surgical management of anterior cerebral artery aneurysms of the proximal (A1) segment. World Neurosurg. 2010;74(4-5):439–440. doi: 10.1016/j.wneu.2010.07.032. [DOI] [PubMed] [Google Scholar]
- [8].Lubicz B, Bruneau M, Dewindt A, Lefranc F, Baleriaux D, De Witte O. Endovascular treatment of proximal anterior cerebral artery aneurysms. Neuroradiology. 2009;51(2):99–102. doi: 10.1007/s00234-008-0474-7. [DOI] [PubMed] [Google Scholar]
- [9].Wakabayashi T, Tamaki N, Yamashita H, Saya H, Suyama T, Mat-sumoto S. Angiographic classification of aneurysms of the horizontal segment of the anterior cerebral artery. Surg Neurol. 1985;24(1):31–34. doi: 10.1016/0090-3019(85)90059-x. [DOI] [PubMed] [Google Scholar]
- [10].Yaşargil MG. Operative anatomy. In: Yasargil MG, editor. Microneu-rosurgery. New York: George Thieme Verlag; 1984. pp. 92–98. [Google Scholar]
- [11].Dashti R, Hernesniemi J, Lehto H, Niemela M, Lehecka M, Rinne J, et al. Microneurosurgical management of proximal anterior cerebral artery aneurysms. Surg Neurol. 2007;68(4):366–377. doi: 10.1016/j.surneu.2007.07.084. [DOI] [PubMed] [Google Scholar]
- [12].de Gans K, Nieuwkamp DJ, Rinkel GJ, Algra A. Timing of aneu-rysm surgery in subarachnoid hemorrhage: a systematic review of the literature. Neurosurgery. 2002;50(2):336–340. doi: 10.1097/00006123-200202000-00018. [DOI] [PubMed] [Google Scholar]
- [13].Nieuwkamp DJ, de Gans K, Algra A, Albrecht KW, Boomstra S, Brouwers PJ, et al. Timing of aneurysm surgery in subarachnoid haemorrhage--an observational study in The Netherlands. Acta Neurochir (Wien) 2005;147(8):815–821. doi: 10.1007/s00701-005-0536-0. [DOI] [PubMed] [Google Scholar]


