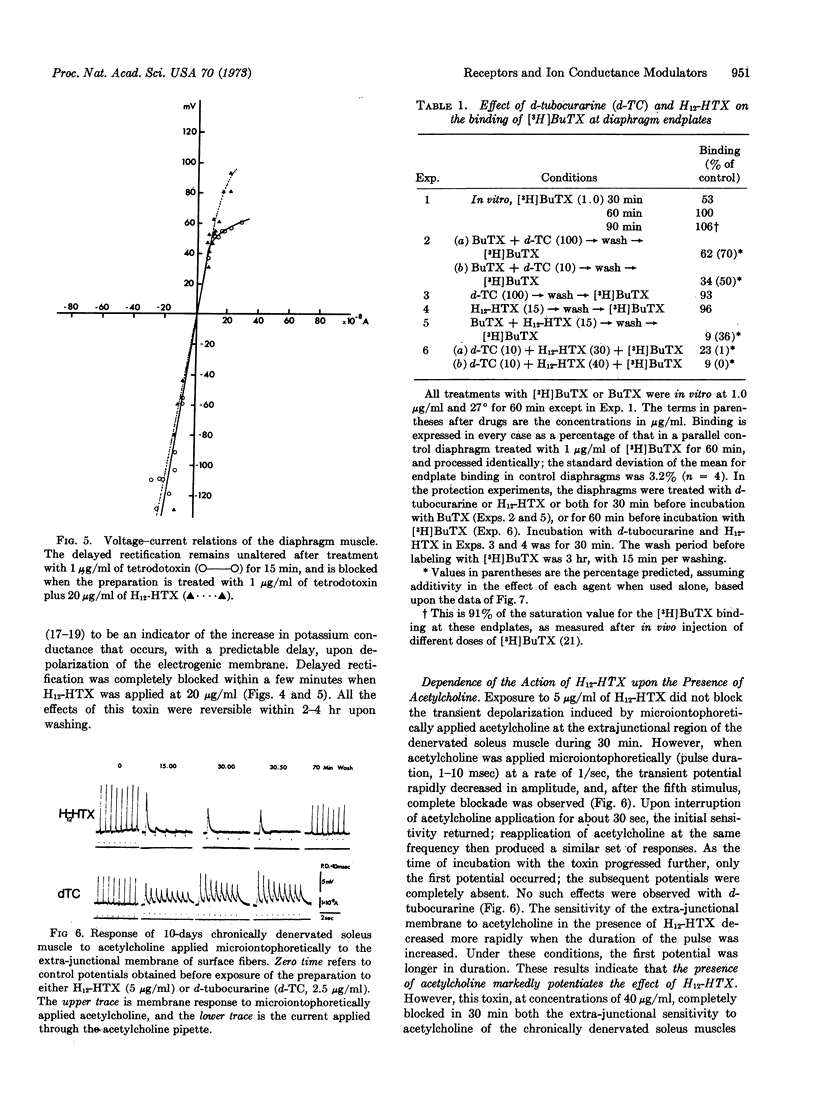
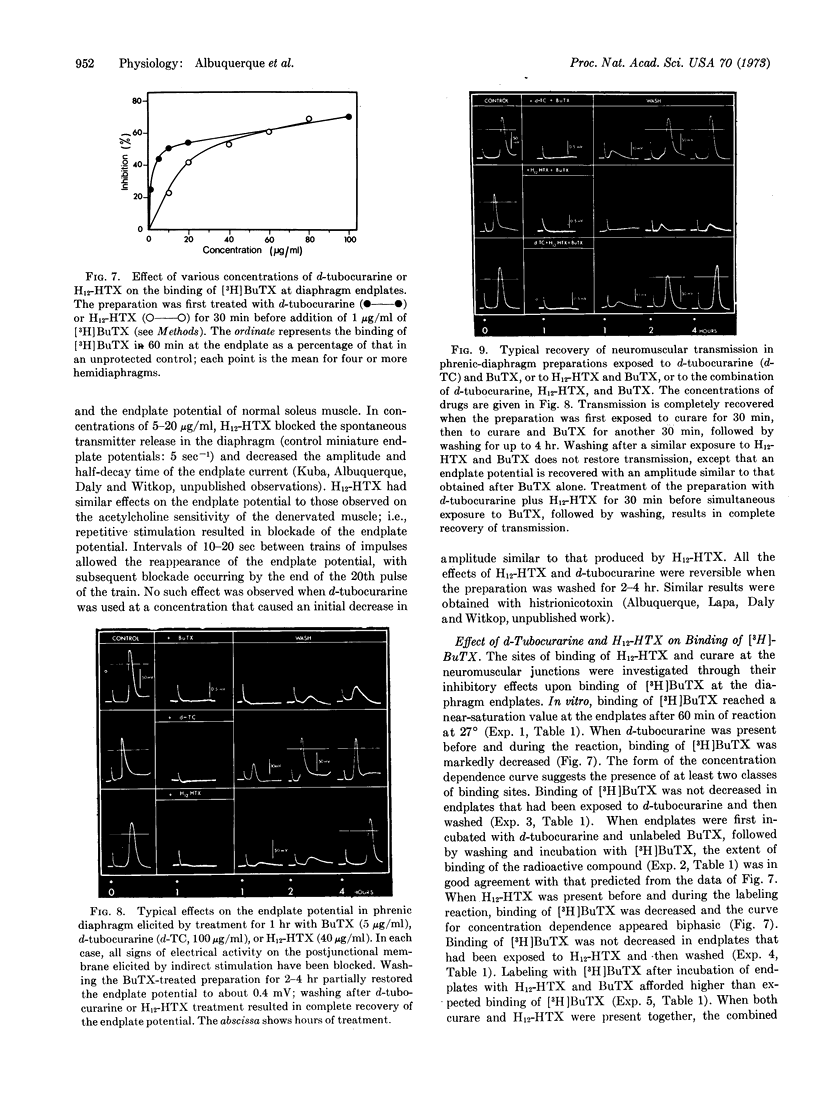
Abstract
The perhydro derivative of histrionicotoxin reversibly blocks the excitatory ionic transduction system in the synaptic and sarcolemmal membranes of mammalian skeletal muscle cells. The efficacy of perhydrohistrionicotoxin as an antagonist at the post-synaptic membrane is increased by the transient presence of acetylcholine in the endplate of innervated muscles and at extrajunctional receptors in denervated muscles. α-Bungarotoxin and [3H]monoacetyl-α-bungarotoxin block the endplate acetylcholine receptors, each binding to the same extent. The effect of bungarotoxin is partially reversible. These electrophysiological results, together with the effects of perhydrohistrionicotoxin and/or d-tubocurarine on the binding of [3H]monoacetyl-α-bungarotoxin at endplates of murine diaphragm muscle and on the bungarotoxin-elicited irreversible blockade of neuromuscular transmission, suggest that at least two types of sites participate in the synaptic excitation by acetylcholine. One site, competitively blocked by bungarotoxin and by curare, is presumably the acetylcholine receptor. Binding of bungarotoxin at this site is responsible for an irreversible blockade of neuromuscular transmission. The second site, competitively blocked by bungarotoxin and perhydrohistrionicotoxin, is proposed to be part of the cholinergic ion conductance modulator. Binding of bungarotoxin to this site does not result in an irreversible blockade.
Keywords: binding sites, perhydrohistrionicotoxin, αbungarotoxin, d-tubocurarine, sodium-potassium conductance, muscle action potential, endplate potential
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Warnick J. E., Sansone F. M. The pharmacology of batrachotoxin. II. Effect on electrical properties of the mammalian nerve and skeletal muscle membranes. J Pharmacol Exp Ther. 1971 Mar;176(3):511–528. [PubMed] [Google Scholar]
- Barnard E. A., Wieckowski J., Chiu T. H. Cholinergic receptor molecules and cholinesterase molecules at mouse skeletal muscle junctions. Nature. 1971 Nov 26;234(5326):207–209. doi: 10.1038/234207a0. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Kelly R. B., Sargent P. B., Williamson P., Hall Z. W. Binding of -bungarotoxin to acetylcholine receptors in mammalian muscle (snake venom-denervated muscle-neonatal muscle-rat diaphragm-SDS-polyacrylamide gel electrophoresis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):147–151. doi: 10.1073/pnas.69.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B. Acetylcholine receptor. I. Identification and biochemical characteristics of a cholinergic receptor of guinea pig cerebral cortex. J Biol Chem. 1972 Jan 10;247(1):130–145. [PubMed] [Google Scholar]
- Daly J. W., Karle I., Myers C. W., Tokuyama T., Waters J. A., Witkop B. Histrionicotoxins: roentgen-ray analysis of the novel allenic and acetylenie spiroalkaloids isolated from a Colombian frog, Dendrobates histrionicus. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1870–1875. doi: 10.1073/pnas.68.8.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. Molecular biology of synaptic receptors. Science. 1971 Mar 12;171(3975):963–971. doi: 10.1126/science.171.3975.963. [DOI] [PubMed] [Google Scholar]
- FUJINO M., YAMAGUCHI T., SUZUKI K. 'Glycerol effect' and the mechanism linking excitation of the plasma membrane with contraction. Nature. 1961 Dec 23;192:1159–1161. doi: 10.1038/1921159a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials without contraction in frog skeletal muscle fibers with disrupted transverse tubules. Science. 1967 Dec 29;158(3809):1702–1703. doi: 10.1126/science.158.3809.1702. [DOI] [PubMed] [Google Scholar]
- Goren H. J., Glick D. M., Barnard E. A. Analysis of carboxymethylated residues in proteins by an isotopic method, and its application to the bromoacetate-ribonuclease reaction. Arch Biochem Biophys. 1968 Aug;126(2):607–623. doi: 10.1016/0003-9861(68)90448-7. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. On the application of "a plausible model" of allosteric proteins to the receptor for acetylcholine. J Theor Biol. 1967 Aug;16(2):306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee C. Y., Tseng L. F. Distribution of Bungarus multicinctus venom following envenomation. Toxicon. 1966 Apr;3(4):281–290. doi: 10.1016/0041-0101(66)90076-6. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., DEGUCHI T., URAKAWA N., OHKUBO Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am J Physiol. 1960 May;198:934–938. doi: 10.1152/ajplegacy.1960.198.5.934. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Chiu T. H., Wieckowski J., Barnard E. A. Types and locations of cholinergic receptor-like molecules in muscle fibres. Nat New Biol. 1973 Jan 3;241(105):3–7. doi: 10.1038/newbio241003a0. [DOI] [PubMed] [Google Scholar]
- Rang H. P. Drug receptors and their function. Nature. 1971 May 14;231(5298):91–96. doi: 10.1038/231091a0. [DOI] [PubMed] [Google Scholar]
- Warnick J. E., Albuquerque E. X., Sansone F. M. The pharmacology of batrachotoxin. I. Effects on the contractile mechanism and on neuromuscular transmission of mammalian skeletal muscle. J Pharmacol Exp Ther. 1971 Mar;176(3):497–510. [PubMed] [Google Scholar]