Abstract
In polytrauma, injuries that may be surgically treated under regular circumstances due to a systemic inflammatory response become life-threatening. The inflammatory response involves a complex pattern of humoral and cellular responses and the expression of related factors is thought to be governed by genetic variations. This aim of this paper is to examine the influence of interleukin (IL) 6 single nucleotide polymorphism (SNP) -174C/G and -596G/A on the treatment outcome in severely injured patients. Forty-seven severely injured patients were included in this study. Patients were assigned an Injury Severity Score. Blood samples were drawn within 24 h after admission (designated day 1) and on subsequent days (24, 48, 72 hours and 7days) of hospitalization. The IL-6 levels were determined through ELISA technique. Polymorphisms were analyzed by a method of Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR). Among subjects with different outcomes, no statistically relevant difference was found with regards to the gene IL-6 SNP-174G/C polymorphism. More than a half of subjects who died had the SNP-174G/C polymorphism, while this polymorphism was represented in a slightly lower number in survivors. The incidence of subjects without polymorphism and those with heterozygous and homozygous gene IL-6 SNP-596G/A polymorphism did not present statistically significant variations between survivors and those who died. The levels of IL-6 over the observation period did not present any statistically relevant difference among subjects without the IL-6 SNP-174 or IL-6 SNP -596 gene polymorphism and those who had either a heterozygous or a homozygous polymorphism.
KEY WORDS: polymorphisms of interleukin-6 genes, IL-6, polytrauma, outcome prognosis
INTRODUCTION
Trauma is the leading cause of death in the developed societies among the population under 40 years of age, and it is the 4th cause of death in general, which therefore makes the understanding of pathophysiological mechanisms exceptionally important [1]. In polytrauma, injuries that may be surgically treated under regular circumstances – due to system overload - become life-threatening. Direct or indirect consequences of trauma (“trauma load”, “antigenic load”) overcome the defence mechanisms of an organism. A physiological “host defence response« switches into a self-destructive “host defence failure disease”, leading to the immune system shutdown, and subsequently to sepsis and progressive multiple organ dysfunction syndrome (MODS), as the most severe complication of polytrauma that usually involves two or more organ systems with the presence of alerted function in acutely ill patients such that homeostasis cannot be maintained without intervention [2]. The inflammatory response involves a complex pattern of humoral and cellular responses and the expression of related factors is thought to be governed by genetic variations. Cytokines are integral components of the host immune response to trauma or infection [3]. Interleukin 6 (IL-6) is a cytokine with both pro- and anti-inflammatory properties and its release into peripheral blood seems to be an early marker of injury severity following trauma. Interleukin 6 is a 21-kDa glycoprotein that is produced by numerous types of cells, particularly lymphocytes, to which it owes its name, and by fibroblasts and monocytes as well. It exhibits various biological effects, including the activation of B and T lymphocytes, induction of acute-phase protein synthesis in the liver, modulation of hematopoiesis, and it belongs to the groups of both pro- and anti-inflammatory cytokines. In addition, it acts as a pyrogen, it activates the coagulation system, and in vitro IL-6 it suppresses the synthesis of TNF-α and IL-1β [4, 5]. The IL-6 gene is located on chromosome 17 and several polymorphisms have been reported. The most frequently studied polymorphism is the single nucleotide polymorphism (SNP) - 174C and -174G in the promoter region, which has been associated with transcription rates of IL-6. The incidence of IL-6-174C alleles is approximately 40% among the general population, and it decreases in patients suffering from inflammatory diseases like juvenile rheumatoid arthritis [6]. The transfection of IL-6-174C alleles into HeLa cells in vitro has resulted in a decrease in IL-6 production compared to that of IL-6 -174G [7]. Additional polymorphisms may affect the IL-6 transcription as well, with complex interaction among certain haplotypes [8], but the published studies in that regard have been few. As far as the -596G/A single-nucleotide polymorphism (SNP) is concerned, relevant literature provides substantially less information on its clinical importance. This aim of this paper is to examine the influence of IL-6 single nucleotide polymorphism (SNP) -174C/G and single nucleotide polymorphism (SNP) -596G/A on the treatment outcome in severely injured patients.
MATERIALS AND METHODS
Patients
Forty-seven severely injured patients were included in this study. All patients were admitted in the intensive care unit due to the trauma severity. Patients were assigned an Injury Severity Score (ISS) [9] by independent evaluators. Exclusion criteria were (I) age of older than 18 years or younger than 65 years, (II) admission more than 8 h after trauma or secondary admission, (III) penetrating injuries, and (IV) any chronic illnesses. Injuries of the various body regions (head and neck, face, thorax, abdomen, extremities, and skin) were classified by usingthe Abbreviated Injury Scale (AIS) [9]. The clinical course was monitored prospectively in all patients. Patients requiring surgical intervention received standard surgical care and postoperative intensive care. We assumed that blood transfusions that were submitted to patients had no significant effect on the results of the study due to the same amount of blood given to the injured patients. Blood samples were drawn within 24h after admission (designated day 1) and on subsequent days (24, 48, 72 hours and 7 days) of hospitalization. Blood (9 ml) was collected in plastic tubes (NH4-heparin tube; Sarsted, Nümbrecht, Germany) along with the routine baseline laboratory work-up and was immediately used for stimulation ex vivo.
DNA isolation
Genomic DNA was isolated from peripheral blood with QIAampDNA Mini Kit (Qiagen GmbH, Hilden, Germany), and stored at -20°C.
Gene variants detection
All analyzed polymorphisms and primers used for their detection, are shown in Table 1. Polymorphisms are denoted by both, common nomenclature (used through the whole article), and HGVS nomenclature.
TABLE 1.
Analyzed gene polymorhisms
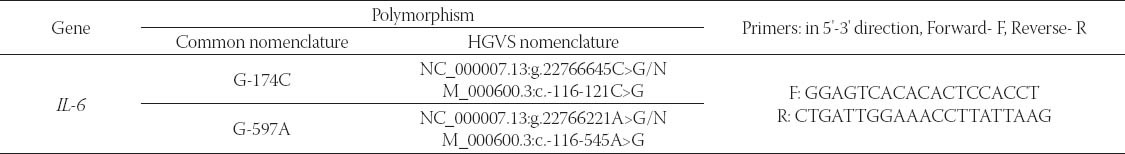
Interleukin-6 G-174C and G-597A detection
The genotyping of the polymorphisms in IL-6 promoter G-174C (rs1800795) and G-597A (rs1800797) was performed using PCR-RFLP technique as previously described [38]. A 527-bp fragment containing both polymorphic sites (-597 and -174) was amplified using one primer set. The PCR conditions were: denaturation step at 94°C for 5 min, 35 cycles of amplification (94°C for 3o sec, 57°C for 3o sec and 72°C for 45 sec) and elongation step of IO min at 72°C. The obtained PCR product was digested with Hin1 II and Fok I (MBI Fermentas, Vilnius, Lithuania). Digestion with Hin1 II (G-174C) gave the following pattern (in base pairs): 331+167+29 for GG genotype, 331+167+29+122+45 for GC genotype and 331+122+45 for CC genotype. Digestion with Fok I (G-597A) gave fragments of the following length (in base pairs): 527 for the GG genotype, 527+461+66 for the GA genotype and 461+66 for the AA genotype.
Statistical analysis
Statistical analyses were performed using the SPSS software v.17.0 (SPSS Inc., Chicago, IL, USA). Descriptive data for all groups and variables were expressed as mean ± standard deviation (SD), mediana, minimum and maximum for continuous measures, or percent of a group for discrete measures. Categorical data were analyzed using the Pearson chi-square test. A normal distribution was tested using the Koglomorov-Smirnov test. If the data were normally distributed, the t-test was used. Non-parametric data were analyzed using the Mann Whitney U test, and Kruskal Wallis test. Differences were considered significant when the p value was less than 0.05 (p<0.05). This study received an approval from the local ethics committee.
RESULTS
A total of forty-seven severely injured patients were included in the final study. Demographic features of patients involved in this research were analyzed. The mean age of patients was 35.5±14; 32 patients were male and 15 females. Patients were stratified acording to outcome to survivors (mean age 34.6±13.17 male patients and 9 female) and non survivors (mean age 36.6±16.15 male and 6 female patients). There was no significant statistical difference between these two groups acording to gender and age (p>0.5) The main mechanism of injury was traffic, followed by fall and assault. The non survivors were the most frequent in the group of patients which were injuried as motorcycle drivers. Using χ2 test no statistically significant difference was recorded between survivors and non-survivors (p=0.609). The Injury Serverity score (ISS) provided an over all score for patients with multiple injuries. Each injury was assigned an Abberviated Inury Scale score (AIS) allocated to one of six body regions (Head, Face, Chest, Abdomen, Extremities, External). Analysing the AIS and ISS score we observed statistically significant higher values of ISS and AIS for head and neck in patients with lethal outcome usin Mann Whitnez U-test (p=0.012 and 0.004, respectively). The IL-6 value analysis during the observation period yielded a statistically relevant difference (Friedman test; p=0.000). The highest values with statistical relevance were measured on admission (Wilcoxon test; p=0.000). The IL-6 level measurement after 24h resulted in statistically relevant lower values compared to those on admission, but these values were higher in turn from the values obtained after 48h (Wilcoxon test; p=0.001), 72h (Wilcoxon test; p=0.001) and after 7 days (Wilcoxon test; p=0.000). For values of IL-6 measured 48h and 72h after admission (Wilcoxon test; p=0.056), 48h and 7 days (Wilcoxon test; p=0.078) and 72h and 7 days after admission, no difference was found between them (Figure 1). A statistically relevant change in IL-6 value over the seven-day observation period was found both in survivors (Friedman test; p=0.000) and in subjects who died (Freidman test; p=0.010). In both of these subject groups, a statistically relevant drop in the values was found (Figure 2). Among subjects who had a different outcome, no statistically relevant difference was noted in the initial 48 hours, while the cytokine levels on day 7 with respect to those obtained 72h after admission were higher in subjects who died (Table 2).
FIGURE 1.
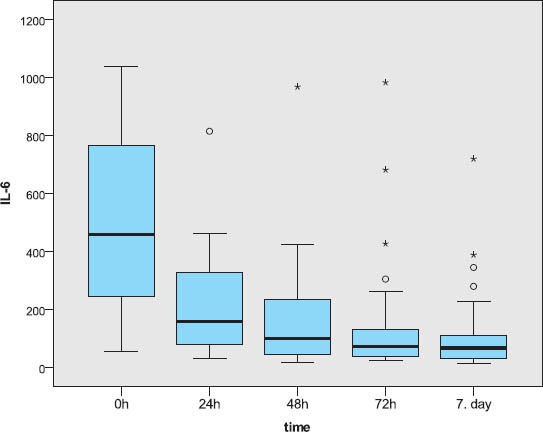
The IL-6 level measurements
FIGURE 2.
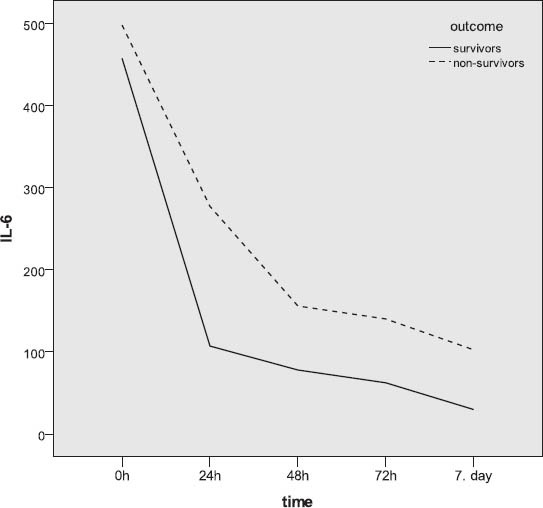
IL-6 levels measurements according to outcome
TABLE 2.
IL-6 levels measurements according to outcome
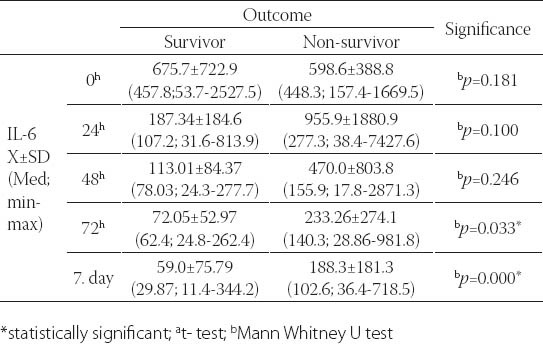
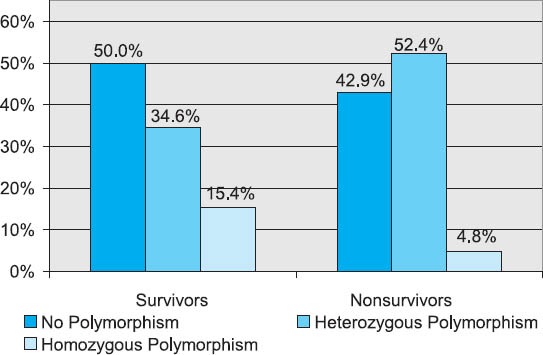
Subjects without the IL-6 SNP-174G/C gene polymorphism (46.8%) and those with heterozygous polymorphism (42.6%) were represented in closely equal number, while trauma patients with homozygous polymorphism were represented in a statistically significantly lower number (χ2-test; p=0.004). Somewhat more than a half of subject did not have the gene IL-6 SNP-596G/A polymorphism, approximately 40% had heterozygous polymorphism, while about 10% had homozygous polymorphism. The subjects with homozygous polymorphism were statistically significantly less represented than the patients without polymorphism or those with heterozygous polymorphism (χ2-test; p=0.002). Among subjects with different outcomes, no statistically relevant difference was found with regards to the gene IL-6 SNP-174G/C polymorphism (Table 3). More than a half of subjects who died had the SNP-174G/C polymorphism, while this polymorphism was represented in a slightly lower number in survivors (Figure 3). The incidence of subjects without polymorphism and those with heterozygous and homozygous gene IL-6 SNP-596G/A polymorphism did not present statistically significant variations between survivors and those who died (Figure 4). Slightly more than a half of subjects who survived had no polymorphism of this gene, close to a third of subjects had heterozygous polymorphism, while 12% of subjects had homozygous polymorphism. In subjects who died, somewhat more than a half had the gene IL-6 SNP-596G/A polymorphism, approximately 43% had heterozygous, while the remaining 9.5% had homozygous form (Figure 4). The IL-6 levels over the observed period of measurement did not present a statistically significant difference between the subjects without the gene IL-6SNP-174G/C polymorphism and those with heterozygous and homozygous polymorphism (Table 4, Figure 5). No statistically relevant difference in IL-6 levels measured on admission, 24h, 48h, 72h and seven days after admission between the subjects without the gene IL-6 SNP-596G/A polymorphism and with the homozygous and heterozygous polymorphism (Table 4). The lowest levels of this interleukin measured during the observation period in the polytrauma group were found in subjects with the homozygous polymorphism of this gene (Figure 6).
TABLE 3.
Gene polymorphism incidence according to outcome
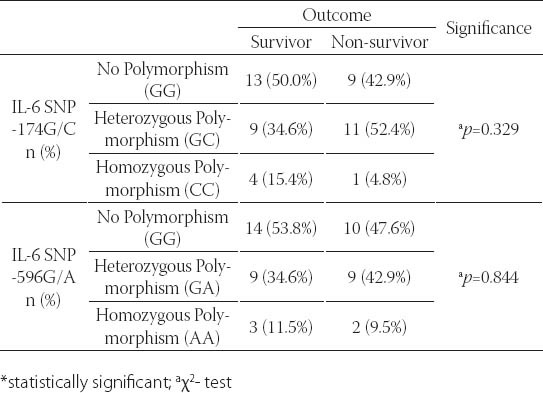
FIGURE 3.
Gene IL-6 SNP-174G/C polymorphism in groups with different treatment outcomes
FIGURE 4.
Gene IL-6 SNP-596G/A polymorphism in groups with different treatment outcome
TABLE 4.
Cytokine IL-6 levels and genetic polymorphism
FIGURE 5.
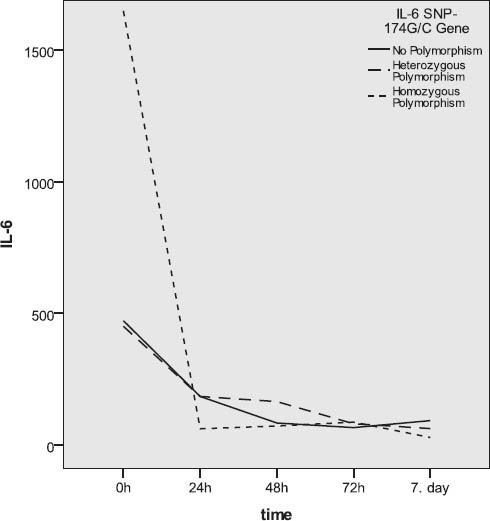
Gene IL-6SNP-174G/C polymorphism and IL-6 levels
FIGURE 6.
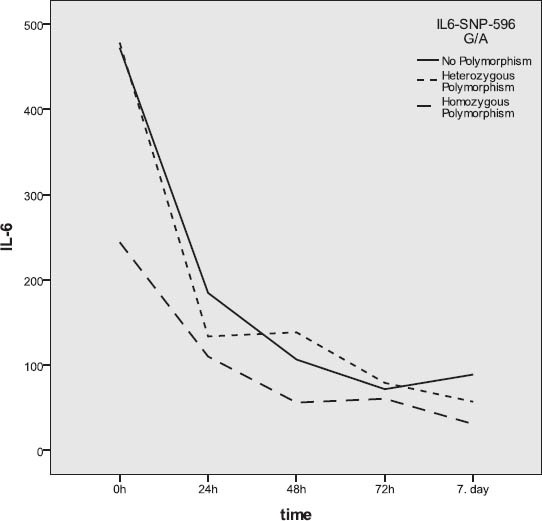
Gene IL-6SNP-596G/A polymorphism and IL-6 levels
DISCUSSION
The term polytrauma involves life-threatening injuries to at least two regions of the body [10]. The incidence of polytrauma in the under-40 age group is 44.9% and it represents the most frequent cause of death [11-13]. The provision of professional and prompt care directly affects mortality, morbidity and functional recovery. A complicated system of cellular interactions and mediators governs the “generalized inflammatory syndrome” in order to induce recovery and control an invasion by external organisms. In some cases of severe trauma, the inflammatory process goes out of control and vital tissue that has not been initially injured sustains damage. As numerous complex mediators and cellular systems are involved, the interpretation of such multiple interactions and their changes over time is highly complicated. IL-6 production is stimulated by TNF-α and IL-1, which was proven in experimental and clinical studies, and it may be found in plasma after these two cytokines [14, 15]. Experimental studies have showed that a high concentration of IL-6 is in correlation with the poor outcome of trauma and sepsis [16]. Jastrow et al. [17] in their analysis of cytokine concentration in serum every 4 hours, 24 hours after trauma, intended to identify a cytokine that could be used as a predictor for the development of MODS. This group of authors analyzed a number of cytokines and they came to the conclusion that IP-10, MIP-1β, IL-10, IL-6, IL-1Ra and eotaxin may be used to predict the development of MODS, as they exhibited higher levels in the period from 4 to 8 hours after trauma compared to the patients who did not get MODS. Considering the IL-6 levels in the period of 4-8 hours after trauma, the AU-ROC was 0.816, cut-off value 4110.2, sensitivity 57%, while the positive predictive value was at 100%, and in the 10-14 hour period after trauma, this value was reduced by 40-60%. Other authors have showed that the increased levels of IL-6 after trauma may be used as predictors for the development of MODS. It was determined that the increased IL-6 values were measured on days 2 and 4 in correlation with the development of MODS [18]. Particular authors described a significant increase in IL-6 on days 1 and 2 and the majority agrees that the sensitivity of this cytokine for the prediction of systemic complications development is reduced after 3 days [19]. In our group, the IL-6 levels were higher in patients who died and exactly on day 3 of observation, the value exhibited a statistically relevant difference. This SNP G>C at position -174 in the promoter region of IL-6 gene is related to the prevalence, incidence and/or prognosis of various diseases like Alzheimer’s disease, atherosclerosis, cardiovascular disease, carcinomas, insulin-independent diabetes mellitus, osteoporosis, sepsis and systemic complications of juvenile chronic arthritis [20-30]. The G allele of -174 SNP is coupled with the increase in transcriptional response to stimuli like endotoxins or IL-1β in vitro [20, 31], while the studies that examined in vivo the -174G>C promoter polymorphism in correlation with the plasma levels of IL-6 yielded contrasting results. In the first study, Fishman et al. [20] demonstrated that the unstimulated levels of IL-6 were coupled with the G allele of healthy individuals. Since then, opposing results related to the combination of IL-6 and SNP-174G>C in healthy and ill individuals have been obtained [31-34]. Thus, the plasma levels of IL-6, stimulated through coronary artery bypass surgery, correlated both with the G allele [31] and with the C allele [32], depending on the blood sample drawing after the procedure, while the administration of endotoxins in healthy volunteers did not confirm the existence of a relation between the IL-6 concentration and -174G>C polymorphism [34]. Banermo et al. [35] studied the role of -174SNP in the induction of plasma concentrations of IL-6 in vivo. They exposed healthy individuals who had the homozygous allele to a standardized inflammatory stimulus by administering a salmonella typhoid vaccine. They came to the conclusion that the G allele -174G>C SNP homozygotes had a stronger IL-6 inflammatory response to s. typhoid vaccination than the C allele homozygotes. The difference was not observed in the serum concentration of TNF-α and IL-1β, which was explained by the fact that this vaccine is an inflammatory stimulus of low potential and a weaker stimulator of cytokine production. Other studies [20,31] have showed that the G allele -174 SNP is coupled with the increased transcription upon endotoxin and IL-1β stimulation. Further ex vivo studies of full healthy lipopolysaccharide-stimulated blood have showed that the haplotypes of IL-6 promoters, including the G allele, are related to the higher levels of IL-6 [35]. Three studies examined the role of IL-6 -174G>C SNP in the plasma IL-6 response in vivo [32-35]. Brull et al. [32] have showed that as opposed to in vitro and ex vivo studies, the patients with homozygous C allele have higher plasma levels of IL-6 up to six hours after CABG stimulation, while a study by Burzotta et al. [31] has demonstrated that CABG stimulation in patients with the G allele induces higher concentrations of IL-6 after 24 and 48h. The conclusion of the third study in vivo is that there is no significant difference in the plasma levels of IL-6 in different genotypes after intravenous endotoxin administration [34]. No adequate explanation for such diverging results has been found, although the different effects caused by the influence of various stimuli on the IL-6 promoter cannot be ruled out, while a possible answer may be the lack of a functional relevance of -174G>C SNP. Arguments speaking against this are the results of research of relation between the G allele and survival of sepsis [30]. It has been demonstrated that the G allele is related to the better survival of sepsis, but not to the incidence of sepsis, which is an indicative of the fact that an increased response of IL-6 in G allele is beneficial when individuals are exposed to an acute infection. On the other hand, an increased response of IL-6 combined with the G allele may be harmful when it comes to the development of chronic diseases like the insulin-independent diabetes mellitus and atherosclerosis, considering that the repeated stimulation with minor stimuli increases the inflammatory stress. For example, it is proven that the insulin resistance is related to the G allele [25]. However, even the polymorphisms of other genes must be taken into account, as other studies have showed that the C allele, when combined with TNF-α SNP, is also related to the development of insulin-independent diabetes mellitus [23]. Several studies have concluded that the IL-6 is of key importance for the development of atherosclerosis, where all but one study [22] suggested that the G allele was related to the atherosclerosis [21, 24, 26]. In addition, several studies pointed to the important role of IL-6 in bone density regulation in pre- and postmenopausal women, as well as in healthy men [27, 28]. The results were that the individuals with C allele had greater bone density when compared to the individuals with G allele. One explanation could be that the prolonged high levels of IL-6 induced by infections and other chronic conditions may cause the loss of bone mass [28, 29]. It appears that it is not only the -174G>C SNP that affects the expression of IL-6 and its production as a response to stimuli. In vitro and in vivo studies have showed that haplotype -174G>C, -373AnTn, -572G>C and -597G>A polymorphisms affect the IL-6 concentration [36]. Haplotype analysis demonstrated that the presence of at least two out of three common haplotypes, including -174G, correlated with the increased IL-6 levels compared to the presence of only one common haplotype, -174C included. It is interesting to note that this study showed that individuals with GG haplotype had lower IL-6 levels, which corresponds to the results obtained by Kelberman et al. [37], and it points to the modulating influence of AnTn on IL-6 response within the -174G>C SNP. As far as IL-6 SNP-174 G>C and IL-6 SNP-596 G>A, Terry et al. determined that the IL-6 transcription positively correlates with these polymorphisms, although the other polymorphism that occurs is in a synergy as far as the effect on the promoter activity is concerned. [8]. Other studies showed that the -597A allele constitutes a potent counterbalance of the -174 C allele, however the limitation to this study was that the information on the origin of IL-6 were not available [36]. Due to conflicting results obtained, further investigation both in vitro and in vivo is required for the purpose of learning more and explaining the IL-6 expression and production. In an analysis of SNP polymorphism IL-6 SNP-174 and SNP-596 in a group of polytrauma patients in our series, no statistically relevant difference in the frequency of a particular outcome, although the -174 polymorphism was less represented in the group of polytrauma patients who survived. In the group of polytrauma subjects, the levels of IL-6, over the 7-day period of observation and cytokine measurement, did not produce a statistically relevant difference between the subjects who did not have the IL6 -174 gene polymorphism and those who had a heterozygous or homozygous polymorphism. The lowest levels of this interleukin in the 7-day observation period were measured in a subject from the group of polytrauma patients, and that subject had a SNP -596 IL-6 of this gene in a homozygous form. In the group of critically ill surgical patients, a statistically significant difference, both for the gene IL-6 SNP-174 and SNP-596 gene polymorphism, was not observed among subjects who had diverse outcomes. The levels of IL-6 over the observation period did not present any statistically relevant difference among subjects without the IL-6 SNP-174 or IL-6 SNP -596 gene polymorphism and those who had either a heterozygous or a homozygous polymorphism (Figures 5 and 6). Furthermore, other polymorphisms may affect the IL-6 transcription with a complex interaction among certain haplotypes [37], although few relevant studies have been published thus far.
CONCLUSION
Based on the results of our study, we concluded that presensce of IL-6 polymorphism (SNP-174 or -596) have no influence to production of IL-6 and due to this inflammatory response to severe trauma, but future studies with more subjects included are necessary to clarify the mechanism of injury and patophysiological response to severe trauma.
DECLARATION OF INTEREST
Authors declare no conflict of interest for this study.
ACKNOWLEDGEMENT
This work was supported by Ministry of Education, Science and Technological Development, Republic of Serbia (Grant No. III41004 and 43007).
REFERENCES
- [1].Butcher N, Balogh ZJ. The definition of polytrauma: the need for international consensus. Injury. 2009;(40 Suppl 4):S12–22. doi: 10.1016/j.injury.2009.10.032. [DOI] [PubMed] [Google Scholar]
- [2].Tsukamoto T, Chanthaphavong RS, Pape HC. Current theories on the pathophysiology of multiple organ failure after trauma. Injury. 2010 Jan;41(1):21–26. doi: 10.1016/j.injury.2009.07.010. [DOI] [PubMed] [Google Scholar]
- [3].Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- [4].Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleu-kin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. 2011 Mar-Apr;26(2):73–87. doi: 10.1177/0885066610384188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Damas P, Canivet JL, de Groote D, Vrindts Y, Albert A, Franchi-mont P, Lamy M. Sepsis and serum cytokine concentrations. Crit Care Med. 1997 Mar;25(3):405–412. doi: 10.1097/00003246-199703000-00006. [DOI] [PubMed] [Google Scholar]
- [6].Barton BE. The biological effects of interleukin 6. Med Res Rev. 1996 Jan;16(1):87–109. doi: 10.1002/(SICI)1098-1128(199601)16:1<87::AID-MED3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [7].Barber RC, Chang LY, Arnoldo BD, Purdue GF, Hunt JL, Horton JW, Aragaki CC. Innate immunity SNPs are associated with risk for severe sepsis after burn injury. clin Med Res. 2006;4(4):250–255. doi: 10.3121/cmr.4.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- [9].Apolone G. The state of research on multipurpose severity of illness scoring systems: are we on target? Intensive Care Med. 2000;26(12):1727–1729. doi: 10.1007/s001340000737. [DOI] [PubMed] [Google Scholar]
- [10].Stelfox HT, Bobranska-Artiuch B, Nathens A, Straus SE. Quality indicators for evaluating trauma care: a scoping review. Arch Surg. 2010;145(3):286–295. doi: 10.1001/archsurg.2009.289. [DOI] [PubMed] [Google Scholar]
- [11].Ekkernkamp A. Journal of Trauma Management & Outcomes: a new platform for interdisciplinary, outcome-oriented research in trauma. J Trauma Manag Outcomes. 2007;1(1):1. doi: 10.1186/1752-2897-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mutschler W, Marzi I. Management of polytrauma. Zentralbl Chir. 1996;121(11):895. [PubMed] [Google Scholar]
- [13].Scorpio RJ, Wesson DE, Smith CR, Hu X, Spence LJ. Blunt cardiac injuries in children: a postmortem study. J Trauma. 1996;41(2):306–309. doi: 10.1097/00005373-199608000-00018. [DOI] [PubMed] [Google Scholar]
- [14].Desborough JP. The stress re sponse to trauma and sur gery. Br J Anaesth. 2000;85:109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- [15].Flohe S, Lendemans S, Schade FU, Kreuzfel der E, Waydhas C. Influence of surgical intervention in the immune response of severely injured patients. Intensive Care Med. 2004;30:96–102. doi: 10.1007/s00134-003-2041-3. [DOI] [PubMed] [Google Scholar]
- [16].Gentile LF, Cuenca AG, Vanzant EL, Efron PA, McKinley B, Moore F, Moldawer LL. Is there value in plasma cytokine measurements in patients with severe trauma and sepsis? Methods. 2013;61(1):3–9. doi: 10.1016/j.ymeth.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jastrow KM, 3rd, Gonzalez EA, McGuire MF, Suliburk JW, Kozar RA, Iyengar S, Motschall DA, McKinley BA, Moore FA, Mercer DW. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209(3):320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [18].Andruszkow H, Fischer J, Sasse M, Brunnemer U, Andruszkow JH, Gansslen A, et al. Interleukin-6 as inflammatory marker referring to multiple organ dysfunction syndrome in severely injured children. Scand J Trauma Resusc Emerg Med. 2014;22(1):16. doi: 10.1186/1757-7241-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Andaluz-Ojeda D, Bobillo F, Iglesias V, Almansa R, Rico L, Gandía F, et al. A combined score of pro- and anti-inflammatory interleu-kins improves mortality prediction in severe sepsis. Cytokine. 2012;57(3):332–336. doi: 10.1016/j.cyto.2011.12.002. [DOI] [PubMed] [Google Scholar]
- [20].Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the in-terleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998 Oct 1;102(7):1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rundek T, Elkind MS, Pittman J. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6 and hepatic lipase genes. stroke. 2002;33:1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chapman CM, Beilby JP, Humphries SE, Palmer LJ, Thompson PL, Hung J. Association of an allelic variant of interleukin-6 with subclinical carotid atherosclerosis in an Australian community population. Eur Heart J. 2003;24:1494–1499. doi: 10.1016/s0195-668x(03)00313-0. [DOI] [PubMed] [Google Scholar]
- [23].Rauramaa R, Väisanen SB, Luong LA, Schmidt-Trücksass A, Pent-tila IM, Bouchard C, et al. Stromelysin-1 and interleukin-6 gene promoter polymorphisms are determinants of asymptomatic carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 2000 Dec;20(12):2657–2662. doi: 10.1161/01.atv.20.12.2657. [DOI] [PubMed] [Google Scholar]
- [24].Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ. The inter-leukin-6-174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J. 2001;22:2243–2252. doi: 10.1053/euhj.2001.2678. [DOI] [PubMed] [Google Scholar]
- [25].Flex A, Gaetani E, Pola R, Santoliquido A, Aloi F, Papaleo P, et al. The-174 G/C polymorphism of the interleukin-6 gene promoter is associated with peripheral artery occlusive disease. Eur J Vasc En-dovasc Surg. 2002 Sep;24(3):264–268. doi: 10.1053/ejvs.2002.1711. [DOI] [PubMed] [Google Scholar]
- [26].Cozen W, Gill PS, Ingles SA, Masood R, Martínez-Maza O, Cock-burn MG, et al. IL-6 levels and genotype are associated with risk of young adult Hodgkin lymphoma. Blood. 2004 Apr 15;103(8):3216–3221. doi: 10.1182/blood-2003-08-2860. [DOI] [PubMed] [Google Scholar]
- [27].DeMichele A, Martin AM, Mick R, Gor P, Wray L, Klein-Cabral M, et al. Interleukin-6 -174GC polymorphism is associated with improved outcome in high-risk breast cancer. Cancer Res. 2003 Nov 15;63(22):8051–8056. [PubMed] [Google Scholar]
- [28].Kubaszek A, Pihlajamäki J, Komarovski V, Lindi V, Lindström J, Eriksson J, et al. Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention. StudyDiabetes. 2003 Jul;52(7):1872–1876. doi: 10.2337/diabetes.52.7.1872. [DOI] [PubMed] [Google Scholar]
- [29].Vozarova B, Fernández-Real JM, Knowler WC, Gallart L, Hanson RL, Gruber JD, et al. The interleukin-6 (-174) G/C promoter polymorphism is associated with type-2 diabetes mellitus in Native Americans and Caucasians. Hum Genet. 2003;112:409–413. doi: 10.1007/s00439-003-0912-x. [DOI] [PubMed] [Google Scholar]
- [30].Kubaszek A, Pihlajamäki J, Punnonen K, Karhapää P, Vauhkonen I, Laakso M. The C-174G promoter polymorphism of the IL-6 gene affects energy expenditure and insulin sensitivity. Diabetes. 2003 Feb;52(2):558–561. doi: 10.2337/diabetes.52.2.558. [DOI] [PubMed] [Google Scholar]
- [31].Chung HW, Seo JS, Hur SE, Kim HL, Kim JY, Jung JH, et al. Association of interleukin-6 promoter variant with bone mineral density in pre-menopausal women. J Hum Genet. 2003;48:243–248. doi: 10.1007/s10038-003-0020-8. [DOI] [PubMed] [Google Scholar]
- [32].Dhamrait SS, James L, Brull DJ, Myerson S, Hawe E, Pennell DJ, et al. Cortical bone resorption during exercise is interleukin-6 genotype-dependent. Eur J Appl Physiol. 2003;89:21–25. doi: 10.1007/s00421-002-0750-x. [DOI] [PubMed] [Google Scholar]
- [33].Garnero P, Borel O, Sornay-Rendu E, Duboeuf F, Jeffery R, Woo P, et al. Association between a functional interleukin-6 gene polymorphism and peak bone mineral density and postmenopausal bone loss in women. Bone. 2002;31:43–50. doi: 10.1016/s8756-3282(02)00810-4. [DOI] [PubMed] [Google Scholar]
- [34].Ferrari SL, Garnero P, Emond S, Montgomery H, Humphries SE, Greenspan SL. A functional polymorphic variant in the interleukin-6 gene promoter associated with low bone resorption in postmenopausal women. Arthritis Rheum. 2001;44:196–201. doi: 10.1002/1529-0131(200101)44:1<196::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [35].Schlüter B, Raufhake C, Erren M, Schotte H, Kipp F, Rust S, et al. Effect of the interleukin-6 promoter polymorphism (-174 G/C) on the incidence and outcome of sepsis. Crit Care Med. 2002 Jan;30(1):32–37. doi: 10.1097/00003246-200201000-00005. [DOI] [PubMed] [Google Scholar]
- [36].Bennermo M, Held C, Stemme S, Ericsson CG, Silveira A, Green FG, et al. Genetic predisposition of the interleukin-6 response to inflammation: implications for a variety of major diseases? Clin Chem. 2004;50(11):2136–2140. doi: 10.1373/clinchem.2004.037531. [DOI] [PubMed] [Google Scholar]
- [37].Rivera-Chavez FA, Peters-Hybki DL, Barber RC, O’Keefe GE. Interleukin-6 promoter haplotypes and interleukin-6 cytokine responses. Shock. 2003 Sep;20(3):218–223. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lorenz E, Frees KL, Schwartz DA. Determination of the TLR4 genotype using allelespecific PCR. Biotechniques. 2001;31(1):22–24. doi: 10.2144/01311bm01. [DOI] [PubMed] [Google Scholar]
- [39].La Par DJ, Rosenberger LH, Walters DM, Hedrick TL, Swenson BR, Young JS, et al. Severe traumatic head injury affects systemic cyto-kine expression. J Am Coll Surg. 2012;214(4):478–486. doi: 10.1016/j.jamcollsurg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


