Abstract
Diabetic cerebrovascular diseases are defined as cerebral vascular diseases induced by diabetes with sugar, fat and a series of nutrient substance metabolic disorders, resulting in intracranial large and small vessel diseases. About 20%-40% patients with type 2 diabetes suffer from cerebral blood vessel diseases. Diabetic cerebrovascular diseases are the main causes of death in patients with diabetes mellitus. The major clinical manifestations are asymptomatic cerebral atherosclerosis, stroke, cerebral small vessel disease and acute cerebral vascular disease. The pathogenesis, clinical characteristics, treatment and prognosis of diabetic cerebrovascular disease are obviously different from non-diabetic cerebral vascular diseases. This paper will focus on the diabetic cerebrovascular disease, including its latest research progress. Diabetic cerebral large vascular disease and diabetic cerebral small vessel disease will be reviewed here.
KEY WORDS: diabetes, cerebrovascular diseases, diabetic cerebral large vascular disease, diabetic cerebral small vessel disease
INTRODUCTION
Diabetes mellitus (DM) is a type of chronic metabolic disease, of which the level of the blood glucose is above the average. The pathogenesis of DM is the insulin resistance or the reduction of the insulin secretion in the impaired pancreas. In the long-term development of DM, the chronic complications in patients are very complicated, including the macrovascular diseases, microvascular diseases, the complications of the nervous system and the diabetes feet. Moreover, the mechanisms of the complications are extremely complex, which are considered to be related to the hereditary susceptibility, obesity, insulin resistance, hyperglycemia, the oxidative stress or the damaged nutrition metabolism, and the factors mentioned above inducing the complications in the DM patients mutually. The cerebrovascular diseases in patients with DM are the most severe complications, especially in patients with type 2 DM. The cerebrovascular diseases include the ischemic stroke and the hemorrhagic stroke, both of which happen in patients with macrovascular disease or microvascular disease. The hyperglycemia in patients with type 2 DM, caused by the insulin resistance or the reduction of the insulin secretion, can induce many risk factors to damage the blood vessel, such as the various cytokines in inflammatory reaction, the metabolic disorders of sugar or lipid, and the changes of hemodynamics. Compared with the group of cerebrovascular diseases without DM, the pathogenesis, clinical characteristics, treatment and the prognosis are more complicated in those with DM.
Pathogenesis of cerebrovascular disease in DM patients
The main mechanism of the cerebrovascular diseases in patients with type 2 DM is the atherosclerosis. However, it has been reported that the atherosclerosis was an inflammatory response in essence [1]. Patients with DM experience some pathologic conditions, such as long-term high blood glucose and multi-substance metabolic disturbance, which damage the blood vessel endothelium for a long time. The hyperglycemia and metabolic disturbance can increase the level of oxidative stress to further impair the endothelia. In the process of the endothelium damage, many cytokines and adhesion molecules are secreted in a high level, then the inflammatory cells (including the T lymphocytes and the mononuclear leucocytes) adhere to the endarterium and move into the vascular wall. After migration into the wall, the mononuclear leucocytes adhere to the vascular wall, then go across the endothelium layer toward the vascular wall and become macrophagocytes. The macrophagocytes phagocytize the low density lipoprotein cholesterol which is increased in the patients with DM, especially in those combined with hyperlipidemia. Then these cells become the xanthoma cells. The blood platelets get together and adhere to the vascular wall in the inflammatory reaction process; at the same time, the smooth muscle cells proliferate and migrate into the endarterium. When the xanthoma cells degenerate and become necrotic cells, the lipid within the cells will be released into the vascular wall, and the extracellular lipid nuclear is formed. After the lipid nuclear increase very high and the macrophagocytes become the major cells in the wall, the inflammatory response turns up (Figure 1). The serum inflammatory factors, such as C-reactive protein (CRP), interleukin-6 and interleukin-17, play a great role in the process of the vascular damage. These factors induce the plaque to become erosive and to break up, then the blood platelets are activated and the thrombus is formed [2]. Afterwards, the vessels become narrow or completely occlusive in the very severe cases. Some researchers found that the incidence of cerebrovascular events was significantly higher in the high CRP group than in the low CRP group. It indicates that CRP may influence the incidence of primary cerebrovascular event in DM patients [3]. Therefore the type 2 DM is a type of low degree chronic systematic inflammatory disease in essence.
FIGURE 1.
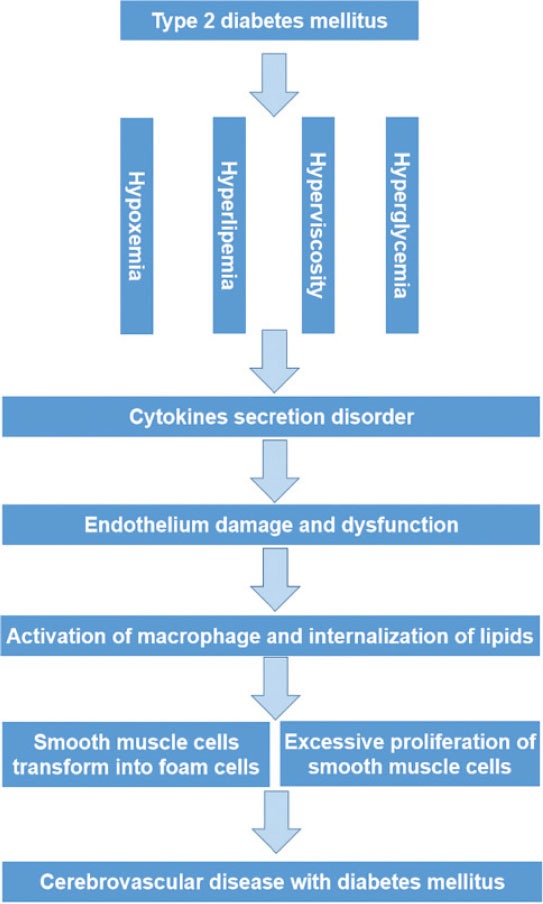
The pathogenesis of cerebrovascular disease in diabetes mellitus.
The level of other biomarkers, such as homocysteine (Hcy), matrix metalloproteinase-9 (MMP-9) and serum uric acid, also rises highly in the type 2 DM patients’ serum. Moreover, these biomarkers are the risk factors of the cerebrovascular diseases in DM patients and take part in the development of the atherosclerosis. Hcy damages the blood vessel endothelium directly by the oxidative stress and endoplasmic reticulum stress [4] or indirectly by the cytokine and the immune response [5]. The MMP-9 acts on the damaged basement membrane and accelerates the unstable plaque rupture in the vessels [6]. Thus the thrombosis is formed and enters into the small vessels in the brain, resulting in the cerebral infarction attack. The high level of the serum uric acid is caused by the metabolic disorder of protein and the hypofunction of the microvessels, which filter the serum uric acid in the kidney. The serum uric acid that can injure the blood vessel endothelium and stimulate the secretion of the cytokines is related to the development of macrovascular diseases in type 2 DM patients [7]. Above all, these biomarkers are sensitive to the vascular damage and can be used to monitor the DM patients’ cerebrovascular complications at the early stage. Hyperglycemia also increases lactate production and exacerbates brain tissue acidosis by increasing the available glucose for anaerobic glucose metabolism and inhibiting mitochondrial respiration. It also causes vasogenic edema, which impairs collateral blood flow, increases the hyperthrombotic state, decreases cerebral blood flow and possibly impairs cerebral autoregulation [8].
Taken together, all these factors probably increase the incidence rate of the cerebrovascular diseases tremendously. The risk of cerebrovascular diseases would be further increased in the DM patients who are also with hyperlipidemia or hypertension. In particular, in the DM patients with hypertension, the rate of cerebrovascular disease is apparently higher and the cerebrovascular risk increases by 2-3 fold in type 2 DM patients with elevated systolic blood pressure [9]. Because of hyperglycemia, inflammation and accumulation of the lactate, the vascular wall is damaged and the endothelial cells become necrotic, then the thrombus forms in the brain. The hyperlidemia in DM patients increases the blood viscosity and changes the haemodynamics, both of which maybe accelerate the development of atherosclerosis. Meanwhile, the hypertension also induces the arteriolosclerosis and fibrinoid necrosis of the wall, and even causes the microaneurysm, which is easily ruptured. All the factors act on the vascular in brain for a long time, resulting in the stroke finally.
Clinical classification and characteristics of cerebrovascular diseases in DM patients
The cerebrovascular diseases complicated in DM patients include the macrovascular disease and microvascular disease. Based on the pathogenesis and the pathology, the brain vessels complications in DM patients can be divided into the ischemic cerebrovascular disease and the hemorrhagic cerebrovascular disease. Through the pathogenesis mentioned above, it is known that the DM can induce the ischemic cerebrovascular disease by multiple factors, such as cerebral infarction and transient ischemic attack. The hemorrhagic cerebrovascular disease is mainly caused by the rupture of the brain blood vessels. The landmark Trial of Org 10172 in Acute Stroke Treatment (TOAST) [10] classified stroke into five subtypes 1) large artery atherosclerosis, 2) cardioembolism, 3) small vessel occlusion (lacunar), 4) stroke of other etiology and 5) stroke of undetermined etiology (Figure 2). The same study found that hyperglycemia worsened outcome in non-lacunar stroke but not in lacunar stroke [11], and cerebral infarction was by far the most common type of stroke, followed by cerebral hemorrhage and subarachnoid hemorrhage [12]. Researchers indicated that the incidence rate of non-lacunar stroke was higher than the lacunar stroke [13]. The macrovascular disease induced by DM mainly affects the carotid artery in which the plaque causes the ischemia (such as the transient ischemic attack) in the brain. If the plaque is erosive and migrates into the small vessels in the brain, the cerebral infarction occurs. Diabetic patients who have a stroke have significantly greater carotid intima-media thickness (CIMT) than both diabetic subjects without stroke and non-diabetic patients [14]. Hence the CIMT can be used to forecast the incidence rate of the cerebral ischemic stroke [15].
FIGURE 2.
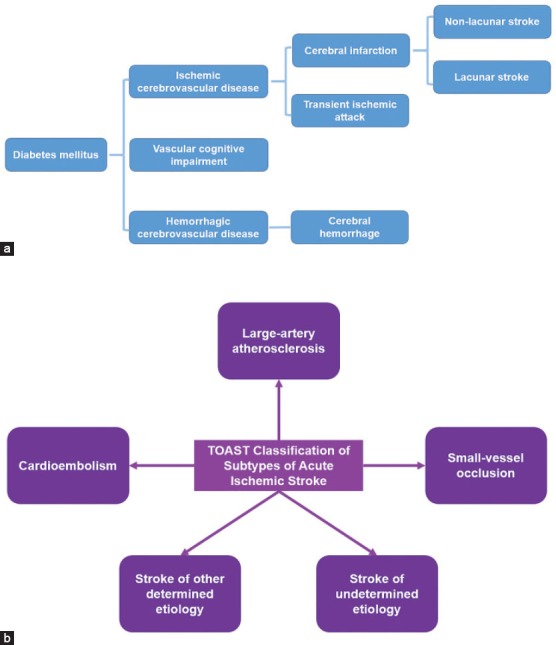
A. The classification of diabetic cerebrovascular diseases. B. The classification of stroke in the landmark Trial of Org 10172 in Acute Stroke Treatment (TOAST) (Adapted from Adams et al., 1993).
The brain vessel disease in DM patients also causes the vascular cognitive impairment. In fact, several studies have evidenced that alterations of insulin homeostasis in pre-diabetes and diabetes increased the risk of developing cognitive decline and dementia, including vascular dementia and Alzheimer's disease (AD). Cognitive impairment due to diabetes mainly occurs at two main periods the first 5–7 years of life when brain system is in development and the period when the brain undergoes neurodegenerative changes due to aging (older than 65 years) [16].
The rate of hemorrhagic cerebrovascular disease in DM patients with the hypertension is higher than those without hypertension. Both hyperglycemia and hypertension can induce the risk factors to act on the brain vessels and to make them easy to be ruptured. A study showed that among those with diabetes ≥10 years, risk of ischemic stroke is 3 times higher than those without diabetes, and it also provided an evidence that the risk of ischemic stroke increased continuously with the duration of diabetes mellitus [17]. It has been well documented that diabetes is associated with an increased risk of ischemic stroke [18]. The disability ratio and mortality ratio are very high in DM patients who complicated with the cerebrovascular diseases. Higher rates of stroke in patients with diabetes, particularly in patients younger than 65, may be associated with a higher burden of disability [19]. The morbidity of the brain complications in DM patients rises in all ages, but younger patients (30 to 44 years old) had a significantly higher risk of stroke [20]. There are some studies showing an increased risk of developing stroke in type 2 DM patients (1.5 to 2 fold in men and 2 to 6.5 fold in women) [21].
Treatment and prognosis of cerebrovascular diseases in DM patients
The treatments of the cerebrovascular diseases in DM patients are divided into three levels: 1) The first level: to prevent the brain vessels complications by controlling the blood glycose positively when the patients are at the stage of insulin resistance or at the early stage of diabetes; to reduce the risk factors caused by the hyperglycemia, which can damage the blood vessels; 2). The second level: to control glucose intensively after the cerebrovascular diseases occur in DM patients. The blood pressure should be reduced to the normal range to avoid the vasculopathy induced stroke; 3). The third level: to improve the cerebral circulation, which may reduce the mortality rate after the stroke in DM patients. Through the three levels’ approach, the health condition and the quality of life could be substantially improved apparently in DM patients. Now, many treatment modalities have been applied to prevent and to treat the cerebrovascular disease in DM patients. These include a tight glycemic control, an appropriate hypertension management, and reduction of LDL-C levels, all of which have been shown to reduce the stroke risk [13].
The most important treatment for DM patients in the first two levels is to prevent the risk factors caused by hyperglycemia. This treatment includes forming a good life style, smoking cessation, controlling the blood pressure and the blood lipid concentration. At the stage of impaired glucose regulation or the preliminary stage of DM, the most effective treatment is to reduce the blood glycose to normal level, to decrease the risk factors that may damage the vessels including the inflammation caused by hyperglycemia. These measures may help prevent the development of the blood vessel complications in DM patients. Oral hypoglycemic agents can control the concentration of sugar in blood, and one study showed that there is a close association between poor glycaemic control and increased revascularization rate in T2DM [22], and normalization of glucose was associated with a reduction in mortality by 4.6 times [23]. Another important way to control the process of atherosclerosis is the antiplatelet therapy, such as the treatment with acetylsalicylic acid (ASA). However, the resistance to ASA occurs in DM patients. A study showed that ASA resistance was significantly higher in men, smokers and insulin users, but significantly lower in beta blocker (BB) users, angiotensin-converting enzyme inhibitor (ACEI) users. However, multivariate analysis showed that insulin usage was the single effective parameter on ASA resistance [24]. When the oral hypoglycemic agents are non-effective in type 2 DM patients, insulin should be applied. At the same time, the development of the atherosclerosis in brain vessels must be monitored timely, because the insulin can induce the ASA resistance. There was also one research assess the endothelial dysfunction (ED) in type 2 diabetic patients and evaluate the flow-mediated vasodilation (FMV) in brachial artery, they thought that estimated factors influencing FMV might be potential therapeutic targets for presented endothelial dysfunction in type 2 diabetic patients with coronary artery disease [25].
Hypertension is the most important single risk factor. This risk can be decreased with antihypertensive agents by 30-40% [26]. ACEIs and angiotensin receptor blocker medications have been shown to be superior to other antihypertensive medications in the prevention of stroke; and the channel blockers have also shown significant benefit in patients with diabetes [27]. A recent study showed that glucagon-like peptide-1 receptor (GLP-1R) activation agonist Ex-4 (exendin-4), a drug for the treatment of T2D (Type 2 diabetes), may also have neuroprotective effects. Further investigation is required of GLP-1R agonists on their neuroprotective action in DM, and their potential use as anti-stroke medication in both diabetic and non-diabetic conditions [28]. Patients with cerebrovascular disease are not recommended to be treated by the combination of aspirin and clopidogrel for prevention of stroke, as there is an added risk of intracerebral hemorrhage and gastrointestinal bleeding without a reduction in ischemic stroke risk [29, 30]. The patients with ischemic stroke should be treated by thrombolytic therapy in the early phase of hyperacute cerebral infarction, and the level of blood glycose should be controlled. However, both hypo-and hyperglycemia seem to carry risks in the setting of an acute ischemic stroke as shown by a J-shaped association between serum glucose levels and functional outcome in acute ischemic stroke [31], so we should avoid the hypoglycemia as much as possible in DM patients. There was a study about acute lacunar stroke indicating that the stroke outcome in patient with controlled normal blood glycose was better [32].
Ischemic stroke caused by carotid atherosclerosis and carotid artery stenosis should consider an operation of carotid endarterectomy, but the operation is limited to the patients whose carotid artery stenosis are over 70% and can be taken only with the evaluation of fewer complications caused by this surgery [33]. In a recent study, the impressive results of reducing stroke risk in DM have been observed with the treatment of statin (such as atorvastatin), which is effective in DM patients even when the LDL-C levels are normal [13].
Because of the complicated environments in vivo, it is very difficult to discover the accurate mechanisms, which induce the complications in cerebral vessels in DM patients. Recent breakthrough of reprogramming somatic cells into stem cells or neurons offers a useful in vitro tool to model disorders in central nervous system [34-45]. However, it will take time to simplify and mimic the in vivo system, especially at the complicated disorder status, such as in DM.
CONCLUSIONS
We conclude that the prevention of DM complications is the best approach at the moment. The preventive measures include good living habits, such as smoking cessation, limiting alcohol and avoiding high-glucose and high-fat diet, is an effective way to prevent the stroke. It helps decrease the morbidity and mortality in stroke patients with DM and improve the patients’ life quality. It is notable that these treatments can only prevent the development of the complications in the brain vessels in DM patients but cannot thoroughly rescue the brain damage. More effective treatments are needed to prevent the occurrence and development of the cerebrovascular disease in DM patients.
DECLARATION OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. http://dx.doi.org/10.1056/NEJM199901143400207 . [DOI] [PubMed] [Google Scholar]
- [2].Portik-Dobos V, Anstadt MP, Hutchinson J, Bannan M, Ergul A. Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. Diabetes. 2001;51(10):3063–3068. doi: 10.2337/diabetes.51.10.3063. http://dx.doi.org/10.2337/diabetes.51.10.3063 . [DOI] [PubMed] [Google Scholar]
- [3].Soejima H, Ogawa H, Morimoto T, Nakayama M, Okada S, Sakuma M, et al. Aspirin possibly reduces cerebrovascular events in type 2 diabetic patients with higher C-reactive protein level: Subanalysis from the JPAD Trial. Journal of Cardiology. 2013;62(3):165–170. doi: 10.1016/j.jjcc.2013.03.015. http://dx.doi.org/10.1016/j.jjcc.2013.03.015 . [DOI] [PubMed] [Google Scholar]
- [4].Zhu WG, Li S, Lin LQ, Yan H, Fu T, Zhu JH. Vascular oxidative stress increases dendritic, cell adhesion and transmigration induced by homocysteine. Cell Immunol. 2009;254(2):110–116. doi: 10.1016/j.cellimm.2008.08.001. http://dx.doi.org/10.1016/j.cellimm.2008.08.001 . [DOI] [PubMed] [Google Scholar]
- [5].Postea O, Krotz F, Henger A, Keller C, Weiss N. Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine 2006. Arterioscler Thromb Vase Biol. 2006;26(3):508–513. doi: 10.1161/01.ATV.0000201039.21705.dc. http://dx.doi.org/10.1161/01.ATV.0000201039.21705.dc . [DOI] [PubMed] [Google Scholar]
- [6].Loftus IM, Naylor AR, Goodall S, Crowther M, Jones L, Bell PR, et al. Increased matrix metallo-proteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke. 2000;31(1):40–47. doi: 10.1161/01.str.31.1.40. http://dx.doi.org/10.1161/01.STR.31.1.40 . [DOI] [PubMed] [Google Scholar]
- [7].Sakai H, Shichiri M, Hirata Y. Hyperuricemia in diabetes mellitus. Nippon Rinsho. 2003;61:390–392. [PubMed] [Google Scholar]
- [8].McCormick M, Hadley D, McLean JR, Macfarlane JA, Condon B, Muir KW. Randomized controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol. 2010;67(5):570–578. doi: 10.1002/ana.21983. [DOI] [PubMed] [Google Scholar]
- [9].Hu G, Sarti C, Jousilahti P, Peltonen M, Qiao Q, Antikainen R, et al. The impact of history of hypertension and type 2 diabetes at baseline on the incidence of stroke and stroke mortality. Stroke. 2005;36(12):2538–2543. doi: 10.1161/01.STR.0000190894.30964.75. http://dx.doi.org/10.1161/01.STR.0000190894.30964.75 . [DOI] [PubMed] [Google Scholar]
- [10].Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. http://dx.doi.org/10.1161/01.STR.24.1.35 . [DOI] [PubMed] [Google Scholar]
- [11].Bruno A, Biller J, Adams HP, Jr, Clarke WR, Woolson RF, Williams LS, et al. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999;52(2):280–284. doi: 10.1212/wnl.52.2.280. http://dx.doi.org/10.1212/WNL.52.2.280 . [DOI] [PubMed] [Google Scholar]
- [12].Icks A, Claessen H, Morbach S, Glaeske G, Hoffmann F. Time-dependent impact of diabetes on mortality in patients with stroke: survival up to 5 years in a health insurance population cohort in Germany. Diabetes Care. 2012;35(9):1868–1875. doi: 10.2337/dc11-2159. http://dx.doi.org/10.2337/dc11-2159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular Complications of Diabetes: Focus on Stroke. Endocrine, Metabolic &Immune Disorders-Drug Targets. 2012;12(2):148–158. doi: 10.2174/187153012800493477. http://dx.doi.org/10.2174/1∋3012800493477 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chlumský J, Charvát J. Echocardiography and carotid sonography in diabetic patients after cerebrovascular attacks. J. Int. Med. Res. 2006;34(6):689–694. doi: 10.1177/147323000603400616. http://dx.doi.org/10.1177/147323000603400616 . [DOI] [PubMed] [Google Scholar]
- [15].Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70(14):1200–1207. doi: 10.1212/01.wnl.0000303969.63165.34. http://dx.doi.org/10.1212/01.wnl.0000303969.63165.34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7(2):184–190. doi: 10.1016/S1474-4422(08)70021-8. http://dx.doi.org/10.1016/S1474-4422(08)70021-8 . [DOI] [PubMed] [Google Scholar]
- [17].Banerjee C, Moon YP, Paik MC, Rundek T, Mora-McLaughlin C, Vieira JR, et al. Duration of Diabetes and Risk of Ischemic Stroke: The Northern Manhattan Study. Stroke. 2012;43(5):1212–1217. doi: 10.1161/STROKEAHA.111.641381. http://dx.doi.org/10.1161/STROKEAHA.111.641381 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haratz S, Tanne D. Diabetes, hyperglycemia and the management of cerebrovascular disease. Current Opinion in Neurology. 2011;24(1):81–88. doi: 10.1097/WCO.0b013e3283418fed. http://dx.doi.org/10.1097/WCO.0b013e3283418fed . [DOI] [PubMed] [Google Scholar]
- [19].Khoury JC, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Diabetes: a Risk Factor for Ischemic Stroke in a Large Bi-Racial Population. Stroke. 2013;44(6):1500–1504. doi: 10.1161/STROKEAHA.113.001318. http://dx.doi.org/10.1161/STROKEAHA.113.001318 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jeerakathil T, Johnson JA, Simpson SH, Majumdar SR. Short-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetes: a population-based cohort study. Stroke. 2007;38(6):1739–1743. doi: 10.1161/STROKEAHA.106.481390. http://dx.doi.org/10.1161/STROKEAHA.106.481390 . [DOI] [PubMed] [Google Scholar]
- [21].Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164(13):1422–1426. doi: 10.1001/archinte.164.13.1422. http://dx.doi.org/10.1001/archinte.164.13.1422 . [DOI] [PubMed] [Google Scholar]
- [22].Çetin S, Öztürk MA, Barındık N, İmren E, Peker Y. Increased coronary intervention rate among diabetic patients with poor glycaemic control: a cross-sectional study. Bosn J Basic Med Sci. 2014;14(1):16–20. doi: 10.17305/bjbms.2014.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gentile NT, Seftchick MW, Huynh T, Kruus LK, Gaughan J. Decreased Mortality by Normalizing Blood Glucose After Acute Ischemic Stroke. Acad Emerg Med. 2006;13(2):174–80. doi: 10.1197/j.aem.2005.08.009. http://dx.doi.org/10.1111/j.1553-2712.2006.tb01668.x . [DOI] [PubMed] [Google Scholar]
- [24].Cetin M, Kiziltunc E, Cetin ZG, Cicekcioglu H, Sahin M, Isik S, et al. Acetylsalicylic acid resistance in patients with Type 2 diabetes mellitus, prediabetes &non-diabetic coronary artery disease. Pak J Med Sci. 2014;30(3):539–544. doi: 10.12669/pjms.303.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bosevski M, Georgievska-Ismail L. Ultrasound measurement of peripheral endothelial dysfunction in type 2 diabetic patients: correlation with risk factors. Bosn J Basic Med Sci. 2010;10(2):84–88. doi: 10.17305/bjbms.2010.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Petznick AM, Shubrook JH. Treatment of specific macrovascular beds in patients with diabetes mellitus. Osteopathic Medicine and Primary Care. 2010;4:5. doi: 10.1186/1750-4732-4-5. http://dx.doi.org/10.1186/1750-4732-4-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Air EL, Kissela BM. Diabetes, the Metabolic Syndrome, and Ischemic Stroke: Epidemiology and Possible Mechanisms. Diabetes Care. 2007;30(12):3131–3140. doi: 10.2337/dc06-1537. http://dx.doi.org/10.2337/dc06-1537 . [DOI] [PubMed] [Google Scholar]
- [28].Darsalia V, Mansouri S, Ortsäter H, Olverling A, Nozadze N, Kappe C, et al. Glucagon-like peptide-1 receptor activation reduces ischemic brain damage following stroke in Type 2 diabetic rats. Clinical Science. 2012;122(10):473–483. doi: 10.1042/CS20110374. http://dx.doi.org/10.1042/CS20110374 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. http://dx.doi.org/10.1161/STR.0b013e3181f7d043. http://dx.doi.org/10.1161/STROKEAHA.111.614933 . [DOI] [PubMed] [Google Scholar]
- [30].Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–337. doi: 10.1016/S0140-6736(04)16721-4. http://dx.doi.org/10.1016/S0140-6736(04)16721-4 . [DOI] [PubMed] [Google Scholar]
- [31].Ntaios G, Egli M, Faouzi M, Michel P. J-shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke. 2010;41(10):2366–2370. doi: 10.1161/STROKEAHA.110.592170. http://dx.doi.org/10.1161/STROKEAHA.110.592170 . [DOI] [PubMed] [Google Scholar]
- [32].Uyttenboogaart M, Koch MW, Stewart RE, Vroomen PC, Luijckx GJ, De Keyser J. Moderate hyperglycaemia is associated with favorable outcome in acute lacunar stroke. Brain. 2007;130(Pt 6):1626–30. doi: 10.1093/brain/awm087. http://dx.doi.org/10.1093/brain/awm087 . [DOI] [PubMed] [Google Scholar]
- [33].Prasad K. Cerebrovascular disease in South Asia – Part II: Risk factors and prevention. J R Soc Med Cardiovasc Dis. 2012;1:21. doi: 10.1258/cvd.2012.012026. http://dx.doi.org/10.1258/cvd.2012.012026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen H, Qian K, Du Z, Cao J, Petersen A, Liu H, et al. Modeling ALS with iPSCs Reveals that Mutant SOD1 Misregulates Neurofilament Balance in Motor Neurons. Cell Stem Cell. 2014;14(6):796–809. doi: 10.1016/j.stem.2014.02.004. http://dx.doi.org/10.1016/j.stem.2014.02.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu J, Liu H, Huang CT, Chen H, Du Z, Liu Y, et al. Generation of Integration-free and Region-Specific Neural Progenitors from Primate Fibroblasts. Cell Rep. 2013;3:1580–1591. doi: 10.1016/j.celrep.2013.04.004. http://dx.doi.org/10.1016/j.celrep.2013.04.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lu J. Progress in Clinical-Grade Induced Human Neural Stem Cells: An Editorial. JSM Genet Genomics. 2013;1:2. [Google Scholar]
- [37].Lu J. Fueling Neuroscience with Human Pluripotent Stem Cells. International Journal of Clinical Therapeutics and Diagnosis. 2014;2:401. [Google Scholar]
- [38].Lu J. Reprogrammed Cells: How Far Away from the Clinical Use? Clon Transgen. 2014;3:e112. http://dx.doi.org/10.4172/2168-9849.1000e112 . [Google Scholar]
- [39].Lu J. Modeling Parkinson's Disease with Human Induced Pluripotent Stem Cells. Clon Transgen. 2014;3:e113. http://dx.doi.org/10.4172/2168-9849.1000e113 . [Google Scholar]
- [40].Lu J, Bradley RA, Zhang SC. Turning Reactive Glia into Functional Neurons in the Brain. Cell Stem Cell. 2014;14(2):133–134. doi: 10.1016/j.stem.2014.01.010. http://dx.doi.org/10.1016/j.stem.2014.01.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lu J, Tan L, Li P, Gao H, Fang B, Ye S, et al. All-trans retinoic acid promotes neural lineage entry by pluripotent embryonic stem cells via multiple pathways. BMC Cell Biol. 2009;10:57. doi: 10.1186/1471-2121-10-57. http://dx.doi.org/10.1186/1471-2121-10-57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Weick JP, Held DL, Bonadurer GF, 3rd, Doers ME, Liu Y, Maguire C, et al. Deficits in human trisomy 21 iPSCs and neurons. Proc Natl Acad Sci USA. 2013;110(24):9962–7. doi: 10.1073/pnas.1216575110. http://dx.doi.org/10.1073/pnas.1216575110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Williams EC, Zhong X, Mohamed A, Li R, Liu Y, Dong Q, et al. Mutant astrocytes differentiated from Rett syndrome patients-specific iPSCs have adverse effects on wild-type neurons. Hum Mol Genet. 2014;23(11):2968–80. doi: 10.1093/hmg/ddu008. http://dx.doi.org/10.1093/hmg/ddu008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. http://dx.doi.org/10.1016/j.cell.2007.11.019 . [DOI] [PubMed] [Google Scholar]
- [45].Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. http://dx.doi.org/10.1126/science.1172482 . [DOI] [PMC free article] [PubMed] [Google Scholar]


