Abstract
Obesity has a protective effect against osteoporosis and this effect has been attributed to a high body fat content. It has been shown that the leptin concentration is higher in obese patients. Leptin, the protein product of obesity gene, is a hormone produced in adipose tissue. Some studies suggest that endogenous leptin might influence bone metabolism in postmenopausal women. In this study, we investigated plasma leptin concentrations in postmenopausal women with osteoporosis and also analyzed the relationship between plasma leptin levels and bone mineral density (BMD) in order to understand the potential role of leptin in maintaining bone mass. Forty-two postmenopausal women with osteoporosis and thirty seven age and BMI-matched healthy postmenopausal women were included in the study. The mean femoral neck BMD value in the patient group was significantly lower than that in the control group (0.691±0.1 g/cm1 and 0.863±0.1 g/cm2, respectively; p<0.001). The mean plasma leptin concentration in the patient group was not significantly different from that in the control group (p>0.05). Plasma leptin levels were correlated with BMI in both groups (p<0.001 in the patient group and p=0.001 in controls). There was also a strong positive correlation between plasma leptin levels and %fat in both groups (p<0.001 in the patient group and p<0.001 in controls). But there was no correlation between plasma leptin levels and femoral neck BMD values in both groups. Our results do not support the hypothesis that leptin itself plays an important role in maintaining bone mass in postmenopausal women.
KEY WORDS: Bone density, leptin, obesity, postmenopausal osteoporosis
INTRODUCTION
Obesity is considered as one of the protecting factors against osteoporosis. Obese women usually show an increased bone mineral density (BMD) [1]. Although the exact mechanism is unclear, bone protective effects of obesity may involve increased aromatization of androgen to estrogen in adipose tissue [2,3], lowered sex hormone binding globulin levels [4], direct effects of high circulating levels of insulin on bone formation [1,5] or mechanical loading on bone tissue [6,7]. Leptin, the protein product of obesity (ob) gene, produced in adipose tissue and plays an important role in regulating of food intake and energy expenditure [8]. It has been shown that the leptin concentration is higher in obese patients, and also, reduction in body weight results in a significant decrease in leptin concentration [9]. Thomas et al. [10] showed that leptin acts on human bone marrow stromal cells to enhance osteoblastic differentiation in their in vitro study. Recent basic and clinical studies suggest that endogenous leptin might play some physiological role in maintaining bone mass and bone quality in postmenopausal women [11, 12]. On the other hand, some studies do not emphasize such an association between serum leptin and bone mineral density in postmenopausal women [13,14]. However, very little is known about the effects of leptin on bone metabolism in adult women.
In this study we assessed plasma leptin concentrations, % fat and BMD values in either osteoporotic and healthy postmenopausal women. We also analyzed the relationship between plasma leptin levels and BMD in order to understand the potential role of leptin to determine bone mass.
MATERIALS AND METHODS
Patients
Forty-two postmenopausal women with osteoporosis (mean ages of 58.2 ± 6.4 years and body mass index (BMI) of 28.6 ± 3.3 kg/m2) who admitted to our outpatient clinic were included in the study. Thirty seven age and BMI-matched healthy postmenopausal women (mean ages of 59.2 ± 7.8 years and BMI of 30.0 ± 3.9 kg/m2) who were selected from the same clinic served as controls. All subjects who had been natural postmenopausal for at least 1 year, gave written informed consent to participate in the study which was carried out in accordance with the Helsinki declaration. We excluded the subjects who had diabetes mellitus, cardiovascular disease, thyroid disorder or metabolic bone diseases and a history of trauma or smoking habits. None were taking medication influencing bone metabolism. Subjects were also excluded if they had vertebral compression fractures on lateral spine radiographs. Height and weight of all patients and controls were measured by standard procedure. BMI was calculated as the weight in kg per height in m2.
Biochemical measurements
Blood sampling
Specimens were collected using standard venipuncture technique as 12-16 h fasting blood and allowed to clot for 30 minutes. Then all samples were separated by centrifugation for 15 minutes at 1500 g and stored at -20°C until analysis day for leptin determinations.
Enzyme-Linked Immunosorbent Assay (ELISA) for quantitative measurement of leptin
The procedure (Diagnostic Systems Laboratories, Inc., Webster, Texas, USA) is an enzymatically amplified ‘two-step’ sandwich-type immunoassay. Intra-assay coefficient of variation is 1.5% for 46.3 ± 0.69 ng/ml.
BMD and body composition measurements
The diagnosis of osteoporosis was based on femoral neck BMD measurements. Patients with femoral neck BMD 2.5 standard deviations below a reference range established using our own data obtained from a Turkish population of normal healthy women using dual energy X-ray absorptiometry (T score less than -2.5) were accepted as having osteoporosis. BMD values at the femoral neck and percentage body fat (fat mass as percentage of body weight) were measured by dual energy X-ray absorptiometry using Norland XR 36-WBL (Fort Atkinson, WI, USA). Coefficient of variation of fat mass measurement was 2.2%.
Statistical analysis
Associations are given as Pearson’s correlation coefficients. Student t-tests were used for comparisons between groups. All tests were two-tailed, and a 5% significance level was maintained. These calculations were performed using the SPSS software, version 16.0 for Windows.
RESULTS
The clinical characteristics of the patient and control groups at baseline are shown in Table 1. The mean femoral neck BMD value in the patient group (0.691 ± 0.1 g/cm2, mean ± SD) was significantly lower than that in the control group (0.863 ± 0.1 g/cm2, mean ± SD; p<0.001). There was no significant difference in plasma leptin concentrations of both groups (p=0.469).
TABLE 1.
Baseline clinical characteristics of the patient and control groups.

Plasma leptin levels were correlated with BMI in both groups (r=0.536, p<0.001 in the patient group and r=0.506, p=0.001 in controls) (Figure 1). There was also a strong positive correlation between plasma leptin levels and percentage body fat in both groups (r=0.798, p<0.001 in the patient group and r=0.648, p<0.001 in controls) (Figure 2). But there was no correlation between plasma leptin levels and femoral neck BMD values in both groups (r=-0.195, p=0.216 in the patient group and r=0.112, p=0.511 in controls). A significant correlation was not observed between percentage body fat and femoral neck BMD values in both groups (r=-0.165, p=0.297 in the patient group and r=0.048, p=0.778 in controls).
FIGURE 1.
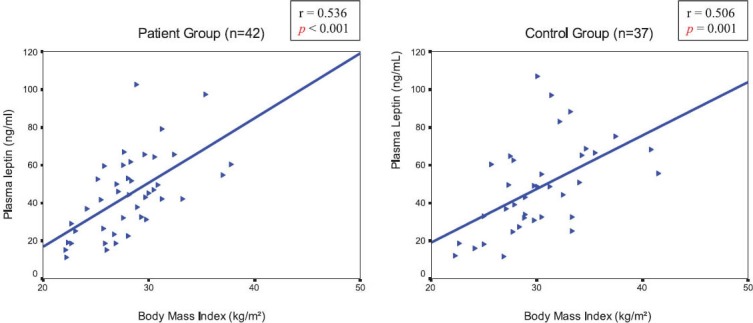
Correlation between plasma leptin levels and Body Mass Index in the patient and control groups.
FIGURE 2.
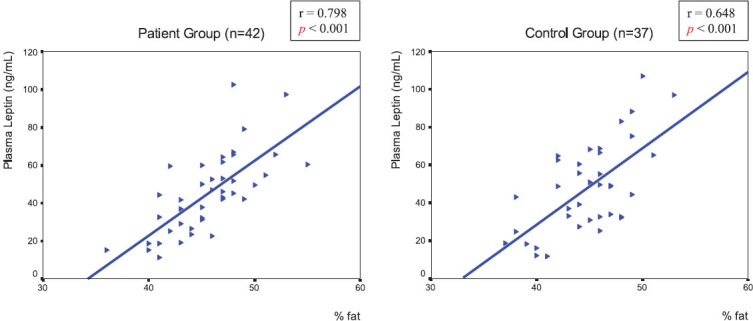
A strong positive correlation between percentage body fat and plasma leptin levels in the patient and control groups.
DISCUSSION
An in vitro study, it has been discovered that human bone marrow stromal cells also express high-affinity receptors for leptin and that leptin induces differentiation of stromal cells toward the osteoblastic lineage [10]. On the contrary, Ducy et al. [15] did not found any leptin receptors among osteoblast cells and intracerebroventricular infusion of leptin in ob/ob, db/db, and wild mice led to a rapid and massive decrease in bone mass, leading to the suggestion that leptin may inhibit bone formation through its binding to leptin receptors in the hypothalamus and to the hypothesis that bone remodeling is under central control. A number of investigators have addressed the role of leptin in human bone physiology. Most of them have found leptin to have no effect. Our present results are in accord with the previous findings by Rauch et al. [16], that none of the indices of bone density was significantly related to leptin serum concentrations before or after adjustment for body mass index. In postmenopausal women, Rauch et al. [16], and Goulding and Taylor [17] found no correlation between circulating leptin levels and markers of bone turnover and thus speculated that leptin played no significant role in the regulation of bone cell activity. Ruhl and Everhart [18] found that increasing levels of leptin were associated with higher BMD levels in 3054 pre- and postmenopausal women from NHANES III (Third U.S. National Health and Nutrition Examination Survey) but the association was no longer significant after adjusting for BMI. In the study of Shaarawy et al. [14], no relationships were found between BMD and serum leptin levels. A study in children has also shown that plasma leptin levels were not related to BMD [19]. There are also some studies suggesting a possible role for leptin in the regulation of human BMD. In a study of 123 postmenopausal women, aged 39-82 years, Martini et al. [20] showed significant positive associations between leptin and BMI (stronger with fat mass than lean mass), as well as with bone turnover markers and bone mass. In a study of 214 healthy nonobese Australian women aged 20-91 years, leptin was positively associated with bone mineral content (BMC) and BMD [21]. Yamauchi et al. [11] also demonstrated an effect of leptin on bone mass in 139 postmenopausal women, independent of percentage fat. In this group, plasma leptin (but not percentage fat) was significantly lower in women with vertebral fractures than in those without fractures.
Thomas et al. [12] demonstrated that serum leptin levels were significantly related to BMD in the pre- and postmenopausal women, but not in the men. Blain et al. [22] reported that leptin was significantly correlated with whole body and femoral neck BMD in a sample of postmenopausal, non-obese women and that this association was independent of the influence years after menopause, fat mass, creatinine clearance, calcium intake, and other hormonal factors exert on BMD. In this study, no correlation with BMDL2-L4 was found. Our data show that there was no significant difference between plasma leptin levels of healthy and osteoporotic postmenopausal women. Besides no significant correlation was observed between plasma leptin levels and bone mineral density values of the femoral neck. These findings suggest that circulating plasma leptin does not have a direct influence on bone mass in postmenopausal women. It is generally accepted that obesity is a major factor protecting against osteoporosis in women. Whether the protective effect is due to the increased weight load or enlarged adipose tissue mass exerting specific effects or to other possibilities, remain an open question. Leptin is an important regulator of the mass of adipose tissue and of body weight; it operates by inhibiting food intake and stimulating energy expenditure. It is suggested that plasma leptin concentrations directly correlate with BMI and the amount of body fat; obese subjects show higher levels than normal subjects and underweight subjects have extremely reduced leptin levels, which rise after partial weight recovery [9, 23, 24]. In similar, we found a strong positive correlation between percentage body fat and plasma leptin levels in both groups. However, we failed to show such an relation between percentage body fat and BMD values. In contrast to our results, as Reid et al. [25] reported that BMD is strongly related to fat mass in post- menopausal women. Chen et al. [26] and Kandeel et al. [27] concluded that bone mineral mass is more closely related to lean tissue mass than to fat tissue mass in postmenopausal women and premenopausal women, respectively. Bedogni et al. [28] concluded that total bone mineral content (BMC) is more associated with lean tissue mass than with fat mass. According to the results of not only in vivo but also in vitro studies, the main effect of leptin on BMD remains controversial. These contradictory results might result from the different population groups. Further studies including testing of multiple genes in both obese and lean subjects, with epidemiologic data on dietary habits in different ethnic groups, are necessary to better understand the role of leptin in regulating weight in human populations. In summary, our results do not support the hypothesis that leptin itself does not have an important role in maintaining bone mass in postmenopausal women.
CONCLUSION
We have investigated plasma leptin concentrations in postmenopausal women with osteoporosis and also analyzed the relationship between plasma leptin levels and bone mineral density in order to understand the potential role of leptin in maintaining bone mass. The results of the present study do not support the hypothesis that leptin itself plays an important role in maintaining bone mass in postmenopausal women. However, future studies on the current topic are recommended.
DECLARATION OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Albala C, Yanez M, Devoto E, Sostin C, Zeballos L, Santos JL. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord. 1996;20:1027–1032. [PubMed] [Google Scholar]
- [2].Frumar AM, Meldrum DR, Geola F, Shamonki IM, Tataryn IV, Deftos LJ, et al. Relationship of fasting urinary calcium to circulating estrogen and body weight in postmenopausal women. J Clin Endocrinol Metab. 1980;50:70–75. doi: 10.1210/jcem-50-1-70. [DOI] [PubMed] [Google Scholar]
- [3].Revilla M, Villa LF, Sanchez-Atrio A, Hernandez ER, Rico H. Influence of body mass index on the age-related slope of total and regional bone mineral content. Calcif Tissue Int. 1997;61:134–138. doi: 10.1007/s002239900310. [DOI] [PubMed] [Google Scholar]
- [4].Reid IR, Evans MC, Ames RW. Volumetric bone density of the lumbar spine is related to fat mass but not lean mass in normal postmenopausal women. Osteoporos Int. 1994;4:362–367. doi: 10.1007/BF01622199. [DOI] [PubMed] [Google Scholar]
- [5].Reid IR, Evans MC, Cooper GJ, Ames RW, Stapleton J. Circulating insulin levels are related to bone density in normal postmenopausal women. Am J Physiol. 1993;265:655–659. doi: 10.1152/ajpendo.1993.265.4.E655. [DOI] [PubMed] [Google Scholar]
- [6].Slemenda CW. Body composition and skeletal density: mechanical loading or something more? J Clin Endocrinol Metab. 1995;80:1761–1763. doi: 10.1210/jcem.80.6.7775618. [DOI] [PubMed] [Google Scholar]
- [7].Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- [9].Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- [10].Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- [11].Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, et al. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in post- menopausal women. Clin Endocrinol (Oxf) 2001;55(3):341–347. doi: 10.1046/j.1365-2265.2001.01361.x. [DOI] [PubMed] [Google Scholar]
- [12].Thomas T, Burguera B, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Riggs BL, et al. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29:114–120. doi: 10.1016/s8756-3282(01)00487-2. [DOI] [PubMed] [Google Scholar]
- [13].Roux C, Arabi A, Porcher R, Garnero P. Serum leptin as a determinant of bone resorption in healthy postmenopausal women. Bone. 2003;33:847–852. doi: 10.1016/j.bone.2003.07.008. [DOI] [PubMed] [Google Scholar]
- [14].Shaarawy M, Abassi AF, Hassan H, Salem ME. Relationship between serum leptin concentrations and bone mineral density as well as biochemical markers of bone turnover in women with post- menopaual osteoporosis. Fertil Steril. 2003;79:919–924. doi: 10.1016/s0015-0282(02)04915-4. [DOI] [PubMed] [Google Scholar]
- [15].Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- [16].Rauch F, Blum WF, Klein K, Allolio B, Schönau E. Does leptin have an effect on bone in adult women? Calcif Tissue Int. 1998;63:453–455. doi: 10.1007/s002239900556. [DOI] [PubMed] [Google Scholar]
- [17].Goulding A, Taylor RW. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 1998;63(6):456–458. doi: 10.1007/s002239900557. [DOI] [PubMed] [Google Scholar]
- [18].Ruhl CE, Everhart JE. Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res. 2002;17(10):1896–1903. doi: 10.1359/jbmr.2002.17.10.1896. [DOI] [PubMed] [Google Scholar]
- [19].Klein KO, Larmore KA, de Lancey E, Brown JM, Considine RV, Hassink SG. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab. 1998;83:3469–3475. doi: 10.1210/jcem.83.10.5204. [DOI] [PubMed] [Google Scholar]
- [20].Martini G, Valenti R, Giovani S, Franci B, Campagna S, Nuti R. Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone. 2001;28:113–117. doi: 10.1016/s8756-3282(00)00408-7. [DOI] [PubMed] [Google Scholar]
- [21].Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM, et al. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab. 2001;86:1884–1887. doi: 10.1210/jcem.86.5.7417. [DOI] [PubMed] [Google Scholar]
- [22].Blain H, Vuillemin A, Guillemin F, Durant R, Hanesse B, de Talance N, et al. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2002;87(3):1030–1035. doi: 10.1210/jcem.87.3.8313. [DOI] [PubMed] [Google Scholar]
- [23].Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, et al. Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3861–3863. doi: 10.1210/jcem.81.11.8923829. [DOI] [PubMed] [Google Scholar]
- [24].Ferron F, Considine RV, Peino R, Lado IG, Dieguez C, Casanueva FF. Serum leptin concentrations in patients with anorexia nervosa, bulimia nervosa and non-specific eating disorders correlate with the body mass index but are independent of the respective disease. Clin Endocrinol (Oxf) 1997;46(3):289–293. doi: 10.1046/j.1365-2265.1997.1260938.x. [DOI] [PubMed] [Google Scholar]
- [25].Reid IR, Ames RW, Evans MC, Sharpe SJ, Gamble GD. Determinants of the rate of bone loss in normal postmenopausal women. J Clin Endocrinol Metab. 1994;79:950–954. doi: 10.1210/jcem.79.4.7962303. [DOI] [PubMed] [Google Scholar]
- [26].Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M. Fat or lean tissue mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res. 1997;12:144–151. doi: 10.1359/jbmr.1997.12.1.144. [DOI] [PubMed] [Google Scholar]
- [27].Kandeel WA, Zaki ME, El Din AMS, Anwar M. Bone mineral density, body composition, and serum leptin in premenopausal Egyptian women. Aust. J. Basic Appl. Sci. 2009;3(3):1964–1971. [Google Scholar]
- [28].Bedogni G, Pietrobelli A, Heymsfield SB, Rountauroli C, Borghi A, Ferrari F, et al. Influence of body composition on bone content in elderly women. A preliminary report. Ann NY Acad Sci. 2000;904:489–490. doi: 10.1111/j.1749-6632.2000.tb06504.x. [DOI] [PubMed] [Google Scholar]


