Abstract
The aim of study was to evaluate endocrine changes in PCOS women during metformin treatment. One hundred women with PCOS, aged 20-40 years were included. A complete hormonal and metabolic pattern was recorded for each subject every 6 months. Metformin treatment after 6 and 12 months significantly reduced weight, BMI, waist circumference, insulin and HOMA-IR (p=0.000) with high differences of variances within repeated measurements. There was significant reduction of PRL, testosterone and estradiol (p=0.000) with small differences within repeated measurements. Metformin did not have effect on TSH. However, results showed important reduction of CRP, LH, LH/FSH, androstendione, DHEA-S and progesterone (p=0.000) with moderate differences within measures. Metformin restored menstrual cyclicity in most participants. At baseline in study group was 69% women with oligomenorrhoea, amenorrhoea or polymenorrhoea. After 12 months of treatment, only 20% PCOS women had irregular menstrual cycle (p=0.000). Hirsutism was also reduced. Intriguingly, during first 6 months of treatment in PCOS women 9 pregnancies occurred (p=0.000), while during last 6 months treatment were 2 pregnancies (p=0.317), in total 11(13%). Multiple regression model revealed that the presence of anovulation in PCOS women was strongly associated with BMI, waist, FSH and age. Insulin resistance was significantly predicted by BMI, cholesterol, progesterone and presence of hirsutism. The metformin therapy significantly improved insulin resistance, imbalance of endocrine hormones, hirsutism and menstrual cyclicity in women with PCOS. The most important predictors for duration of metformin treatment in PCOS women were testosterone, progesterone, FSH, CRP and presence of anovulation.
KEY WORDS: endocrine changes, PCOS, metformin treatment
INTRODUCTION
The aetiology of the neuroendocrine irregularities in women with PCOS remains uncertain; though, latest studies have shown decreased sensitivity of the gonadotropin-releasing hormone (GnRH) pulse generator to inhibition by ovarian steroids, mostly progesterone [1]. Women with polycystic ovary syndrome (PCOS) need higher levels of progesterone to slow the frequency of GnRH pulse secretion, resulting in insufficient plasma follicle-stimulating hormone (FSH) synthesis and persistent plasma luteinizing hormone (LH) stimulation of ovarian androgens. In hyperandrogenemic girls certain to develop PCOS, increase in ovarian steroids may not be adequate to suppress the GnRH pulse generator, leading to a persistently rapid LH pulse frequency, reduced FSH production, and insufficient follicular development [2]. PCOS is connected with nearly 75% of women who suffer from infertility due to anovulation [3]. PCOS is often accompanying to irregular gonadotropin levels, lower levels of insulin growth factor-binding protein-1 (IGF-BP1), increased insulin resistance and increased ovarian 17-hydroxiprogester- one (17-OHP) and androgen answers to GnRH-agonists [4]. The main disturbances in this syndrome are: 1. Abnormal morphology of the ovary, determined by more than 12 small follicles per ovary measuring 2-9 mm in diameter with increased central stroma or an ovarian volume over 10 ml on transvaginal ultrasound examination of the ovaries [5, 6, 7]; 2. Abnormal steroidogenesis - hyperandrogenism [8]; 3. Oligo-anovulation [9]. Insulin resistance is a common piece of PCOS, and there is known association between high insulin concentrations and anovulatory infertility [10]. Hyperinsulinemia existed in about 80% of obese PCOS women and in 30–40% of normal weight women with PCOS [11]. Management of PCOS depends on the symptoms [12]. Therapy for PCOS becomes necessary in adults in order to induce ovulatory cycles and fertility, and to improve cosmetic appearance [13]. Metformin is an insulin sensitizing drug that has been recently presented for treating PCOS women [14]. Metformin increases insulin sensitivity in the liver by reducing gluconeogenic enzyme activities, inhibiting hepatic uptake of lactate and alanine, increasing the conversion of pyruvate to alanine and inhibiting glucose output [15]. Clinical trials have shown that metformin can effectively reduce androgens, improve insulin sensitivity, and simplify weight loss in patients with PCOS [16]. However, a recent large randomized study of more than 600 women reported no improvement in fertility with use of extended release metformin in women with PCOS compared with clomiphene [17]. If pregnancy still escapes women with PCOS after initial pharmacologic treatments, gonadotropin therapy by itself or in conjunction with assisted reproductive therapy is considered [18]. There are claims that metformin use during pregnancy reduces miscarriage rates and that neonatal and infant outcomes are equivalent to those of the general population, although widespread use of metformin in early pregnancy should be discouraged until more data are available [19]. The aim of this study was to evaluate clinical, hormonal and metabolic changes in PCOS women during metformin treatment.
MATERIALS AND METHODS
Patients
A total of 137 women with PCOS diagnosed last two years were interweaved for inclusion in the study. One hundred women with PCOS, aged 20-40 years were included. After 6-months of the metformin treatment, 5 women were excluded because of non-compliance. There were eleven pregnancies and these patients excluded from the study, but not stopped with metformin during pregnancy. Therefore, the number of women with PCOS available for final statistical analysis after 12 months of treatment was 84.
Procedures
The procedures used were in accordance with the guidelines of the Helsinki Declaration on Human Experimentation and the Good Clinical Practice (CGP) guidelines. PCOS diagnosis was made in accordance with Rotterdam criteria. Patients to be included in the study were required not to have received any medication for PCOS, or for other conditions associated with insulin resistance, within the last 6 months. Exclusion criteria were considered as: age less than 20 or higher than 40 years, presence of neoplastic, endocrine, metabolic, hepatic and cardiovascular disorders or other concurrent medical illnesses. Biochemical, clinical, and ultrasounds data, performed at baseline, at 6-months and 12-months follow-up were collected. Fasting serum glucose, basal insulin, HOMA-IR index, FSH, LH, PRL, TSH, total testosterone, DHEA-S, androstendione, CRP and lipid profile were determined at follicular phase. Every 6 months all parameters were re-evaluated. Homeostasis model analysis (HOMA) [fasting glucose (mmol/l) × fasting insulin (μU/ml)/22.5] was also calculated. Anthropometric measurements (height, weight, BMI and waist), Ferriman-Gallwey score and ultrasonography data were noted for each subject. Two months before starting metformin treatment, into all patients were included lifestyle changes by diet and exercise. All patients have taken metformin two or three times a day during meals (1000-1500 mg/day) and followed during 12 months. The treatment was well tolerated. Normal ovulatory status was defined by plasma progesterone assay (>22 nmol/l) performed seven days before the expected menses and by the presence of regular menstrual bleedings in three consecutive evaluations.
Statistical analysis
The normal distribution of continuous variables was evaluated by using the Kolmogorov-Smirnov test, and continuous data were expressed as mean ± standard deviation (SD). Variables without normal distribution were square root transformed to normalize distributions. The difference of repeated measures of continuous variables were analysed with the oneway analysis of variance (ANOVA) for repeated measures with Bonferroni test for the post-hoc analysis. The Friedman test was performed for testing difference of repeated measures of categorical variables. Multiple regression analysis was performed to identify independent predictors. The results were expressed as odds ratios (ORs) with 95% confidence intervals. The Statistics Package for Social Science (SPSS 20) was used; p value <0.05 was considered statistically significant.
RESULTS
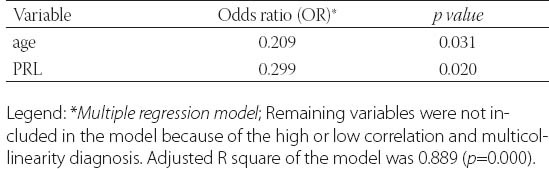
Clinical data from patients in this study was detailed in Table 1. and Table 2. Metformin treatment after 6 and 12 months significantly reduced weight, body mass index (BMI) and waist circumference (p=0.000). Metformin has very strong effect on weight, BMI and waist (Partial Eta Squared 0.75, 0.70 and 0.78). Evaluated differences of variances for weight, BMI and waist within repeated measurement were statistical significant (Wilks’ lambda 0.25, 0.31 and 0.22). Clinical and hormonal values were square root transformed to normalize distributions. Continuous variables were analysed with the one-way analysis of variance for repeated measures with Bonferroni test for the post-hoc analysis. Fasting glucose, cholesterol and triglycerides also significantly decreased (p=0.000) but with not to strong metformin’s effect on these variables. Level of fasting insulin and HOMA-IR index decreased very significantly (p=0.000). Multiple regression analysis sugests very strong effect of metformin on insulin and IR (0.87) with high differences within measures at the same time (Wilks’ lambda 0.14 and 0.13). The data presented significant reduction of the levels of PRL, total testosterone and estradiol (p=0.000) but with not to strong metformin’s effect (Partial Eta Squared 0.20, 0.22 and 0.32) and with small differences within groups (Wilks’ lambda 0.80, 0.78 and 0.68). Metformin did not have effect on TSH (p=0.252, Partial Eta Squared 0.03) and there was strong difference within groups (Wilks’ lambda 0.97). Similar effect was and on FSH (Partial Eta Squared 0.09) with significant difference within measures. Furthermore, results showed important reduction of CRP, LH, LH/FSH, androstendione, DHEA-S and progesterone (p=0.000; Partial Eta Squared 0.71, 0.63, 0.52, 0.46, 0.40 and 0.75) with moderate differences within groups (0.29, 0.37, 0.48, 0.54, 0.60 and 0.25). Bonferroni test is used to adjust for multiple comparisons. Metformin restored menstrual cyclicity in most participants (Table 2). At baseline in study group was 69% women with oligomenorrhoea/amenorrhoea/polymenorrhoea, after 6 months that number was 45% and after 12 months only 20% PCOS women had irregular menstrual cycle (p=0.000) with significant differences between all measures (p=0.000). Hirsutism FG score was also reduced during treatment with differences between second and third (p=0.003) and first and third measure (p=0.004) and without differences between first and second measure (p=0.86). At beginning of the research, 52% of PCOS patients had bilateral PCO. Ovarian morphology and volume changed in 12% of subjects after 6 months and in 19% of subjects after 12 months of treatment. Ultrasonography showed no difference between first and second examination (0.70) but with significant difference in frequency of PCO between second and third and between first and third examination (p=0.000) Eighty six of these subjects remained anovulatory despite treatment. After six months of treatment, this number became 40% and at the end of 12 months treatment this number reduced to 21% (p=0.000). Lower difference was between second and third evaluation (p=0.014) than between first and second (p=0.000) and between first and third evaluation (p=0.000). Intriguingly, during first 6 months of metformin treatment in PCOS women 9 (11%) pregnancies occurred (p=0.000), while during last 6 months of treatment happened 2 pregnancies (p=0.317), in total 11 (13%). Between second and third evaluation there was no difference. There was one reduces miscarriage rate, due to stop of metformin treatment during pregnancy. There was no congenital anomaly at birth and eleven infants developed well at one year follow-up. Multiple regression model was performed to evaluate the anovulation risk in PCOS women as a function of anthropometric, metabolic and hormonal values (Table 3). Because of the high correlation between anovulation and progesterone (r =0.9), it was not included in the model. On the other side, due to very low correlations between anovulation and age, FSH, LH, TSH, PRL, androgens, CRP, triglycerides, they were not included in the model. Multicollinearity diagnosis for glucose, insulin and IR, excluded them from the model. Adjusted R square of the model was 0.948 (p=0.000). It revealed that the presence of anovulation in PCOS women was associated with BMI, waist, FSH and age as independent predictors. The most important factors for metformin treatment duration in PCOS women were testosterone and progesterone (p=0.000), presence of anovulation (p=0.000), FSH and CRP (Table 4.). Because of the high correlation between glucose, insulin and IR, they were excluded from the regression model. Remaining variables excluded from the model because of low correlation or multicollinearity diagnosis. Adjusted r square of the model was 0.939 (p=0.000). Insulin resistance was significantly predicted by BMI, cholesterol, progesterone and presence of hirsutism (Table 5). Because of the high correlation (r >0.7) between glucose, insulin and IR, they were not included in the model. Due to very low correlations (r<0.3) between insulin and age, FSH, LH, TSH, PRL, androgens, CRP, triglycerides, they were also not included in the model. Adjusted R square of the model was 0.772 (p=0.000). Pregnancy depends on balance of all metabolic, endocrine and anthropometric parameters (p=0.000). Adjusted R square of the model was 0.889 (p=0.000). In the final model multicollinearity analysis showed only age and level of PRL as independent factors for pregnancy in women with PCOS treated with metformin (Table 6).
TABLE 1.
Clinical and laboratory characteristics of the study population with test of variances for repeated measures
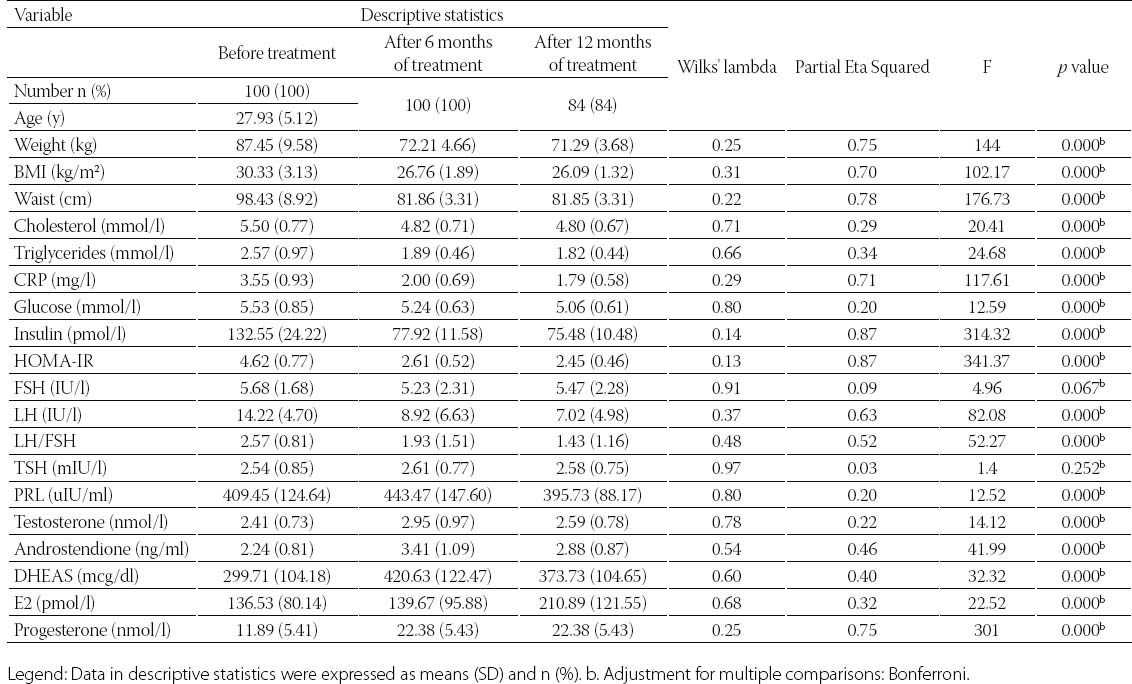
TABLE 2.
Friedman test of mean differences for dependent categorical variables
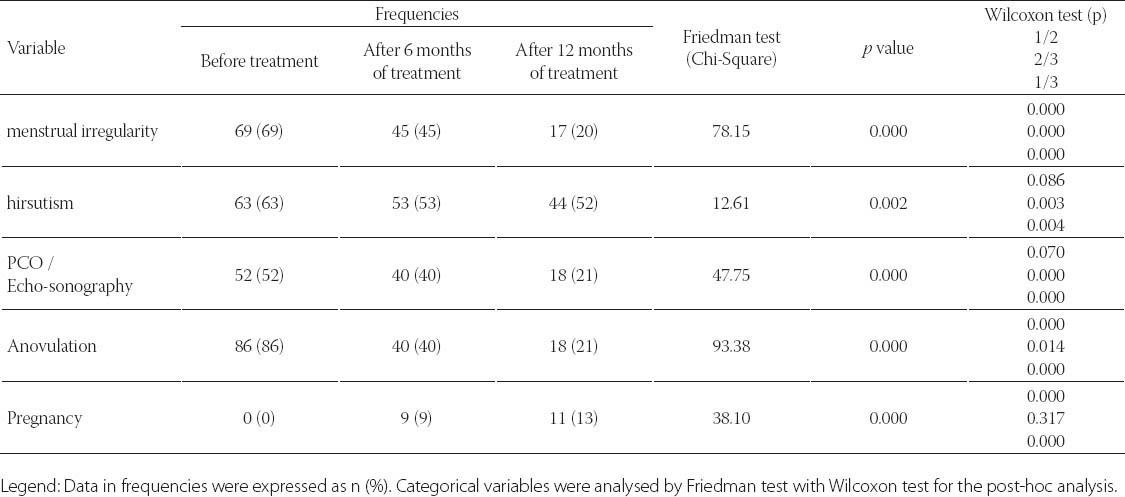
TABLE 3.
Anovulation risk in PCOS women as a function of anthropometric, metabolic and hormonal markers
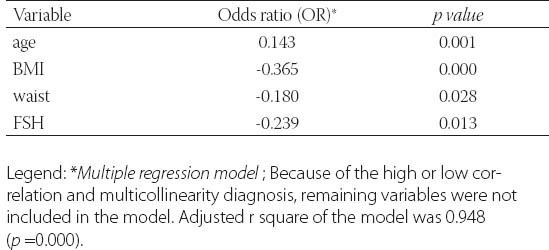
TABLE 4.
The predictors of metformin treatment duration in PCOS women
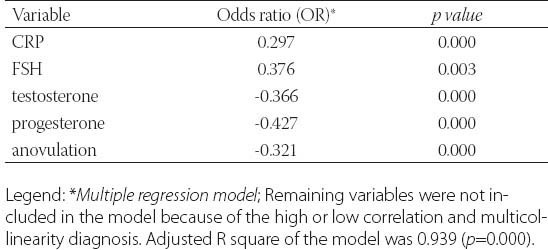
TABLE 5.
The predictors of insulin resistance in PCOS women
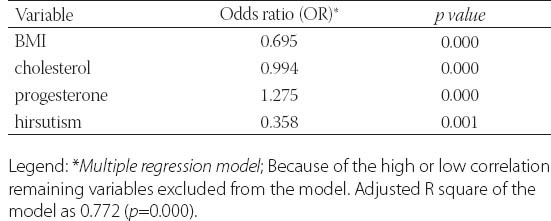
TABLE 6.
Independent factors of pregnancy in PCOS women treated with metformin
DISCUSSION
The effect of metformin on endocrine, metabolic and anthropometric parameters in patients with PCOS has been described in several studies. Furthermore, to our knowledge, this is one of the rarely studies describing all these parameters in the same time, and exploring its relationship with duration of metformin treatment. A recent position statement from the Androgen Excess and PCOS Society recommended that women with PCOS, regardless of weight, to be screened for IGT or type 2 DM by an oral glucose tolerance test at their initial presentation and every 2 years thereafter. However, this statement noted that the use of metformin to treat or prevent the progression of IGT could be considered but should not be mandated at this point in time, as well-designed randomized controlled trials demonstrating efficacy have yet to be conducted [20]. In the present research, metformin treatment after 6 and 12 months significantly reduced weight, BMI and waist circumference (p=0.000). High waist value suggested visceral adiposity.
Hyperinsulinemia is an important parameter in deciding whether or not to initiate metformin therapy to PCOS women with the expectation of preventing the onset of type 2 diabetes mellitus [21].
Fasting insulin and IR in present research, significantly decreased during metformin treatment in PCOS women (p=0.000). Insulin resistance was significantly predicted by BMI, waist, cholesterol, progesterone and presence of hirsutism.
In meta-analysis of Salpeter et al. [22] women with PCOS experienced a 5.3% decrease in BMI, a 2.6% mean decrease in fasting glucose, and an 19.7% improvement in IR as assessed by the HOMA. Fasting insulin decreased by 5.7%, although the difference did not reach significance. In addition, HDL cholesterol increased by a mean of 9.4% whereas triglycerides decreased by 11.9%. These results were of similar magnitude to those of non-PCOS, except the measurement of fasting insulin, which in non-PCOS was improved by a mean of 16.1% [22]. Cardiovascular risk including markers of sub-clinical inflammation, and dyslipidemia may also be improved by metformin therapy [21].
Likewise, we also observed significantly reduction of CRP and lipids level by metformin therapy (p=0.000). Metformin plays significant role in improving ovulation induction in women with PCOS through a reducing insulin levels and altering the effect of insulin on ovarian androgen biosynthesis, theca cell proliferation, and endometrial growth. Likewise, potentially through a direct effect, it inhibits ovarian gluconeogenesis and consequently reduces ovarian androgen production [23]. Metformin increased menstrual cyclicity, improved ovulation, and a reduction in circulating androgen levels in PCOS women [24].
Present study described significantly reduction of anovulatory menstrual cycle, menstrual irregularity, hirsutism and PCO morphology in PCOS women treated with metformin (p=0.000). The presence of anovulation in PCOS women was strongly associated with BMI, waist, FSH and age. Therefore, the data of this research presented significantly reduction of the levels of total testosterone, DHEA-S, androstendione and PRL (p=0.000). Metformin modulates the reproductive axis, affecting the release of GnRH and LH. Tosca et al. [25] concluded that, in rat pituitary cells, metformin decreases gonadotropin secretion and MAPK3/1 phosphorylation induced by GnRH and FSH release. In a prospective, controlled and randomized trial Billa et al. [26] determined that the metformin administration lowered LH activity in all PCOS women and in ovulatory responders and also compromised PRL stimulated secretion in the latter cases. These findings have indicated an effect of metformin on pituitary activity. Present results described significant decreasing of LH and LH/FSH level in PCOS women during metformin treatment. Palomba et al. concluded that in patients with PCOS, who are at high risk for ovarian hyperstimulation syndrome (OHSS) and who have been stimulated with gonadotropins for in-vitro fertilisation (IVF) cycles, metformin reduces the risk of OHSS by modulating the ovarian response to the stimulation [27]. Cycle cancellation rate under metformin resulted with significant influence predisposed by interaction with BMI, age and basal FSH levels [28]. According to current study result metformin did not have effect on TSH.
Contrary to present results one of the studies showed that metformin treatment has a TSH-lowering effect in hypothyroid patients with PCOS, both treated with l-thyroxin and untreated [29]. Pregnancy depends on balance of the most metabolic, endocrine and anthropometric parameters (p=0.000). Perhaps, this is some kind of answer on the question how long to treat PCOS with metformin.
CONCLUSIONS
The metformin therapy significantly improved insulin resistance, imbalance of endocrine hormones, hirsutism and menstrual cyclicity. Pregnancy depends on balance of the metabolic, endocrine and anthropometric parameters. The most important factors for duration of metformin treatment in PCOS women were testosterone, progesterone, FSH, CRP and presence of anovulation. Further prospective studies are needed to explore relationship of endocrine changes and long-term metformin treatment of PCOS women.
DECLARATION OF INTEREST
Author declares that there is no conflict of interest.
REFERENCES
- [1].Pastor CL, Griffm-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. doi: 10.1210/jcem.83.2.4604. [DOI] [PubMed] [Google Scholar]
- [2].McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20(4):317–326. doi: 10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- [3].Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- [4].Carmina E, Orio F, Palomba S, Longo RA, Lombardi G, Lobo RA. Ovarian size and blood flow in women with polycystic ovary syndrome and their correlations with endocrine parameters. Fertil Steril. 2005;84(2):413–419. doi: 10.1016/j.fertnstert.2004.12.061. [DOI] [PubMed] [Google Scholar]
- [5].Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [6].Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- [7].Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9:505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- [8].Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Esco-bar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- [9].Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- [10].Norman RJ, Noakes M, Wu R, Davies MJ, Moran L, Wang JX. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update. 2004;10:267–280. doi: 10.1093/humupd/dmh018. [DOI] [PubMed] [Google Scholar]
- [11].Dunaif A, Segal K, Futterweit W, Dobrjansky A. Profound peripheral resistance independent of obesity in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- [12].Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3:25–35. doi: 10.2147/IJWH.S11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Geller D, Pacaud D, Gordon C, Misra M. Emerging Therapies: the use of insulin sensitizers in the treatment of adolescents with polycystic ovary syndrome (PCOS) Int J Pediatr Endocrinol 2011. 2011:9. doi: 10.1186/1687-9856-2011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Palomba S, Falbo A, Zullo F, Orio F., Jr Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009;30(1):1–50. doi: 10.1210/er.2008-0030. [DOI] [PubMed] [Google Scholar]
- [15].Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosi- glitazone and metformin stimulate AMP-activated protein kinase through distinct signalling pathways. J Biol Chem. 2002 Jul 12;277(28):25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- [16].La Marca A, Artensio AC, Stabile G, Volpe A. Metformin treatment of PCOS during adolescence and the reproductive period. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):3–7. doi: 10.1016/j.ejogrb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [17].Vanky E, Stridsklev S, Heimstad R, Romundstad P, Skogøy K, Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010;95(12):E448–455. doi: 10.1210/jc.2010-0853. [DOI] [PubMed] [Google Scholar]
- [18].McFarland C. Treating polycystic ovary syndrome and infertility. MCN Am J Matern Child Nurs. 2012;37(2):116–121. doi: 10.1097/NMC.0b013e31824239ce. [DOI] [PubMed] [Google Scholar]
- [19].Norman RJ. Editorial: Metformin--comparison with other therapies in ovulation induction in polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(10):4797–800. doi: 10.1210/jc.2004-1658. [DOI] [PubMed] [Google Scholar]
- [20].Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome-- a position statement of the Androgen Excess Society. Clin Endocrinol Metab. 2007;92(12):4546–4556. doi: 10.1210/jc.2007-1549. [DOI] [PubMed] [Google Scholar]
- [21].Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199(6):596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- [22].Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: met-formin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121:149–157. doi: 10.1016/j.amjmed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- [23].Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3:25–35. doi: 10.2147/IJWH.S11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- [25].Tosca L, Rame C, Chabrolle C, Tesseraud S, Dupont J. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction. 2010;139:409–418. doi: 10.1530/REP-09-0351. [DOI] [PubMed] [Google Scholar]
- [26].Billa E, Kapolla N, Nicopoulou SC, Koukkou E, Venaki E, Milin-gos S, et al. Metformin administration was associated with a modification of LH, prolactin and insulin secretion dynamics in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2009;25(7):427–434. doi: 10.1080/09513590902770172. [DOI] [PubMed] [Google Scholar]
- [27].Palomba S, Falbo A, Carrillo L, Villani MT, Orio F, Russo T, et al. METformin in High Responder Italian Group. Metformin reduces risk of ovarian hyperstimulation syndrome in patients with polycystic ovary syndrome during gonadotropin-stimulated in vitro fertilization cycles: a randomized, controlled trial. Fertil Steril. 2011;96(6):1384–1390. doi: 10.1016/j.fertnstert.2011.09.020. [DOI] [PubMed] [Google Scholar]
- [28].Palomba S, Falbo A, Russo T, Di Cello A, Morelli M, Orio F, et al. Metformin administration in patients with polycystic ovary syndrome who receive gonadotropins for in vitro fertilization cycles:10-year experience in a large infertile population. Gynecol Endocrinol. 2012;28(2):81–86. doi: 10.3109/09513590.2011.588749. [DOI] [PubMed] [Google Scholar]
- [29].Rotondi M, Cappelli C, Magri F, Botta R, Dionisio R, Iacobello C, De Cata P, Nappi RE, Castellano M, Chiovato L. Thyroidal Effect of Metformin Treatment in Patients With Polycystic Ovary Syndrome. Clin Endocrinol. 2011;75(3):378–38. doi: 10.1111/j.1365-2265.2011.04042.x. [DOI] [PubMed] [Google Scholar]


