Abstract
Lesatropane, a synthesized chiral tropane (3S, 6S-isomer of satropane), is a novel muscarinic agonist, and is being under preclinical development in China for the treatment of primary glaucoma. The reports concerning that activation of muscarinic acetylcholine receptors (mAChRs) could protect cells against apoptosis prompted us to study the neuroprotective effects of lesatropane and the mechanism. We found that lesatropane could protect PC12 cells from glutamate-induced neurotoxicity and reverse the decreased ERK1/2 activation caused by glutamate. Atropine or pirenzepine, antagonist of mAChR or M1 mAChR, antagonized the protective effects of lesatropane respectively and suppressed the lesatropane’s effects on ERK1/2. Furthermore, chelerythrine, a PKC inhibitor, partially suppressed ERK1/2 activation induced by lesatropane. The results indicated that the specific M1 mAChR via PKC-ERK1/2 pathway might be involved in the neuroprotective effects of lesatropane. While M1 mAChR is a therapeutic target of Alzheimer’s disease (AD), the results of this paper contribute to further information concerning the activation of M1 mAChR as a therapeutic target in AD.
KEY WORDS: muscarinic agonist, neuroprotective effects, glutamate, PC12 cells
INTRODUCTION
Tropane derivatives (atropine, scopolamine, etc.) usually serve as muscarinic antagonists. Interestingly, lesatropane (Figure 1.), a chiral tropane derivative (3S, 6S-isomer of satropane) synthesized in our laboratory, is a novel muscarinic agonist [1,2], and is being under preclinical development in China for the treatment of primary glaucoma [3]. Muscarinic acetylcholine receptors (mAChRs) have five subtypes (M1-M5). It has been reported that activation of the Gq/11-coupled mAChRs (M1, M3, M5) could protect cells against apoptosis induced by DNA damage, oxidative stress, impaired mitochondrial function, serum deprivation or UV irradiation [4-6]. Glutamate is a major excitatory neurotransmitter in the brain and also could lead to neuronal injury or cell death when it released in excess or overstimulated its membrane receptors [7]. Previously we reported that activation of the M1 mAChR could protect rat retinal ganglion cells against glutamate-induced neuronal apoptosis [8]. It is interesting to confirm the neuroprotective effects mediated through M1 mAChR using PC12 cells, since PC12 cells as a common model to evaluate the neuroprotective effects of certain compounds, endogenously express mAChRs, predominantly the M1 mAChR. In this paper, we show that lesatropane protects PC12 cells from glutamate - induced neurotoxicity and the M1 mAChR is mainly responsible for the neuroprotective effects partially through the PKC-ERK1/2 pathway. Because of the important role of M1 mAChR in cognitive processes, it has long been aimed at a high-profile target for the treatment of neurodegenerative disorders like Alzheimer’s disease (AD) [9]. The study of activation of M1 mAChR against glutamate-induced neurotoxicity may help to validate selective M1 mAChR agonist for the treatment of AD.
FIGURE 1.
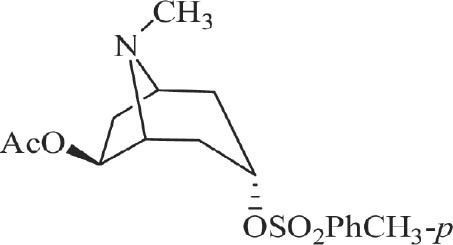
The chemical structure of lesatropane [2].
MATERIALS AND METHODS
Chemicals
RPMI Medium 1640 was purchased from Gibco (Now York, U.S.). Lesatropane was synthesized in our group, as previously described [10]. Its purity and optical purity checked by HPLC were over 98%. Atropine, pirenzepine dihydro-chloride, N,N-dimethyl sulfoxide (DMSO), 2,3,5-triphenyl-tetrazolium chloride solution (TTC), 3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and L-glutamic acid monosodium salt were purchased from Sigma (St. Louis, U.S.). Hoechst 33258 was purchased from Beyotime (Zhejiang, China). Chelerythrine chloride was from Alexis (Pennsylvania, U.S.) and U0126 was from Upstate (Massachusetts, U.S.). Non-Radioactive Protein Kinase Assay Systems was purchased from Promega (Madison, U.S).
Cell culture and drug treatment
PC12 cells obtained from the Shanghai Cell Culture Center (Shanghai, China) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum in a humidified atmosphere with 5% CO2 at 37°C. The cells in the medium without serum were pre-incubated with lesatropane for 24 h before being exposed to glutamate. Atropine or pirenzepine was used at 1 μM. U0126 was used at 20 μM and chelerythrine was at 5 μg/ml. They were used respectively 30 min before addition of lesatropane.
Measurement of cell viability
PC12 cells were plated in 96-well culture plates at 1.0×104-1.5×104 cells/well, and pre-incubated with lesatropane for 24 h before being exposed to glutamate for 48 h. Cell viability assays were performed using MTT method. MTT in PBS was added to the cultures at a final concentration of 0.5 mg/ml. After further incubation at 37°C for 4 h, the medium was carefully removed and formazan crystals were dissolved in 150 μl DMSO per well. The absorbance at 490 nm was measured on a plate reader (Bio-tek, Vermont, U.S.). Preparation of brain slices and drug treatment The brain slices were made as described before [11]. SD rats with a mean weight 80 g were decapitated. Whole brains were quickly removed and immersed in iced ACSF, which had the following composition (in mM): NaCl 119, KCl 2.5, CaCl2 2, MgSO4 1, NaH2PO4 1.25, NaHCO2 26.2, glucose 10 (final pH 7.4). Brains were cut coronally into 400 μm thick sections with a vibrating tissue slicer (ZQP-86, Xiangshan, China). Cortical slices were quickly isolated from the sections. Before being transferred to an experimental chamber, all slices were incubated in ACSF bubbled with 95% O2 and 5% CO2 at 37°C for 90 min recovery. After the process, brain slices were transferred to experimental chambers and randomly assigned to one of the following groups: (I) control group, in which slices were incubated in oxygenated MgfACSF with the following composition (in mM): NaCl 143, KCl 5.4, CaCl2 1.8, NaH2PO4 1.0, HEPES 2.4, glucose 5.6 (final pH 7.4) for 1 h; (II) glutamate injury group, in which slices were pre-incubated in oxygenated MgfACSF for 30 min, and then were incubated in oxygenated MgfACSF containing 1 mM glutamate for 20 min; (III) glutamate injury + drug group, in which slices were incubated with 1 μM lesatropane in oxygenated MgfACSF 30 min prior to and during glutamate application. All slices were transferred to oxygenated ACSF for 2 h recovery, and the activity of the slices was evaluated by using TTC staining method.
LDH measurement
The plasma membrane damage of the PC12 cells was assessed by the release of LDH into the culture medium. LDH leakage was calculated of the percentage of LDH in the medium versus total LDH activity in the cells. After the treatment, the LDH released into the culture medium was examined by using an assay kit (Jiancheng, Nanjing, China) according to the manufacturer’s protocol on a plate reader (Bio-tek, Vermont, U.S.).
Hoechst 33258 staining
PC12 cells were plated on coverslips in 24 culture plates and treated as before. After the treatment, the cells were fixated with cold 4% paraformaldehde for 10 min, and stained with Hoechst 33258 (10 μg/mL) for 15 min under dim light. For each coverslip, cells were observed under a fluorescence microscope (Zeiss, Oberkochen, Germany) with a UV-2A filter, and the apoptotic cells were identified.
Immunoblots
For analysis of the phosphorylated (activated) ERK1/2 and total ERK1/2, immunoblotting was performed with anti-pERK1/2 and anti-ERK 1/2 antibodies. After the treatment, cells were washed with PBS and lysed with a lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 4 μg/ml leupeptin, 1 μg/ml aprotinin and 100 μg/ m1 PMSF). Proteins were loaded onto 10% SDS-PAGE gels, transferred to nitrocellulose membranes (Amersham, U.S.) and blocked at 37°C for 1 h in 5% (w/v) non-fat milk in TBST (20 mM Tris-HCl, pH 7.6, 500 mM NaCl, 0.1% (v/v) Tween). The blots were incubated overnight at 4°C in the primary antibody dilution. The membranes were incubated with pERK1/2 and ERK1/2 primary antibodies (1:1000, Cell signaling, Maryland, U.S.). After washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5000, KPL, Maryland, U.S.) and visualized with the ECL detection kit (Pierce Biotechnology, Illinois, U.S.) according to the manufacturer’s instructions.
PKC activity measurement
The non-radioactive protein kinase assay was used to detect the Protein kinase C (PKC). After the treatment, cells were suspended in cold PKC extraction buffer and homogenized using a cold homogenizer. Each sample was combined with the peptag, PKC reaction buffer, peptag C1 peptide, the sonicated PKC activator buffer, and was incubated at 30°C for 30 min. After the separation of phosphorylated and nonphosphorylated peptag peptides by electrophoresis, the kinase activity was quantitated by densitometric methods.
Statistical analysis
The data are presented as the mean ± standard error of the mean. The difference between the groups was assessed by using Student-Newman-Keuls test, where p<0.05 indicated a significant difference.
RESULTS
Lesatropane protects PC12 cells and cortical slices against glutamate neurotoxicity.
Treatment of PC12 cells with 1 mM glutamate for 48 h resulted in a decrease of survival rate by around 20% relative to the untreated group, which was consistent with the previous report [12]. Pre-treatment with 0.001-0.1 μM lesatropane for 24 h significantly prevented PC12 cell death caused by over-mentioned glutamate neurotoxicity, and the maximal effect was observed at 0.01 μM lesatropane (Figure 2A). The neuroprotective effects of lesatropane on cortical slices were also observed. Cortical slices were subjected to 1 mM glutamate for 20 min and following 2 h recovery. The survival activity of the brain slices was reduced to 31.44±5.18% and the pre-treatment with 0.1 μM lesatropane increased the activity to 66.80±8.22% (Figure 2B).
FIGURE 2.
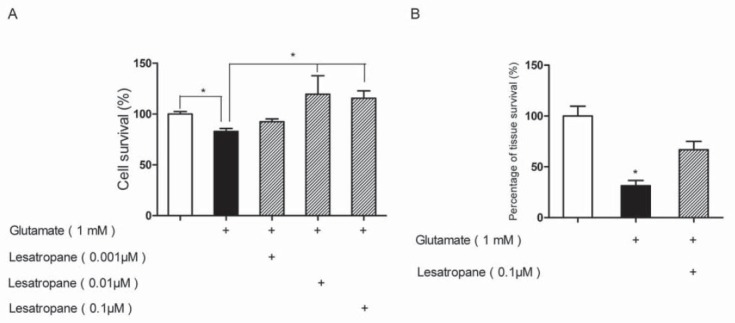
Effects of lesatropane on glutamate injury in PC12 cells or cortical slices. (A) PC12 cells were pre-treated for 24 h with lesatropane (1 nM -0.1 μM) followed by treatment with 1 mM glutamate for 48 h. Cell survival rates were evaluated by MTT. (B) Cortical slices were incubated with 0.1 μM lesatropane 30 min prior to and during the treatment of 1 mM glutamate. The viability of cortical slices was evaluated by using TTC method. Values are expressed as mean ± S.E. of n=5 independent observations. * p<0.05 vs. glutamate injury group.
Neuroprotective effects of lesatropane are mediated through M1 mAChR in PC12 cells
In order to find out if the activation of M1 mAChR was responsible for the neuroprotection observed, PC12 cells were pre-treated with atropine (a nonselective mAChR antagonist) or pirenzepine (a selective M1 mAChR antagonist) for 30 min respectively before 0.01 μM lesatropane treatment. Then, the cell viability, LDH release, and the apoptotic status of PC12 cells were observed respectively. The treatment of PC12 cells with 1 mM glutamate for 48 h reduced cell viability to 82.91±2.88 % and increased in 75.03±21.61% LDH release compared with the untreated groups, which were consistent with the previous report [12]. Typical apoptotic nuclei condensation of PC12 cells was also observed under the same conditions by nuclear staining. However, if the cells were pre-treated with 0.01 μM lesatropane, cell viability increased significantly by 36.55 ± 8.23% (Figure 3A), and the release of LDH reduced by 61.06±10.03% (Figure 3B). The number of apoptotic cells was significantly decreased in the lesatropane group. But, when the PC12 cells were pre-treated with 1 μM atropine, or 1 μM pirenzepine for 30 min before using lesatropane, the protective effects of lesatropane on glutamate neurotoxicity were suppressed (Figs. 3A-C), suggesting that M1 mAChR was involved in lesatropane’s neuroprotective effects.
FIGURE 3.
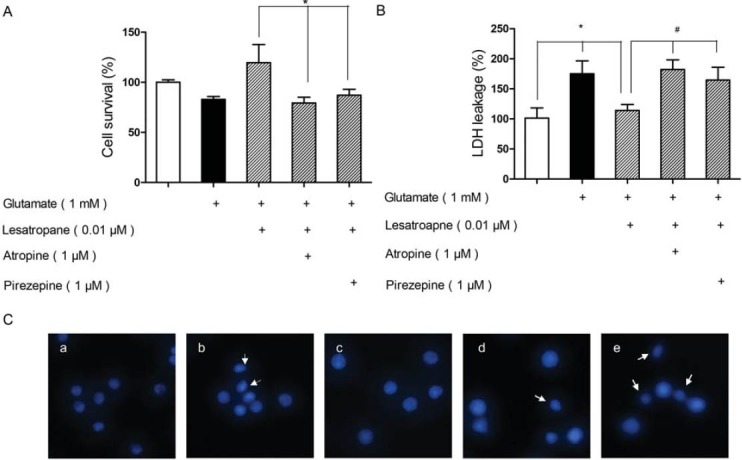
Neuroprotective effects of lesatropane are through M1 mAChR in PC12 cells. Muscarinic antagonist atropine or M1 mAChR antagonist pirenzepine was pre-treated 30 min before the treatement of 0.01 μM lesatropane for 24 h and 1 mM glutamate for 48 h. (A) Cell survival rate evaluated by MTT; (B) The measurement of lactate dehydrogenase release; (C) Cell nuclear morphology stained by Hoechst 33258. Cells were (a): Non-treated (control); (b): treated with glutamate for 48 h; (c) pre-treated with 0.01 μM lesatropane, then incubated with 1 mM glutamate for 48 h; Cells were pre-treated by (d) atropine or (e) pirenzepine 30 min before treatment of lesatropane for 24 h and then treatment with 1 mM glutamate for 48 h. Values are expressed as mean± S.E. of n=5 independent observations. * p<0.05 vs. glutamate injury group, # p<0.05 vs. lesatropane group.
Activation of ERK1/2 pathway is involved in the neuroprotective effects of lesatropane
We interested in the intracellular MAPK/ERK1/2 signaling pathway for the neuroprotective effects of lesatropane, as ERK1/2 is an effecter of mAChRs in PC12 cells [13,14] and the importance of ERK1/2 in AD has been recognized [15,16]. Since the activity of ERK1/2 is dependent on its phosphorylation [17], the phosphorylation of ERK1/2 in PC12 cells was detected by immunoblot. Treatment of cells with 1 mM glutamate led to a significant decrease of the ERK1/2 activity. Lesatropane (0.01 μM) could activate ERK1/2 and prevent the decrease of ERK1/2 activity caused by glutamate (Figure 4A). The lesatropane-induced ERK1/2 activation can be inhibited by atropine or pirenzepine (Figure 4B). In the presence of MEK inhibitor U0126 (20 μM), the effect of lesatropane against glutamate-induced cell death was reduced by 26.62% (Figure 4C). In all, ERK1/2 signaling plays a beneficial protective role in PC12 cells against glutamate neurotoxicity and lesatropane exerts its neuroprotective effects via M1 mAChR-ERK1/2 pathway.
FIGURE 4.
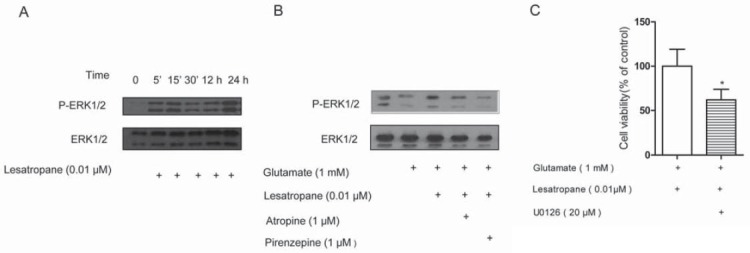
Activation of ERK1/2 pathway was involved in the neuroprotective effects of lesatropane. (A) lesatropane stimulated activation of ERK1/2 in PC12 cells. Cells were treated with 0.01 μM lesatropane for 5 min, 15 min, 30 min, 12 h, 24 h, then harvested and lysed. The activated ERK1/2 (pERK1/2) and total ERK were determined (Western blotting). (B) The activation of ERK1/2 was measured in PC12 cells which were treated with or without 1 μM atropine or pirenzepine 30 min before addition 0.01 μM lesatropane, then 1 mM glutamate. (C) PC12 cells were pre-treated with U0126 (20 μM) 30 min before addition of 0.01 μM lesatropane, then 1 mM glutamate. Cell viability was quantified using the MTT assay after 48 h. * p<0.01 vs lesatropane group. Data represent the mean ± S.E. of n=5 independent observations.
Lesatropane-induced ERK1/2 activity is partially dependent on PKC
M1 mAChR-mediated MAPK activation occurs in different mechanisms. Some of them are protein kinase C (PKC) dependent, others not [13,18]. Treatment of PC12 cells with 1 mM glutamate for 5 min resulted in a decrease of PKC activity to 27.44% relative to the untreated group. If the cells were pre-treated with 0.01 μM lesatropane for 24 h and followed by glutamate, PKC activity could increase to 47.79% (Figure 5A). When chelerythrine (a PKC inhibitor, 5 μg/ml) was added 30 min before the treatment with lesatropane, it could somewhat reduce ERK1/2 activation (Figure 5B) and the neuroprotective effect of lesatropane by 62.0% (Figure 5C). These results implied that the effect of lesatropane against glutamate-induced decrease of ERK1/2 activity was partially dependent on PKC.
FIGURE 5.
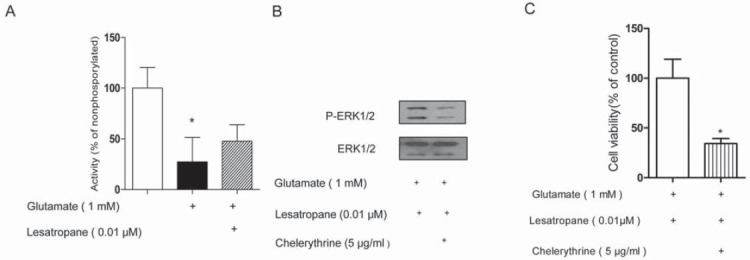
The lesatropane-induced ERK1/2 activity and neuroprotective effects are partially dependent on PKC. PC12 cells were pretreated with 5 μg/ml chelerythrine 30 min before the addition of 0.01 μM lesatropane, then 1 mM glutamate. (A) PKC activity measurement by non-radioactive protein kinase assay. (B) ERK1/2 activation was determined by Western blotting. (C) Cell viability was quantified using the MTT assay after 48 h. * p<0.01 vs lesatropane group. Data represent the mean± S.E. of n=5 independent observations.
DISCUSSION
Base on the couple of different G proteins, the mAChRs are generally considered to divide into two distinct classes and the Gq/11-coupled M1 and M3 mAChRs are mainly reported for the pro-survival response in a variety of stimuli [19-22]. Here, we found that lesatropane significantly reversed the decreased PC12 cell viability caused by glutamate. The protective effects of lesatropane against glutamate-induced PC12 cell death should be principally mediated by the M1 mAChR, since M1 mAChR antagonist pirenzepine can inhibit lesatropane’s effects and PC12 cells principally express M1 mAChR at the mRNA level (data not shown). Although the activation of ERK1/2 known as an effecter of mAChRs, is usually considered to promote neuronal cell survival or memory preservation [15, 23-26], there are still some evidences implicating the opposite effects [27-31]. In order to clarify the mechanism of neuroprotective effects of lesatro-pane, it is critical to identify the role of ERK1/2 pathway in activation of M1 mAChR in glutamate-induced PC12 cell death. ERK1/2 activation was markedly reduced after treatment with glutamate and the pre-treatment with lesatropane could reverse the decrease of ERK1/2 activation. Atropine or pirenzepine inhibited the rescued activation of ERK1/2 by lesatropane. Meanwhile, MEK inhibitor U0126 abolished lesatropane’s effects. So here we found that ERK1/2 played a pro-survival role in PC12 cells during glutamate neurotoxicity and the neuroprotective effects of lesatropane should be mediated by activating ERK1/2. Considering that M1 mAChR activating MAPK was through PKC in PC12 cells [13,32], we further examined the role of PKC in lesatropane induced ERK1/2 activation and finally peculated that lesat-ropane exerted the neuroprotective effects by activation of M1 mAChR via PKC-mediated phosphorylation of ERK1/2. Glutamate neurotoxicity associated with numerous neurodegenerative disorders including AD is considered as a key factor in the pathogenesis. While acetylcholinesterase inhibitor was involved in a neuroprotective cascade of glutamate neurotoxicity [33], the present study contributes the view of action of cholinergic activity could promote neuron survival during glutamate-induced cell death. More importantly, the neuroprotective effects of lesatropane are mainly mediated by M1 mAChR which is well accepted target in AD. In fact, a number of M1 mAChR agonists were reported to relieve the symptoms and intervene in the pathological process [34-36], our studies may help validate truly selective M1 mAChR agonists for the treatment of AD.
CONCLUSION
This paper indicated lesatropane could protect glutamate induced neurotoxicity through the specific M1 mAChR via PKC-ERK1/2 pathway. While M1 mA-ChR is a therapeutic target of AD, the results of this paper may provide further information of the activation of M1 mAChR as a therapeutic target in AD.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Founding of China (No. 30672441, 30873057, 81171245), Key Basic Project of Shanghai Municipal Science and Technology Commission (No. 08JC1413600, 11JC1406600), Scientific and Technological Support Projects of Shanghai Municipal Science and Technology Commission (No. 12431900604).
DECLARATION OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Niu YY, Yang LM, Liu HZ, Cui YY, Zhu L, Feng JM, et al. Activity and QSAR study of baogongteng A and its derivatives as muscarinic agonists. Bioorg Med Chem Lett. 2005;15(21):4814–4818. doi: 10.1016/j.bmcl.2005.07.045. [DOI] [PubMed] [Google Scholar]
- [2].Zhu L, Yang LM, Cui YY, Zheng PL, Niu YY, Wang H, et al. Stereoselectivity of satropane, a novel tropane analog, on iris muscarinic receptor activation and intraocular hypotension. Acta Pharmacol Sin. 2008;29(2):177–184. doi: 10.1111/j.1745-7254.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- [3].Fu J, Feng X, Yuan H, Yan L, Kuang X, Xia Z, et al. Study of ocular pharmacokinetics of in situ gel system for S(-)-satropane evaluated by microdialysis. J Pharm Biomed Anal. 2008;48(3):825–833. doi: 10.1016/j.jpba.2008.06.001. [DOI] [PubMed] [Google Scholar]
- [4].Leloup C, Michaelson DM, Fisher A, Hartmann T, Beyreuther K, Stein R. M1 muscarinic receptors block caspase activation by phosphoinositide 3-kinase-and MAPK/ERK-independent pathways. Cell Death Differ. 2000;7(9):825–833. doi: 10.1038/sj.cdd.4400713. [DOI] [PubMed] [Google Scholar]
- [5].Lindenboim L, Pinkas-Kramarski R, Sokolovsky M, Stein R. Activation of muscarinic receptors inhibits apoptosis in PC12M1 cells. J Neurochem. 1995;64(6):2491–2499. doi: 10.1046/j.1471-4159.1995.64062491.x. [DOI] [PubMed] [Google Scholar]
- [6].Murga C, Laguinge L, Wetzker R, cuadrado A, Gutkind JS. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinasegamma. J Biol Chem. 1998;273(30):19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- [7].Olney JW, de Gubareff T. Glutamate neurotoxicity and Hunting-ton's chorea. Nature. 1978;271(5645):557–559. doi: 10.1038/271557a0. [DOI] [PubMed] [Google Scholar]
- [8].Zhou W, Zhu X, Zhu L, Cui YY, Wang H, Qi H, et al. Neuroprotection of muscarinic receptor agonist pilocarpine against glutamate induced apoptosis in retinal neurons. Cell Mol Neurobiol. 2008;28(2):263–275. doi: 10.1007/s10571-007-9251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117(2):232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- [10].Yang L, Wang H. The preparation and bioactivities of chiral analogs of baogongteng A. Yao Xue Xue Bao. 1998;33(11):832–835. [PubMed] [Google Scholar]
- [11].Wang ZJ, Liang CL, Li GM, Yu CY, Yin M. Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices. Chem Biol Interact. 2006;163(3):207–217. doi: 10.1016/j.cbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [12].Kogo J, Takeba Y, Kumai T, Kitaoka Y, Matsumoto N, Ueno S, et al. Involvement of TNF-alpha in glutamate-induced apoptosis in a differentiated neuronal cell line. Brain Res. 2006;1122(1):201–208. doi: 10.1016/j.brainres.2006.09.006. [DOI] [PubMed] [Google Scholar]
- [13].Berkeley JL, Levey AI. Muscarinic activation of mitogen-activated protein kinase in PC12 cells. J Neurochem. 2000;75(2):487–493. doi: 10.1046/j.1471-4159.2000.0750487.x. [DOI] [PubMed] [Google Scholar]
- [14].Wotta DR, Wattenberg EV, Langason RB, el-Fakahany EE. M1, M3 and M5 muscarinic receptors stimulate mitogen-activated protein kinase. Pharmacology. 1998;56(4):175–186. doi: 10.1159/000028196. [DOI] [PubMed] [Google Scholar]
- [15].Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9(5):544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- [16].Zhu X, Lee HG, Raina AK, Perry G, Smith MA. The role of mitogen-activated protein kinase pathways in Alzheimer's disease. Neurosignals. 2002;11(5):270–281. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- [17].Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- [18].Haring R, Fisher A, Marciano D, Pittel Z, Kloog Y, Zuckerman A, et al. Mitogen-activated protein kinase-dependent and protein kinase C-dependent pathways link the m1 muscarinic receptor to beta-amyloid precursor protein secretion. J Neurochem. 1998;71(5):2094–2103. doi: 10.1046/j.1471-4159.1998.71052094.x. [DOI] [PubMed] [Google Scholar]
- [19].Budd DC, McDonald J, Emsley N, Cain K, Tobin AB. The C-terminal tail of the M3-muscarinic receptor possesses anti-apoptotic properties. J Biol Chem. 2003;278(21):19565–19573. doi: 10.1074/jbc.M211670200. [DOI] [PubMed] [Google Scholar]
- [20].Budd DC, Spragg EJ, Ridd K, Tobin AB. Signalling of the M3-muscarinic receptor to the anti-apoptotic pathway. Biochem J. 2004;381(Pt 1):43–49. doi: 10.1042/BJ20031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tobin AB, Budd DC. The anti-apoptotic response of the Gq/11- coupled muscarinic receptor family. Biochem Soc Trans. 2003;31(Pt 6):1182–1185. doi: 10.1042/bst0311182. [DOI] [PubMed] [Google Scholar]
- [22].De Sarno P, Shestopal SA, King TD, Zmijewska A, Song L, Jope RS. Muscarinic receptor activation protects cells from apoptotic effects of DNA damage, oxidative stress, and mitochondrial inhibition. J Biol Chem. 2003;278(13):11086–11093. doi: 10.1074/jbc.M212157200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fukunaga K, Miyamoto E. Role of MAP kinase in neurons. Mol Neurobiol. 1998;16(1):79–95. doi: 10.1007/BF02740604. [DOI] [PubMed] [Google Scholar]
- [24].Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- [25].Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- [26].Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004;271(11):2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- [27].Satoh T, Nakatsuka D, Watanabe Y, Nagata I, Kikuchi H, Namura S. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neurosci Lett. 2000;288(2):163–166. doi: 10.1016/s0304-3940(00)01229-5. [DOI] [PubMed] [Google Scholar]
- [28].Stanciu M, DeFranco DB. Prolonged nuclear retention of activated extracellular signal-regulated protein kinase promotes cell death generated by oxidative toxicity or proteasome inhibition in a neuronal cell line. J Biol Chem. 2002;277(6):4010–4017. doi: 10.1074/jbc.M104479200. [DOI] [PubMed] [Google Scholar]
- [29].Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12(10):1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- [30].Zhu D, Wu X, Strauss KI, Lipsky RH, Qureshi Z, Terhakopian A, et al. N-methyl-D-aspartate and TrkB receptors protect neurons against glutamate excitotoxicity through an extracellular signalregulated kinase pathway. J Neurosci Res. 2005;80(1):104–113. doi: 10.1002/jnr.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275(16):12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- [32].Messing RO, Stevens AM, Kiyasu E, Sneade AB. Nicotinic and muscarinic agonists stimulate rapid protein kinase C translocation in PC12 cells. J Neurosci. 1989;9(2):507–512. doi: 10.1523/JNEUROSCI.09-02-00507.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Takada Y, Yonezawa A, Kume T, Katsuki H, Kaneko S, Sugimoto H, et al. Nicotinic acetylcholine receptor-mediated neuroprotection by donepezil against glutamate neurotoxicity in rat cortical neurons. J Pharmacol Exp Ther. 2003;306(2):772–777. doi: 10.1124/jpet.103.050104. [DOI] [PubMed] [Google Scholar]
- [34].Hock C, Maddalena A, Heuser I, Naber D, Oertel W, von der Kammer H, et al. Treatment with the selective muscarinic agonist talsaclidine decreases cerebrospinal fluid levels of total amyloid beta-peptide in patients with Alzheimer's disease. Ann N Y Acad Sci. 2000;920:285–291. doi: 10.1111/j.1749-6632.2000.tb06937.x. [DOI] [PubMed] [Google Scholar]
- [35].Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist A F102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 2000;48(6):913–918. [PubMed] [Google Scholar]
- [36].Hock C, Maddalena A, Raschig A, Muller-Spahn F, Eschweiler G, Hager K, et al. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer's disease. Amyloid. 2003;10(1):1–6. doi: 10.3109/13506120308995249. [DOI] [PubMed] [Google Scholar]


