Abstract
YKL-40 is a novel inflammatory protein. Elevated serum levels of YKL-40 have been reported in patients with atherosclerosis and other cardiovascular diseases, but the circulating profile of YKL-40 in patients with cerebrovascular disease has been less investigated. This prospective observational study aimed to determine serum levels of YKL-40 in patients with different subtypes and severities of cerebrovascular disease. Eighty patients with acute ischemic stroke, 30 patients with acute hemorrhagic stroke, 15 patients with transient ischemic attack (TIA) and 18 age- and gender-matched healthy control subjects were recruited. Blood was sampled. Serum levels of YKL-40 were measured by ELISA. In healthy control subjects, serum levels of YKL-40 were 45.09±31.41 ng/ml, significantly lower than those in patients with acute ischemic stroke (178.58±127.78 ng/ml), hemorrhagic stroke (105.32±87.35 ng/ml) and TIA (148.09±108 ng/ml) respectively (p<0.05). When the 80 acute ischemic stroke cases were stratified into four Oxfordshire Community Stroke subtypes, serum levels of YKL-40 were significantly higher in patients with total anterior (n=16), partial anterior (n=25) and posterior (n=12) circulation infarctions respectively than in those with lacunar (n=27) infarction (p<0.05). Moreover, 63 of 80 patients with acute ischemic stroke survived. Circulating levels of YKL-40 in these stroke survivors were associated with the United States National Institutes of Health Stroke Scale (NIHSS) scores of neurological deficit. In summary, serum levels of YKL-40 were elevated in patients with cerebrovascular disease in lesion subtype- and severity-dependent manners. These observations suggest a potential for YKL-40 as a diagnostic/prognostic biomarker for cerebrovascular disease.
KEY WORDS: YKL-40, cerebrovascular disease, stroke, infarction, diagnostic/prognostic biomarker
INTRODUCTION
Cerebrovascular disease, often referred to as stroke, is a heterogeneous group of disorders in which areas of the brain are transiently or permanently affected by ischemia or bleeding. Stroke-associated burden and death rates vary considerably among countries, depending on the prevalence of cardiovascular disease risk factors and socioeconomic characteristics [1]. While stroke is the number one killer in middle-income countries, it is the 5th most common cause of death in low-income countries [2]. In China, stroke claims 1.6 million lives and affects approximately 2.5 million new individuals annually [3], whereby posing a significant health problem.
Stroke is believed to be preventable and treatable through management of risk factors; an accurate and timely diagnosis is the key to optimizing the patient outcome [4]. Recently, serum biomarkers have been suggested to play an important role in the diagnosis of acute cerebral ischemia and in the subsequent implementation of timely reperfusion strategies [5]. Nevertheless, to date, no reliable biomarkers have been identified and clinically validated for cerebrovascular abnormalities.
YKL-40, named as a combination of the letter codes of its first three N-terminal amino acids, tyrosine (Y), lysine (K) and leucine (L), and its molecular weight (40 kD) [6], is a novel proinflammatory protein. Since its first identification in the media of human osteoblastic cell cultures [7], YKL-40 has been demonstrated to be produced in many other cell types as well [8,9]. Although YKL-40 is detectable in the peripheral blood of healthy individuals [10-12], its serum concentration is significantly increased in a number of disease conditions including cardiovascular disorders [13]. However, little information is available regarding blood levels of YKL-40 in Chinese patients with cerebrovascular disease. The present study was therefore conducted to assess changes in serum levels of YKL-40 in Chinese patients with cerebrovascular disease of different subtypes and severities, aiming at establishing the possible use of YKL-40 as a diagnostic/prognostic biomarker for cerebrovascular disease.
MATERIALS AND METHODS
Ethical Statement
The study was reviewed and approved by the Institutional Ethics Committee of Sun Yat-sen University in Guangzhou, China and conducted in compliance with the Helsinki Declaration. Written informed consent was obtained from all subjects.
Patients
A total of 125 patients with acute cerebral vascular disease who were referred to the Neurological Division in the Department of Emergency Medicine, the First Affiliated Hospital of Sun Yat-sen University consecutively between June 2010 and July 2011 were recruited. Patients with concomitant inflammatory disease such as infection and autoimmune disorder, liver or kidney disease, myocardial infarction or malignancies were excluded. Eighteen age- and gender-matched healthy volunteers were included as control subjects.
Diagnosis of Cerebrovascular Disease
All patients were diagnosed by a set of neurological examinations including cranial CT and/or MRI, while TIA that has been redefined recently as a brief (less than 24 h) episode of neurologic dysfunction caused by focal brain, spinal cord, or retinal ischemia by the American Heart Association and American Stroke Association without acute infarction [14] was determined based on the medical history of the event. In the 125 patients, 80 were diagnosed with acute ischemic stroke within 48 h of symptom onset, 30 with acute hemorrhagic stroke within 24 h of symptom onset, and the remaining 15 with TIA within 72 h of symptom onset. Based on the CT and/or MRI findings, the 80 acute ischemic stroke patients were stratified into four subtype groups: total anterior circulation infarcts (TACI, n=16), partial anterior circulation infarcts (PACI, n=25), posterior circulation infarcts (POCI, n=12) and lacunar infarcts (LACI, n=27) by the Oxfordshire Community Stroke Project (OCSP) criteria [15].
By day 14 of hospitalization, 17 out of 80 acute ischemic stroke patients died. The neurological deficit in the 63 ischemic stroke survivors was assessed using the United States National Institutes of Health Stroke Scale (NIHSS). Depending on the NIHSS score, the deficit was defined as mild (a score 1-4), moderate (a score 5-20) and severe (a score 21-42) respectively.
Measurement of Major Biochemical Variables and YKL-40 in the Blood
Blood was drawn by venipuncture into pyrogen-free tubes with no anticoagulant within 48 h after hospital admission. After clotting at room temperature for 3 h, the blood sample was centrifuged at 3,000×g for 5 min. Serum was collected and stored at −80°C until analysis.
Blood laboratory tests were performed using an autoanalyzer as instructed; white blood cell and platelet counts as well as serum concentrations of total cholesterol, triglycerides, HDL (high density lipoprotein) cholesterol and LDL (low density lipoprotein) cholesterol were measured. The measurement of serum levels of YKL-40 was performed with a commercial enzyme immunoassay (EIA) kit from Quidel Corporation (San Diego, California, USA) as previously described [16].
Statistical Analysis
Quantitative data were expressed as mean±standard deviation (SD) and analyzed by t-test or one way ANOVA together with Bonferroni test. Categorical data were expressed as percentages and analyzed by χ2-test. Differences were considered statistically significant when p<0.05. All analyses were performed with the statistical software SPSS 13.0 (Chicago, IL, USA).
RESULTS
Basic Demographics
The median age of patients in the three cerebrovascular disease subgroups (i.e. acute ischemic stroke, n=80; acute hemorrhagic stroke, n=30; and TIA, n=15) was respectively 69.5 (39-92), 60 (36-84) and 66 (43-76) years, which were similar to that of the 18 healthy control subjects (56 years, rang 35-80 years). A similar male to female ratio was also observed in the three disease subgroups (46/34, 20/10 and 8/7, respectively) and control (11/7) group (Table 1).
TABLE 1.
Major biochemical variables in patients with different subtypes.
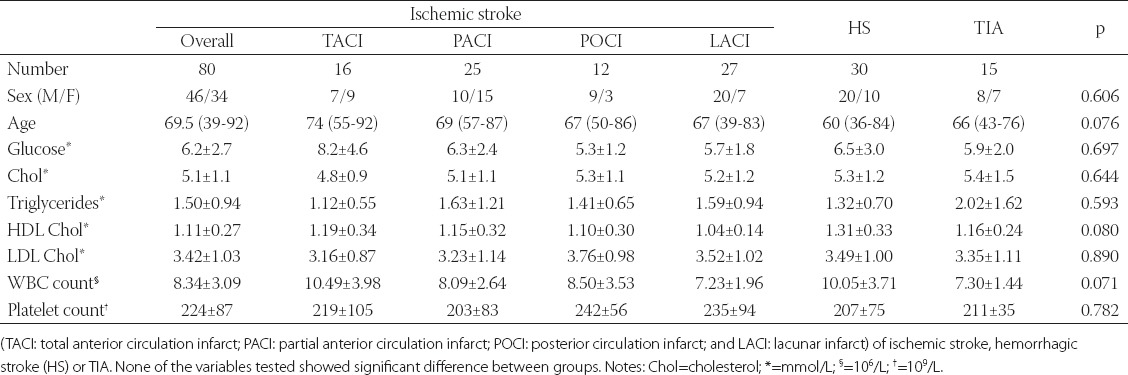
The median age in the four subtypes of ischemic stroke (TACI, PACI, POCI and LACI) was respectively 74 (rang 55-92), 69 (rang 57-78), 67 (rang 50-86), and 67 (rang 39-83) years; there were no significant differences between these subtypes. In contrast, the male to female ratio was slightly different among TACI (7/9), PACI (10/15), POCI (9/3) and LACI (20/7) patients (Table 1).
Biochemical Parameters
Serum levels of glucose, total cholesterol, triglycerides, HDL and LDL cholesterols, white blood cell count and platelet counts were measured in the 125 patients. As shown in Table 1, no significant differences (P>0.05) in these variables were detected either between ischemic stroke, hemorrhagic stroke and TIA groups or between the four subgroups (TACI, PACI, POCI and LACI) of ischemic stroke patients.
Serum Levels of YKL-40
Presented in Figure 1 are the results of serum YKL-40 concentration measurements. The average serum levels of YKL-40 in 18 healthy volunteers were 45.09±31.41 ng/ml. Compared to this baseline level, a significant elevation was observed in patients with acute ischemic stroke (178.58±127.78 ng/ml), acute hemorrhagic stroke (148.7±108 ng/ml) or acute TIA (105.32±87.35 ng/ml) (p<0.05). Serum levels of YKL-40 were not significantly different between ischemic stroke, hemorrhagic stroke and TIA (p>0.05).
FIGURE 1.
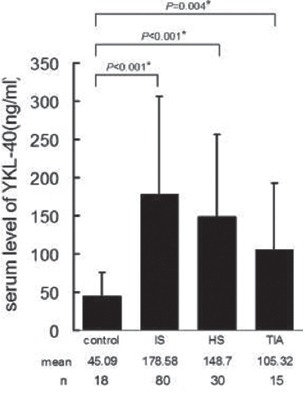
Average serum levels of YKL-40 in healthy control subjects and patients with acute ischemic stroke (IS), acute hemorrhagic stroke (HS) and transient ischemic attack (TIA) respectively. A single asterisk (*) indicates a significant difference from the control subjects at α=0.05
In the 80 ischemic stroke patients, stroke subtype-dependent changes in the average serum levels of YKL-40 were observed (Figure 2). LACI patients (n=27) had a level very close to the baseline level in normal control subjects. Compared to the level in LACI patients, significantly higher levels were detected in TACI (P<0.01), PACI (p<0.05) and POCI (p<0.05) patients.
FIGURE 2.
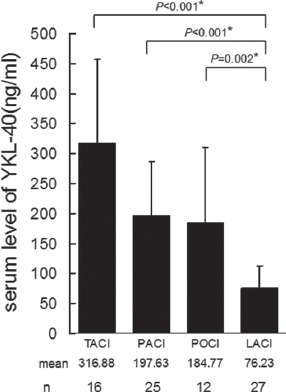
Average serum levels of YKL-40 in subtypes of ischemic stroke classified by the Oxfordshire Community Stroke Project criteria. TACI: total anterior circulation infarct (n=16); PACI: partial anterior circulation infarct (n=25); POCI: posterior circulation infarct (n=12); and LACI: lacunar infarct (n=27). A single asterisk (*) indicates a significant difference from the LACI group at α=0.05
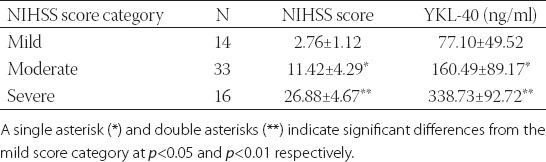
Out of the 80 ischemic stroke patients, 63 survived and were subjected to the NIHSS assessment 14 days after admission. Presented in Table 2 are the results of this assessment together with the data on serum YKL-40 measured within 48 h after admission. Apparently, the more severe the neurologic deficit was, the higher the average serum level of YKL-40 in the acute ischemic stroke survivors.
TABLE 2.
Serum levels of YKL-40 in association with the Unites States National Institute of Health Stroke Scale (NIHSS) score of neurologic deficits in 63 patients who survived acute ischemic stroke at two weeks after hospital admission.
DISCUSSION
As a gene localized in a highly conserved region on human chromosome 1q31-q32 [17], YKL-40 is expressed in a relatively stable level across healthy human individuals of different races. In a Danish study involving a total of 245 healthy subjects (134 women and 111 men) ranging from 18 to 79 years in age, Johansen et al. [12] reported that the baseline serum levels of the YKL-40 protein in normal Scandinavians were 43 ng/ml, which increased with age (r=0.45, p<0.0001) but showed little diurnal rhythm-, physical activity-, and gender-associated variations. In this study, there were no significant differences in age and gender. Average serum levels of YKL-40 in the 18 healthy Chinese subjects in the present study were 45.09±31.41 ng/ml, similar to the baseline levels detected in the Scandinavian population but lower than that (62.64 ng/ml) reported in the study of Zheng et al. [18] on 248 healthy Chinese subjects and higher than that (37.85±14.12) reported in another Chinese study involving on 80 healthy subjects [19]. Apparently, the baseline levels of YKL-40 in the circulation needs to be further established in studies involving a larger sample size and perhaps more standardized assay protocols.
YKL-40 is a protein that shares a high degree of sequence similarity with chitinase 3 but lacks the classical chitinolytic activity. The physiological function of YKL-40 remains unclear. Nevertheless, recent studies have demonstrated that circulating levels of YKL-40 are elevated in numerous human diseases including coronary artery disease [20] and acute myocardial infarction [21], suggesting a diagnostic/prognostic role for this molecule as a biomarker of these diseases. In the present study, we demonstrated that the serum levels of YKL-40 were also elevated significantly (p<0.05) in Chinese patients with cerebrovascular disease as compared with the levels in the healthy control subjects and that the elevation did not significantly differ between ischemic stroke, hemorrhagic stroke and TIA (p>0.05). These observations support an association between circulating levels of YKL-40 and cerebrovascular disease subtypes and severities as previously reported [22].
We further assessed the relationship between the serum level of YKL-40 and the OCSP ischemic stroke subtypes. The results showed that the levels in LACI patients were similar to the baseline levels in normal control subjects, but were significantly elevated in TACI (P<0.01), PACI (P<0.05) and POCI (p<0.05) patients. Given a previous report that OCSP class-different patients are different significantly in mortality, morbidity, length of hospital stay, and complications (e.g. respiratory tract infection and seizures) [23], the association of serum levels of YKL-40 with the OCSP classification observed in the present study suggests that YKL-40 may be useful in predicting the severity of infarct lesions in acute ischemic stroke patients. A wide spectrum of acute and chronic infections as well as many exogenous and intrinsic sources of inflammation are associated with an increased risk for ischemic stroke and YKL-40 may play a role in tissue remodeling during inflammation [24]. These may partially explain the association between YKL-40 levels and cerebrovascular disease subtypes and severities, but the mechanism underlying the role for YKL-40 in the development of cerebrovascular lesions warrant further investigation.
Moreover, we observed that serum levels of YKL-40 were positively associated with the NIHSS score of neurological deficit. This finding is in agreement with the results of a recent study by Park and colleagues that the circulating YKL-40 level in 105 Korean patients with acute ischemic stroke was correlated with the severity of neurological deficit as assessed by the NIHSS [22].
At present, the cellular origin of increased circulatory YKL-40 in cerebrovascular disease remains unclear. Given that inflammation plays an important role in the secondary brain injury following ischemic and hemorrhagic strokes [25] and this inflammatory response involves immediate infiltration of blood components (e.g. red blood cells, leukocytes, and macrophages) as well as microglia and astrocyte activation at the injury site [26], it is likely that all these cells involved may contribute to the increased YKL-40 production in cerebrovascular disease. Nevertheless, this speculation warrants more intensive investigations.
CONCLUSION
In summary, we have demonstrated that circulating levels of YKL-40 are significantly elevated in patients with acute cerebral vascular disease including acute ischemic and hemorrhagic strokes and TIA. We have also demonstrated that YKL-40 in the circulation is associated with the severity of neurological deficit in acute ischemic stroke patients. These observations suggest that YKL-40 may potentially offer a novel diagnostic and/or prognostic biomarker for cerebrovascular disease.
ACKNOWLEDGMENTS
This study was supported by the Science and Technology Planning Project of Guangdong Province, China (No. 2007B030704001) and the Fundamental Research Funds for the Central Universities (11ykpy14). The authors would like to express thanks to Dr. Ziming Yu for proofreading the manuscript.
DECLARATION OF INTEREST
None of the authors have any commercial associations or sources of support that might pose a conflict of interest.
REFERENCES
- [1].Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012;22(4):530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8(4):345–354. doi: 10.1016/S1474-4422(09)70023-7. [DOI] [PubMed] [Google Scholar]
- [3].Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- [4].Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42(12):3651–3654. doi: 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- [5].Caplan LR, Searls DE, Hon FK. Cerebrovascular disease. Med Clin North Am. 2009;93(2):353–369. doi: 10.1016/j.mcna.2008.09.004. viii. [DOI] [PubMed] [Google Scholar]
- [6].Kernagis DN, Laskowitz DT. Evolving role of biomarkers in acute cerebrovascular disease. Ann Neurol. 2012;71(3):289–303. doi: 10.1002/ana.22553. [DOI] [PubMed] [Google Scholar]
- [7].Hauschka PV, Mann KG, Price P, Termine JD. Report of the Ad Hoc Committee on Nomenclature and Standards for Bone Proteins and Growth Factors. J Bone Miner Res. 1986;1(5):485–486. doi: 10.1002/jbmr.5650010513. [DOI] [PubMed] [Google Scholar]
- [8].Johansen JS, Williamson MK, Rice JS, Price PA. Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res. 1992;7(5):501–512. doi: 10.1002/jbmr.5650070506. [DOI] [PubMed] [Google Scholar]
- [9].Nishikawa KC, Millis AJ. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res. 2003;287(1):79–87. doi: 10.1016/s0014-4827(03)00069-7. [DOI] [PubMed] [Google Scholar]
- [10].Bojesen SE, Johansen JS, Nordestgaard BG. Plasma YKL-40 levels in healthy subjects from the general population. Clin Chim Acta. 2011;412(9-10):709–712. doi: 10.1016/j.cca.2011.01.022. [DOI] [PubMed] [Google Scholar]
- [11].Johansen JS, Hvolris J, Hansen M, Backer V, Lorenzen I, Price PA. Serum YKL-40 levels in healthy children and adults. Comparison with serum and synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br J Rheumatol. 1996;35(6):553–559. doi: 10.1093/rheumatology/35.6.553. [DOI] [PubMed] [Google Scholar]
- [12].Johansen JS, Lottenburger T, Nielsen HJ, Jensen JE, Svendsen MN, Kollerup G, et al. Diurnal, weekly, and long-time variation in serum concentrations of YKL-40 in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2603–2608. doi: 10.1158/1055-9965.EPI-07-2766. [DOI] [PubMed] [Google Scholar]
- [13].Rathcke CN, Vestergaard H. YKL-40--an emerging biomarker in cardiovascular disease and diabetes. Cardiovasc Diabetol. 2009;8:61. doi: 10.1186/1475-2840-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia;Council on Cardiovascular Radiology and Intervention;Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- [15].Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- [16].Roslind A, Johansen JS. YKL-40: a novel marker shared by chronic inflammation and oncogenic transformation. Methods Mol Biol. 2009;511:159–184. doi: 10.1007/978-1-59745-447-6_7. [DOI] [PubMed] [Google Scholar]
- [17].Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15(2):194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- [18].Zheng JL, Lu L, Hu J, Zhang RY, Zhang Q, Chen QJ, et al. Increased serum YKL-40 and C-reactive protein levels are associated with angiographic lesion progression in patients with coronary artery disease. Atherosclerosis 2010. 2010;(2):590–595. doi: 10.1016/j.atherosclerosis.2009.12.016. [DOI] [PubMed] [Google Scholar]
- [19].Ma WH, Wang XL, Du YM, Wang YB, Zhang Y, Wei DE, et al. Association between human cartilage glycoprotein 39 (YKL-40) and arterial stiffness in essential hypertension. BMC Cardiovasc Disord. 2012;12:35. doi: 10.1186/1471-2261-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S. Serum YKL-40 levels in patients with coronary artery disease. Coron Artery Dis. 2007;18(5):391–396. doi: 10.1097/MCA.0b013e328241d991. [DOI] [PubMed] [Google Scholar]
- [21].Hedegaard A, Ripa RS, Johansen JS, Jorgensen E, Kastrup J. Plasma YKL-40 and recovery of left ventricular function after acute myocardial infarction. Scand J Clin Lab Invest. 2010;70(2):80–86. doi: 10.3109/00365510903518191. [DOI] [PubMed] [Google Scholar]
- [22].Park HY, Jun CD, Jeon SJ, Choi SS, Kim HR, Choi DB, et al. Serum YKL-40 levels correlate with infarct volume, stroke severity, and functional outcome in acute ischemic stroke patients. PLoS One. 2012;7(12):e51722. doi: 10.1371/journal.pone.0051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pittock SJ, Meldrum D, Hardiman O, Thornton J, Brennan P, Moroney JT. The Oxfordshire Community Stroke Project classification: correlation with imaging, associated complications, and prediction of outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2003;12(1):1–7. doi: 10.1053/jscd.2003.7. [DOI] [PubMed] [Google Scholar]
- [24].Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation. 2010;7:34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. 2009;22(3):294–301. doi: 10.1097/wco.0b013e32832b4db3. [DOI] [PubMed] [Google Scholar]
- [26].Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27(5):894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]


