Abstract
Liver ischemia reperfusion injury (IRI) is an important pathologic process leading to bodily systemic effects and liver injury. Our study aimed to investigate the protective effects of diosmin, a phlebotrophic drug with antioxidant and anti-inflammatory effects, in a liver IRI model. Forty rats were divided into 4 groups. Sham group, control group (ischemia-reperfusion), intraoperative treatment group, and preoperative treatment group. Ischemia reperfusion model was formed by clamping hepatic pedicle for a 60 minute of ischemia followed by liver reperfusion for another 90 minutes. Superoxide dismutase (SOD) and catalase (CAT) were measured as antioaxidant enzymes in the liver tissues, and malondialdehyde (MDA) as oxidative stress marker, xanthine oxidase (XO) as an oxidant enzyme and glutathione peroxidase (GSH-Px) as antioaxidant enzyme were measured in the liver tissues and the plasma samples. Hepatic function tests were lower in treatment groups than control group (p<0.001 for ALT and AST). Plasma XO and MDA levels were lower in treatment groups than control group, but plasma GSH-Px levels were higher (p<0.05 for all). Tissue MDA levels were lower in treatment groups than control group, but tissue GSH-Px, SOD, CAT and XO levels were higher (p<0.05 for MDA and p<0.001 for others). Samples in control group histopathologically showed morphologic abnormalities specific to ischemia reperfusion. It has been found that both preoperative and intraoperative diosmin treatment decreases cellular damage and protects cells from toxic effects in liver IRI. As a conclusion, diosmin may be used as a protective agent against IRI in elective and emergent liver surgical operations.
KEY WORDS: diosmin, ischemia-reperfusion, flavonoid, liver
INTRODUCTION
Ischemia reperfusion injury (IRI) is an important clinical issue which is a concern to many organs including brain, heart, kidneys, and liver [1,2]. Hepatic ischemia-reperfusion periods may take place during hepatic tumor resection, trauma surgery of liver, vessel reconstruction surgery, and liver transplantation. Furthermore, hepatic circulation is known to be affected by hemorrhagic shock, advanced sepsis, and severe trauma, independent of the surgery [3]. Different mechanisms such as hypoxic response, inflammatory reaction, free radical injury, and apoptosis play role in development of cellular damage in liver during ischemic period following reperfusion [4]. Diosmin is a hesperidin-derivative bioflavonoid. Flavonoids have been demonstrated to exert anti-lipo-peroksidant, anti-tumoral, anti-platelet, anti-ischemic, anti-allergic, and anti-inflammatory activities [5]. In this study we aimed to investigate the protective effect of diosmin on oxidative stress and cellular damage in IRI.
MATERIALS AND METHODS
Animals
Forty female Wistar-Albino rats of 230±30 gr weight were kept in separate wire cages at constant room temperature (21 ± 2°C) in cycle of light and darkness of 12 hours. They were fed with water and rat feed. They were kept off food by 12 hours prior to the surgery. They were allowed to drink water until 2 hours prior to the surgery. No parenteral or enteral antibiotics were administered in any stage of the study. This study was conducted in accordance with National Guide for Care and Use of Laboratory Animals after approval of Ethical Committee of Ankara Research and Education Hospital. Rats were randomly divided into 4 groups each containing 10 rats:
SHAM group. Rats in this group, hepatic pedicule was mobilized.
Control group (ischemia-reperfusion). Rats in this group, IRI was generated. Any treatment was given.
Intraoperative treatment group. Rats in this group, IRI was generated. Rats in this group were given diosmin 50 mg/kg in in the form of gavage per orogastric tube (7 Gauge feeding tube) immediately following induction of ischemia to reach of plasma concentration of diosmin at the beginning of reperfusion.
Preoperative treatment group. Rats in this group were given diosmin in the form of gavage per orogastric tube at a dose of 50 mg/kg/day for ten days prior to operation. The tube was removed after daily drug was administered. IRI was generated in operation time.
All rats were sacrificed simultaneously. No rat died during the study period. At the end of these procedures blood and liver tissue samples were obtained for biochemical and histopathologic assessment.
Procedure of IRI
The animals were anesthetized by administering ketamine hydrochloride 80 mg/kg and xylasine 20 mg/kg. After abdomen was shaved and disinfected a midline incision was made. Ischemic period was generated by clamping hepatic pedicle with micro vascular bulldog clamp for 60 minutes. Following ischemic period liver was reperfused by declamping and reperfusion was continued until 90 minute.
Drugs and Chemicals
Ketamine hydrochloride (Ketalar®; Parke-Davis, Istanbul, Turkey); Xylasine (Rompun® Bayer, Istanbul, Turkey); Diosmin (Vendios®; Bilim, Istanbul, Turkey). All other chemicals are of analytical grade.
Biochemical analysis
Liver tissue samples were kept at -80°C for assessment of oxidative stress. Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gama glutamyl transferase (GGT) levels were measured using Olympus Au 640 autoanalysator to assess liver functions. To assess oxidative injury, blood malondialdehyde (MDA) levels, glutathion peroxidase (GSH-Px), and xanthine oxidase (XO) enzymatic activities were studied. MDA levels, GSH-Px, XO, superoxide dismutase (SOD), and catalase (CAT) enzymatic activities were measured in liver tissue samples.
Assessment of oxidative stress
Following sacrifice of animals liver samples were obtained and exposed to ice bath until homogenization. Liver samples were first washed with distilled water, tissues were homogenized with physiologic saline (20% w/v, approximately 1g in 5 ml for each). Later, supernatants were centrifuged at 4000 rpm, for 15 minutes. All procedures were completed at +4°C. Protein concentrations of supernatants obtained from tissue homogenates were measured with Lowry’s method for protein measurement [6]. The obtained supernatants were separated to be studied in different measurement devices. MDA Measurement: As an end product of lipid peroxidation, MDA is used as a marker of oxidation. By measuring the absorbance level of the complex formed by MDA and thiobarbituric acid at a wave length of 532 nm, which is the basis of the Dahle’s spectrophotometric method, the results were expressed in terms of nmol/mg tissue weight [7]. SOD Determination: Measurement of SOD is based on the principle of reduction of nitroblue tetrazolium (NBT) compound found in the reaction medium when superoxide radical formed by xanthine-xanthine oxidase system cannot be removed by SOD enzyme; the results were expressed in terms of U/mg. One unit of SOD was expressed as the substance amount causing 50% inhibition in the NBT reduction rate [8]. GSH-Px Determination: Glutathione Peroxidase activity was measured according to Paglia method [9]. GSH-Px activity was calculated with the absorbance decrease during oxidation of NADPH to NADP+ read at 340 nm and the results were expressed in terms of milli-international unit/milligram (mIU/mg) tissue protein. CAT Determination: Catalase activity was measured according to Aebi method [10]. Measurement of catalase enzyme activity was based on the principle of spectro-photometric observation of decrease of absorbance level of hydrogen peroxide at a wave length of 240 nm and the results were expressed in terms of IU/mg. XO Determination: Xanthine oxidase enzyme activity was measured by spectrophotometric determination of absorbance level of uric acid formation from xanthine at a wave length of 293 nm and the results were expressed in terms of mIU/mg [11].
Histopatologic assessment
For light microscopic analysis, liver tissue samples obtained from the animals were fixed by keeping in 10% neutral buffered formalin for 2 days. Tissues were washed with water and dehydrated by treating with ethanol with increasing concentrations (50%, 75%, 96%, and 100%). Following dehydration, specimens were immersed in xylene to obtain transparency. They were then immersed in paraffin and infiltrated. Sections with a thickness of 5 μm from paraffin-immersed tissues were obtained using Leica RM 2125 RT. Sections which were systematically chosen in a random manner were stained with hematoxylin-eosin (H&E) and Periodic Acid-Schiff (PAS). Histopathologic examination was performed by two histologists blinded to the study. Study photographs were taken with Nikon eclipse E 600 and marked.
Statistical analysis
Data were analyzed using SPSS 15.0 package program. Data were given as mean± standard deviation. The differences between the groups were compared with One-Way ANOVA or Kruskal Wallis variance analysis. When p value was significant, Mann-Whitney U multi variance analysis was used to detect the group creating the difference. While evaluating the histopathology results, Student’s t-test variance analysis was used. A p< 0.05 was considered statistically significant.
RESULTS
Table 1 shows the liver function test results (AST, ALT and GGT) of the groups. There was a significant difference between SHAM group and controls in terms of AST and ALT levels (p<0.001). GGT levels were significantly lower in SHAM group compared to control group, albeit statistically non-significant (p=0.393). SHAM group and intraoperative treatment group were significantly different in terms of AST and GGT levels (p<0.001 for AST, p<0.05 for GGT). There was a significant difference between SHAM and preoperative groups in terms of AST and Alt levels (p<0.001 for all). There was a significant difference between control and intraoperative treatment group in terms of all liver function tests (p<0.05 for AST and ALT, p<0.001 for GGT). Control and preoperative treatment group were significantly different in terms of AST and ALT levels (p<0.001 for all). GGT levels were lower in preoperative treatment group, albeit statistically non-significant (p=0.436). There was no difference between treatment groups in terms of AST levels. However, there were significant differences between treatment groups in terms of ALT and GGT levels (p<0.05). ALT levels were lower in preoperative treatment group and GGT levels were lower in intraoperative treatment group. Plasma MDA, GSH-Px and XO results are summarized in Table 2. Control group and other groups were significantly different in terms of all results (p<0.05 for all). Plasma XO and MDA levels were lower in treatment groups than control group, but plasma GSH-Px levels were higher (p<0.05 for all). Tissue MDA levels were lower in treatment groups than control group, but tissue GSH-Px, SOD, CAT and XO levels were higher (p<0.05 for MDA and p<0.001 for others). Treatment groups did not differ significantly in terms of MDA and XO levels, however, GSH-Px levels were significantly higher in treatment group (p<0.001). Liver tissue MDA, GSH-Px, SOD, CAT and XO results are summarized in Table 3. SHAM group and control group differed significantly in terms of all results (p<0.001 for all). SHAM group and treatment groups had significant differences in MDA, GSH-Px, CAT and XO levels (p<0.05 for MDA and XO, p<0.001 for GSH-Px and CAT). Control group and treatment groups were significantly different in terms of all results (p<0.05 for MDA and XO, p<0.001 for GSH-Px, SOD and CAT). MDA, SO, and XO levels were not different in treatment groups, however, GSH-Px and CAT levels were significantly higher in intraoperative treatment group (p<0.001). Tissues from the SHAM group presented no morphological alterations in the normal lobular structure of liver tissue and portal tract (Figure 1A1). The sinusoids can just be seen as pale-stained spaces between the plates of liver cells (Figure 1A2). The hepatic asinus is a more physiologically useful model of liver structure and lies between two or more terminal hepatic venules and blood flows from the portal tracts through the sinusoids to the venules. The asinus is divided into zones 1, 2, and 3. The glycogen in hepatocytes which, being polysaccharide, is PAS-positive was found homogenous in the hepatocyte cytoplasm of all three zones (Figure 1A3). The control group, showed multiple and extensive areas of portal inflammation with a moderate increase in the level of inflammatory cell infiltration (Figure 1B1). We observed an massive congestion in the parenchyma of the liver and dilatation of the sinusoidal spaces (Figure 1B2). The PAS stained sections showed heterogeneous distribution of glycogen in the hepatic asinus. In consequence of the glycolysis there was a significant reduction in the store of glycogen. This reduction was especially evident in the zone 3 which was the mostly affected region of the ischemia in the hepatic asinus. The cytoplasm of the hepatocytes of the zone 3 were homogeneous due to the glycogen loss (Figure 1B3). The marked congestion in the distended sinusoids seen in the control group was restricted to rare areas in the treatment groups (Figure 1C2). The other diagnosis of the isch-aemia-reperfusion group was not observed in both single and ten days dose treated groups (Figure 1C, 1D). As a conclusion; the histological features shown in Figure 1A, 1C and 1D suggests the livers from the sham and diosmin treated animals have a normal liver lobular architecture and cell structure. However, the liver sections obtained from the control group showed portal inflammation, sinusoidal dilatation and congestion and glycogen depletion (Figure B).
TABLE 1.
Liver function tests
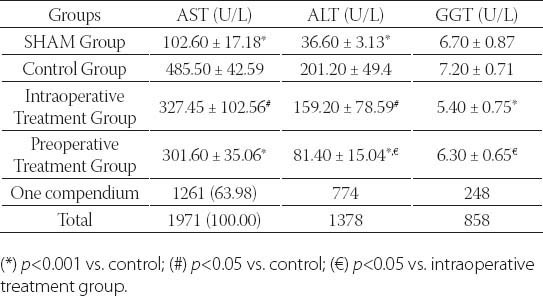
TABLE 2.
Plasma oxidative stress activities of the groups
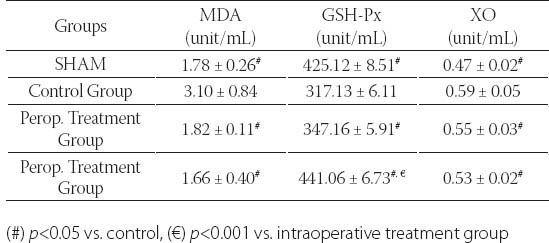
TABLE 3.
Tissue oxidative stress activities of the groups
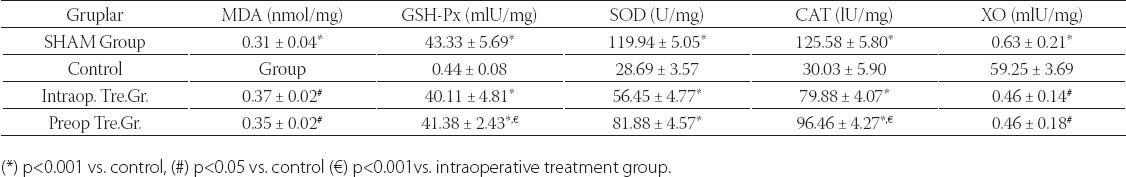
FIGURE 1.
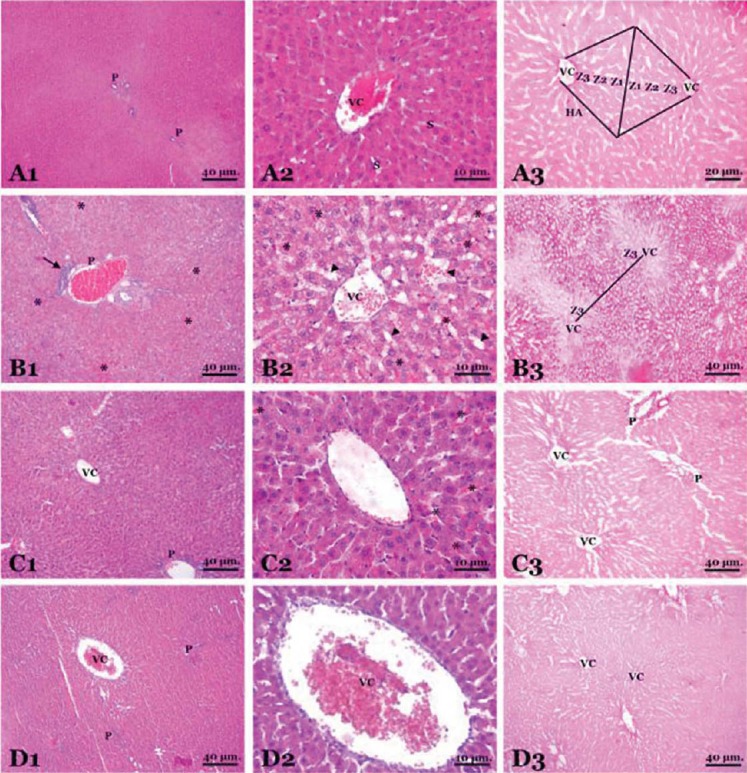
Histopathological findings in groups (A micrographs on the first line is SHAM group, B micrographs on the second line is control group, C micrographs on the third line is intraoperative treatment group and, D micrographs on the fourth line is preoperative treatment group)
Figure Legend:
This panel of the rat liver is stained by hematoxylin and eosin (1st and 2nd micrographs of the each group on the left two columns of the panel) and Periodic Acid-Schiff reaction (3rd micrograps of the each group on the right column of the panel).
A micrographs shows the structure of the liver composed of tightly packed, pink-staining plates of hepatocytes (H). Portal tracts (P) which contain the main blood vessels, hepatic (centrilobular) venule “vena centralis” (VC), the sinusoids (S) are lined by flat endothelial lining cells. The hepatic asinus (HA) lies between two terminal hepatic venules and divided into zones 1,2 and 3 (Z1, Z2, Z3).
B micrographs, inflammatory cell infiltration (arrow) in the portal tract. Sinusoidal dilatation (arrow head) and congestion (*).
C, D micrographs, shows the diosmin treated groups in close morphology to the regular structure of liver except the mild congestion (*) in certain regions.
DISCUSSION
IRI is an important clinical problem involving many organs including brain, heart, kidneys, and liver [1, 2]. Ischemia reperfusion leads to a series of pathologic reactions resulting in cellular death and organ dysfunction. Different mechanisms take part in hepatic cellular damage during ischemia and after reperfusion. Despite it is not known which mechanism is important in the pathogenesis of ischemic cellular damage, oxygen deprivation is the most commonly accused factor. During ischemia, multiple different cellular and subcellular dysfunctions such as mitochondrial dysfunction, dysfunction in cell membrane, and decreased protein synthesis arise. Recent studies have suggested that the most detrimental factor in cellular necrosis after temporary and permanent hepatic ischemia is the reperfusion injury [5]. The most common reasons for IRI in liver are resection surgery for large hepatic tumors, transplantation, and trauma surgery. In addition, hepatic circulation is known to be affected by hemorrhagic shock, advanced sepsis, and severe trauma, independent of the surgery [5, 12]. Hepatic blood flow has to be partially or completely interrupted during surgeries for extensive hepatic damage and large tumor resections. Various experimental studies have demonstrated that liver can tolerate ischemic periods of 90-120 minutes [13]. Recovery of liver function have been reported following vessel clamping up to 65 minutes in hepatic resections [14]. We designed an injury time of 60 minutes for ischemia and 90 minutes for reperfusion, consistent with the literature. Many complex mechanisms such as lipid peroxidation, free radical injury, and increased inflammatory response play a role in ischemic cellular damage. Thus, to prevent negative effects of hepatic ischemia various agents and methods have been employed, including melatonin, L-arginine, allopurinol, and TNF-α [15, 16]. Diosmin, is a hesperidine-derivative bioflavonoid [9]. Flavonoids have antibacterial, antiviral, anti-inflammatory, anti-allergic, and vasodilatory effects. Studies have shown that they also inhibit lipid peroxidation, throm-bocyte aggregation, capillary permeability, and various enzymatic systems including cyclooxygenase and lipooxygenase. In addition, diosmin inhibits formation of free oxygen radicals both in vivo and in vitro [17]. Diosmetin is the active metabolite of diosmin and is rapidly absorbed. Its half-life is 26-43 hours. It reaches peak levels one hour following oral intake and plasma concentration starts to fall by 2 hours [18]. In studies investigating the effects of mikronized purified flavonoid fraction (MPFF) on microcirculation it has been shown that MPFF intercellular adhesion molecule expression, leucocyte adhesion and migration, formation of free oxygen radicals, synthesis of prostoglandin E2, F2α, and tromboksan B2, thrombocyte functions, and increased micro vascular permeability in ischemia-reperfusion. Furthermore, MPFF has favorable effects on lymphatic drainage [19]. Diosmin reinforces venous tonus by prolonging parietal norepinephrine activity. The protective effect of diosmin-hesperidin complex in ischemia reperfusion injury may be explained by preservation of mean arteriolar and venular diameter [20, 21]. Diosmin also has antioxidant effect. Flavonoids inhibit oxidation of low density lipoproteins in vitro. In addition, they exert antioxidant effect against peroxyl and hydroxyl radicals [22]. In a study exploring the effect of diosmin-hesperidin on oxidative stres Unlu et al. [23] showed that, in addition to antioxidant effect, periglomerular and perivascular leucocyte infiltration is significantly lower in rats given diosmin-hesperidin complex. Diosmetin, the main metabolite of diosmin, has been shown to exert protective effect on hepatocytes against cellular damage induced by erithromycine estolate and tert-butylhydroperoxide in humans [24]. Moreover, in another study we conducted in our clinics diosmin decreased small intestinal damage and exerted a protective effect in ischemia reperfusion injury [25]. Liver IRI occurs in a number of clinical settings in general surgery such as trauma, transplantation, hepatic resection and is associated with increased morbidity and mortality. Reactive oxygen radicals and reactive nitrogen species play a major role in the pathophysiology of IRI. The antioxidant defence system is a complex and it normally controls the production their. Oxidative stress occurs when there is significant imbalance between production and removal their. This condition accelerates degradation of membrane phospholipids by damage of lipids, proteins, carbohydrates, and nucleic acids. Hepatocytes tend to be resistant to injury by reactive oxygen and nitrogen species, since they contain high intracellular antioxidants’ concentrations such as GSH-Px, SOD, CAT and lipid soluble antioxidants [26]. MDA, an interval metabolite of the lipid degradation and polyunsaturated fatty acid preoxidation, is a sensitive indicator of IRI [27]. Adenine nucleotides are catabolized to hypoxanthine during ischemic insult. When perfusion of ischemic organ is restored hypoxanthine is oxidized to xanthine by the enzyme xanthine oxidase, releasing free oxygen radicals which cause cell membrane damage by peroxidizing fatty acids found in the structure of phospholipid layer of cell membranes [3]. The XO pathway has been implicated as an important route in the oxidative injury to tissues, especially after ischemia-reperfusion. It scavenges reactive oxygen species and reactive nitrogen species [28]. We observed a significantly lower levels of MDA and XO, markers of tissue lipid peroxidation, in treatment groups. All aerobic creatures are subject to physiologic oxidative stress during metabolism. Body glutathion is an important component of the antioxidant system and protects the cell against oxidative damage by reacting with free radicals and peroxides. Hepatic GSH concentrations have been shown to decrease during hepatic ischemia reperfusion injury. Our study revealed that GSH-Px levels which were measured to assess GSH levels, were significantly high in both treatment groups, particularly so in the group given diosmin preoperatively. CAT, an endogenous antioxidant, and SOD which protects oxygen-metabolizing cells against detrimental effects such as lipid peroxidation of superoxide free radical and has a role in intracellular killing of phagocyted bacteria were also high in the treatment group. The degree of damage ischemia and reperfusion inflict in liver at a cellular basis is best reflected by serum enzyme levels such as ALT, AST, and GGT as well as histopathologic changes [29]. Uhlmann et al. [29] found an AST level of 130 U/L and an ALT level of 78 U/L after reperfusion following a 90-minute partial ischemia in sham-operated rats while the same numbers following reperfusion were 1330 U/L and 750 U/L, respectively. Our study demonstrated significantly lower AST, ALT, and GGT levels in treatment groups, consistent with the literature. Under light microscopy hepatic ischemia reperfusion is characterized by neutrophil infiltration, regional hemorrhage and necrosis, congestion, sinusoidal enlargement, regional hepatocellular vacuolization, hepatocyte swelling while under ultra-structural examination it is evident by distorted mitochondrial structure, swelling, staining differences, and neutrophil aggregation [30]. Crockett et al. [30] observed sinusoidal congestion, cytoplasmic vacuolization, hepatocellular necrosis, neutrophil infiltration, and a high ALT level in hepatic ischemia reperfusion group. We histopathologically investigated dilatation in vena porta branches and vena centralis, sinusoidal congestion, parenchymal congestion, sinusoidal dilatation, and portal inflammatory cell infiltration. We found that SHAM group and diosmin-treated groups had a normal liver and cellular structure in terms of histologic features observed in SHAM, intraoperative diosmin and preoperative diosmin administered groups whereas we found portal inflammation, sinusoidal dilatation, congestion, and glycogen deficiency in tissue sections obtained from control group.
CONCLUSION
In conclusion, we found that diosmin administered both pre-operatively and intraoperatively decreases cellular damage and protects cells against harmful effects during hepatic IRI. However, we also found that this effect is more pronounced in the group treated preoperatively. We think that the protective effect of diosmin may be due to its anti-inflammatory and antioxidant effects. In addition, we think that diosmin treatment can decrease morbidity and mortality by preventing free radical-induced oxygen injury in hepatic ischemia reperfusion. According to findings obtained from our study, we think that diosmin can be used as a protective agent against IRI in both elective and emergent liver surgeries. Nevertheless, further studies are needed to obtain better outcomes.
DECLARATION OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Mccord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- [2].Gasanov F, Aytac B, Vuruskan H. The effects of tadalafil on renal ischemia reperfusion injury: an experimental study. Bosn J Basic Med Sci. 2011;11(3):158–162. doi: 10.17305/bjbms.2011.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Powner DJ. Factors during donor care that may affect liver transplantation outcome. Prog Transplant. 2004;14(3):241–247. doi: 10.1177/152692480401400310. [DOI] [PubMed] [Google Scholar]
- [4].Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125(3):917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- [5].Terao J, Piskula M, Yao Q. Protective effect of epicatechin gallate, and guercetin on lipid peroxidation in phospholipid bilayers. Arch Biochem Biophys. 1994;308(1):278–284. doi: 10.1006/abbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- [6].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- [7].Dahle LK, Hill EG, Holman RT. The thiobarbituric acid reaction and the autoxidations of polyunsaturated fatty acid methyl esters. Arch Biochem Biophys. 1962;98:253–261. doi: 10.1016/0003-9861(62)90181-9. [DOI] [PubMed] [Google Scholar]
- [8].Durak I, Canbolat O, Kavutçu M, Oztürk HS, Yurtarslani Z. Activities of total, cytoplasmic, and mitochondrial superoxide dismutase enzymes in sera and pleural fluids from patients with lung cancer. J Clin Lab Anal. 1996;10(1):17–20. doi: 10.1002/(SICI)1098-2825(1996)10:1<17::AID-JCLA4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [9].Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- [10].Aebi H. Catalase. In: Bergmeyer U, editor. Methods of enzymatic analysis. New York and London: Academic Press; 1974. pp. 673–677. [Google Scholar]
- [11].Hashimoto S. A new spectrophotometric assay method of xanthine oxidase in crude tissue homogenate. Anal Biochem. 1974;62(2):426–435. doi: 10.1016/0003-2697(74)90175-4. [DOI] [PubMed] [Google Scholar]
- [12].Imamovic S, Ljuca F, Imamovic G, Iljazagic Halilovic F, Krdzalic A, Hasukic S, Mesic D, Zerem E. Influence of donor age on renal graft function in first sevenpost transplant days. Bosn J Basic Med Sci. 2010;10(1):73–77. doi: 10.17305/bjbms.2010.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nordlinger B, Douvin D, Javaudin L, Bloch P, Aranda A, Boschat M, Huguet C. An experimental study of survival after two hours of normothermic hepatic ischemia. Surg Gynecol Obstet. 1980;150(6):859–864. [PubMed] [Google Scholar]
- [14].Delva E, Barberousse JP, Nordlinger B, Ollivier JM, Vacher B, Guil-met C, Huguet C. Hemodynamic and biochemical monitoring during major liver resection with use of hepatic vascular exclusion. Surgery. 1984;95(3):309–318. [PubMed] [Google Scholar]
- [15].Kim SH, Lee SM. Cytoprotective effects of melatonin against necrosis and apoptosis induced by ischemia/reperfusion injury in rat liver. J Pineal Res. 2008;44(2):165–171. doi: 10.1111/j.1600-079X.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- [16].Chattopadhyay P, Verma N, Verma A, Kamboj T, Khan NA, Wahi AK. L-arginine protects from pringle manoeuvere of ischemia-re- perfusion induced liver injury. Biol Pharm Bull. 2008;31(5):890–892. doi: 10.1248/bpb.31.890. [DOI] [PubMed] [Google Scholar]
- [17].Tian X, Yang X, Wang K, Yang X. The efflux of flavonoids morin, isorhamnetin-3-O-rutinoside and diosmetin-7-O-beta-D-xylopy- ranosyl-(1-6)-beta-D-glucopyranoside in the human intestinal cell line caco-2. Pharm Res. 2006;23(8):1721–1278. doi: 10.1007/s11095-006-9030-5. [DOI] [PubMed] [Google Scholar]
- [18].Cova D, De Angelis L, Giavarini F, Palladini G, Perego R. Pharma-cokinetics and metabolism of oral diosmin in healthy volunteers. Int J Clin Pharmacol Ther Toxicol. 1992;30(1):29–33. [PubMed] [Google Scholar]
- [19].Lyseng-Williamson KA, Perry CM. Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63(1):71–100. doi: 10.2165/00003495-200363010-00005. [DOI] [PubMed] [Google Scholar]
- [20].Jones SM, Thurman RG. 1-arginine minimizes reperfusion injury in a low-flow, reflow model of liver perfusion. Hepatology. 1996;24(1):163–168. doi: 10.1002/hep.510240127. [DOI] [PubMed] [Google Scholar]
- [21].Bouskela E, Cyrino FZGA, Lerond L. Effects of oral administration of different doses of purified micronized flavonoid fraction on microvascular reactivity after ischemia/reperfusion in the hamster cheek pouch. Br J Pharmacol. 1997;122(8):1611–1616. doi: 10.1038/sj.bjp.0701554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22(5):749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- [23].Unlü A, Sucu N, Tamer L, Coskun B, Yücebilgiç G, Ercan B, et al. Effects of Daflon on oxidative stress induced by hindlimb ischemia/reperfusion. Pharmacol Res. 2003;48(1):11–15. [PubMed] [Google Scholar]
- [24].Villa P, Bégué JM, Guillouzo A. Erythromycin toxicity in primary cultures of rat hepatocytes. Xenobiotica. 1985;15(8-9):767–773. doi: 10.3109/00498258509047439. [DOI] [PubMed] [Google Scholar]
- [25].Tanrikulu Y, Kismet K, Serin Kilicoglu S, Devrim E, Erel S, Sen Tanrikulu C, Dinc S, et al. Diosmin ameliorates intestinal injury induced by hepatic ischemia reperfusion in rats. Bratisl Lek Listy. 2011;112(10):545–551. [PubMed] [Google Scholar]
- [26].Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005;11(9):1031–1047. doi: 10.1002/lt.20504. [DOI] [PubMed] [Google Scholar]
- [27].Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [28].Sanhueza J, Valdes J, Campos R, Garrido A, Valenzuela A. Changes in the xanthine dehydrogenase/xanthine oxidase ratio in the rat kidney subjected to ischemia-reperfusion stress: preventive effect of some flavonoids. Res Commun Chem Pathol Pharmacol. 1992;78(2):211–218. [PubMed] [Google Scholar]
- [29].Uhlmann D, Glasser S, Gaebel G, Armann B, Ludwig S, Tannapfel A, Hauss J, et al. Improvement of postischemic hepatic microcir-culation after endothelinA receptor blockade--endothelin antagonism influences platelet-endothelium interactions. J Gastrointest Surg. 2005;9(2):187–197. doi: 10.1016/j.gassur.2004.06.006. [DOI] [PubMed] [Google Scholar]
- [30].Crockett ET, Galligan JJ, Uhal BD, Harkema J, Roth R, Pandya K. Protection of early phase hepatic ischemia-reperfusion injury by cholinergic agonists. BMC Clin Pathol. 2006;6(3) doi: 10.1186/1472-6890-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


