Abstract
Epilepsy is one of the most common neurological disorders, characterized by recurrent seizures, which may increase the content of reactive oxygen and nitrogen species. The objective of this study was to investigate the effects of Neuropeptide Y on oxidative and nitrosative balance and brain-derived neurotrophic factor levels induced by pentylenetetrazole (a standard convulsant drug) in the hippocampus of Wistar rats. Three groups of seven rats were treated intraperitoneally as follows: group 1 (saline + saline) 1 ml saline, group 2 (salin + Pentylenetetrazole) 1 ml saline 30 min before Pentylenetetrazole; and group 3 (Neuropeptide Y + Pentylenetetrazole) 60 μg/kg Neuropeptide Y 30 min before 60 mg/kg Pentylenetetrazole. After 24 h, the animals were euthanized by decapitation. Hippocampus were isolated to evaluate the malondi-aldehyde, glutathione, nitric oxide, and brain-derived neurotrophic factor levels in three rat groups. The results of this study demonstrated that while intraperitoneally administered neuropeptide Y did not result in a statistically significant difference in BDNF levels, its administration caused a statistically significant decrease in malondialdehyde and nitric oxide levels and an increase in glutathione levels in rats with pentylenetetrazole-induced epileptic seizure. Neuropeptide Y were able to reduce nitroxidative damage induced by pentylenetetrazole in the hippocampus of Wistar rats.
KEY WORDS: epileptic seizure, neuropeptide Y, brain-derived neurotrophic factor, oxidative stress, nitrosative stress
INTRODUCTION
Generalized epilepsy is a chronic disorder characterized by recurrent seizures, which has been shown to cause an increase in reactive oxygen species (ROS) in the brain and thus increased oxidative stress [1]. Oxidative stress and mitochondrial dysfunction can occur as a consequence of prolonged epileptic seizure and may contribute to seizure-induced brain damage [2]. Nitric oxide (NO) free radical is a molecular messenger involved in numerous physiological processes. However, several studies on the complex role of NO in the nervous system have demonstrated that NO plays a role in the pathophysiology of brain damage in brain ischemia, trauma, and chronic epilepsy and contributes to neurotoxicity and neural damage associated with several neurodegenerative diseases [3, 4]. Brain-derived neurotrophicfactor (BDNF) is a small dimeric protein that belongs to the neurotrophin family and was originally identified as a crucial neuronal survival factor [5]. BDNF is abundant in the brain. In addition to its ability to promote neuronal proliferation and differentiation, BDNF influences the shape and number of dendritic spines and thus affects the morphological development of neurons [6-8]. Neuropeptide Y (NPY) is widely expressed in the central nervous system where it is involved in various functions including the regulation of circadian rhythms, blood pressure, feeding behaviour, anxiety, memory processing, and cognition [9]. In the hippocampus, NPY is specifically expressed in a subset of GABAergic neurons in the pyramidal cell layer and in the dentate hilus where it is probably released by the activation of intrinsic and projection pathways [10]. There is also increasing evidence that the peptide exerts powerful anticonvulsant activities and a related neuroprotective effect on cornu ammonis (CA) pyramidal neurons [9, 11, 12]. For these reasons, we studied the acute effects of exogenous intraperitoneally (ip) administered NPY in adult rats following pentylenetetrazole (PTZ) intoxication, particularly during the early phases that are reasonably crucial in the establishment of brain injury. Changes in the hippocampal malondialdehyde (MDA), glutathione (GSH), NO, and BDNF levels were studied after single-dose NPY administration in rats with PTZ-induced epileptic seizure.
MATERIALS AND METHODS
Chemicals
NPY (human, rat, catalog number H-6375) was purchased from Bachem AG, Switzerland. It was reconstituted in sterile distilled H2O at a concentration of 0.1 mM, filter-sterilized, and stored at -20°C. PTZ was obtained from Sigma Chemical Co. (St. Louis, MO, USA). NPY (60 μg/kg) and PTZ (60 mg/kg) were dissolved in saline and injected ip.
Animals
Male Wistar rats weighing 250–300 g were housed in groups with free access to water and standard rat food and kept under a 12-h light/dark cycle. All experiments were conducted after approval from the Kocaeli University Ethics Committee. Efforts were made to minimize animal pain and discomfort.
Experimental design
Three groups of seven rats were treated as follows: group 1 [saline + saline (SF + SF)] 1 ml saline ip; group 2 [(saline + PTZ (SF + PTZ)] 1 ml saline ip 30 min before PTZ; and group 3 (NPY + PTZ) 60 μg/kg NPY ip 30 min before PTZ [13, 14]. A convulsive dose of 60 mg/kg PTZ was used for injection, and the rats were housed in Plexiglas cages (50 cm × 50 cm × 40 cm). Following injections, rats were monitored for seizure activity for 30 min. Animals were sacrificed 24 h after inducing seizures or administering saline. Brains were immediately removed and the hippocampus was dissected. To prepare a 10% (w/v) homogenate, the hippocampus was weighed and homogenized in cold Tris–HCl buffer (pH 7.4). Next, BDNF, MDA, NO, and GSH levels were measured. Lipid peroxide levels, expressed in terms of MDA, were determined according to the method of Buege and Aust [15]. GSH was measured according to the method of Ellman [16]. Nitrite and nitrate were estimated as an index of NO production [17]. The method for determining nitrite and nitrate levels was based on the Griess reagent, which consists of sulfanilamide and N-(1-naphthyl) ethylenediamine. Protein concentrations of hippocampal homogenates were determined by the method of Lowry et al. [18]. The hippocampal BDNF levels were measured using an ELISA kit (BOSTER ELISA; Boster Biological Technology., Ltd., CA, USA) according to the manufacturer’s instructions. All measurements were performed in duplicate.
Statistical analysis
Statistical analysis was performed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA) for Windows (version 13.0). The values were expressed as the mean ± standard deviation (SD). The significance of differences among the three groups was assessed using oneway analysis of variance (ANOVA) with the post-hoc Tukey test. p < 0.05 was considered statistically significant.
RESULTS
The mean MDA levels in the hippocampus were significantly higher in the SF + PTZ group than in the SF + SF and SF + NPY groups (20.13 ± 5.19, 13.51 ± 2.58, 9.65 ± 3.13 nmol/100 mg protein, p < 0.05 and p < 0.001, respectively) (Figure 1). Hippocampal NO levels were significantly higher in the SF + PTZ group than in the SF + SF and SF + NPY groups (7.60 ± 0.73, 3.98 ± 1.18, 3.28 ± 1 μmol/100 mg protein, p < 0.001, respectively) (Figure 2).
FIGURE 1.
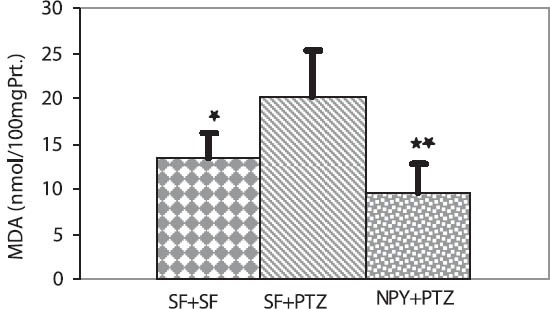
Effect of Neuropeptide Y on MDA levels in the hippocampus of Wistar rats. MDA levels were significantly higher in the SF + PTZ group than in the SF + SF and the NPY + PTZ groups (respective values: *p < 0.05, **p < 0.001).
FIGURE 2.
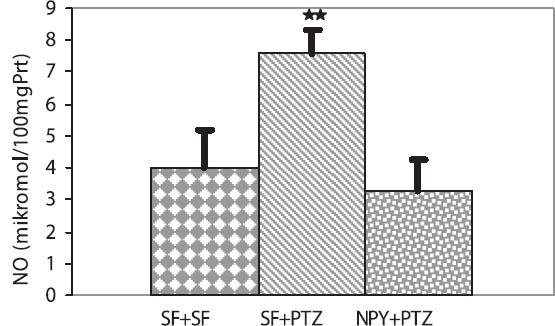
Effect of Neuropeptide Y on NO levels in the hippocampus of Wistar rats. NO levels were significantly higher in the SF + PTZ group than in the SF + SF and the NPY + PTZ groups (respective values: **p < 0.001, **p < 0.001).
In contrast the mean GSH levels were significantly lower in the SF + PTZ group than in the SF + SF, and SF + NPY groups. (14.97 ± 1.65, 26.43 ± 5.86, 30.96 ± 6.02 nmol/ mg protein, p < 0.001 and p < 0.001, respectively) (Figure 3). The mean BDNF levels were significantly higher in the SF + PTZ group than in the SF + SF group (377.9 ± 27.5, 285.8 ± 53.6 ng/g protein, p < 0.001, respectively) (Figure 4). The intra- and inter-assay coefficients of variation for BDNF were 6.84 % and 7.45 %, respectively.
FIGURE 3.

Effect of Neuropeptide Y on GSH levels in the hippocampus of Wistar rats. GSH levels were significantly lower in the SF + PTZ group than in the SF + SF and the NPY + PTZ groups (respective values: **p < 0.001, **p < 0.001).
FIGURE 4.
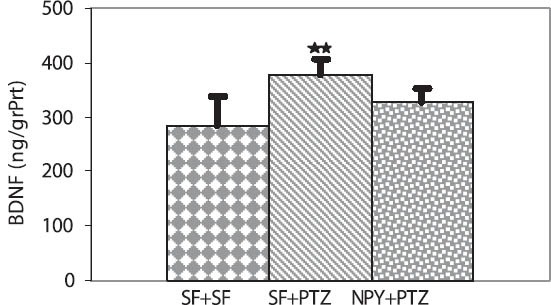
Effect of Neuropeptide Y on BDNF levels in the hippocampus of Wistar rats. BDNF levels were significantly higher in the SF + PTZ group than in the SF + SF group (**p < 0.001).
DISCUSSION
The PTZ-induced clonic seizure paradigm represents an animal model of myoclonic seizures and is very sensitive to changes in seizure susceptibility [19]. Disinhibition of the inhibitory neurotransmitter GABA through specific interactions with the GABA-gated chloride ionophores and/or activation of N-methyl-D-aspartate (NMDA) receptors appears to be among the factors involved in the initiation and generalization of PTZ-induced seizures [20, 21]. ROS/reactive nitrogen species (RNS) have been implicated in the pathogenesis of various neurological disorders including epilepsy [22]. Excitotoxicity and disrupted energy metabolism act in a synergistic manner, leading to nerve cell death in neurodegenerative disorders [23]. These cooperative pathways trigger oxidative stress by free radical formation [24], and ROS/RNS cause lipid peroxidation with high levels of MDA, resulting in damage to biological membranes [25]. Epileptic activity causes excessive production of ROS/RNS, a factor believed to be involved in the mechanisms leading to neurodegeneration and cell death [26, 27]. In the present study, we found that the mean hippocampal MDA levels in rats treated with PTZ to induce epileptic seizures were significantly higher than the control group (SF + SF) and the NPY + PTZ groups. In contrast, statistical analysis showed that PTZ-treated rats had significantly lower GSH levels than saline- and NPY-treated rats. The increased levels of MDA and reduced concentrations of GSH observed in the SF + PTZ group indicated the presence of oxidative stress in epileptic seizure. In the group treated with NPY, there was a statistically significant decrease or increase in MDA or GSH levels, respectively. These findings demonstrate that in addition to its anticonvulsive effects on pre-synaptic Y2 receptors, NPY contributes to the oxidant–antioxidant balance [9, 28, 29]. In the present study, statistically significant levels of NO were detected in the SF + PTZ group, whereas NO levels decreased significantly in the NPY-treated group. NO produced from increased action of the excitatory neurotransmitter glutamate is postulated to be involved in pathophysiology of many models of epilepsy [27, 30, 31]. In neurons, NO synthesis is stimulated by Ca2+ influx, which is induced by activation of glutamate receptors, preferentially by the NMDA receptors [27, 32]. Bal-Price et al. [33] stated that neurons in culture were sensitive to cell death induced by NO, NO donors, or NO-producing cells. A major cause of this sensitivity is inhibition of mitochondrial function by NO, glutamate release, and subsequent excitotoxic death of neurons [33, 34]. High levels of NO lead, in general, to a mixture of nitrosative, nitrative, and oxidative stress, which is referred to as nitroxidative stress [35]. The actions of NO are generally dependent on the following factors: (a) the concentration of NO, (b) the time course of exposure to NO, (c) the presence or absence of a particular ROS at effective levels, and (d) the presence or absence of particular pathways in particular cells. This indicates that NO is cytoprotective at low levels and toxic at high levels [34, 36, 37]. NO can also be harmful under oxidative stress and conditions caused by the oxidation and nitrotyrosination of functional proteins [38]. In our present study, in addition to an increase observed in NO levels, we detected an increase in oxidation levels (high MDA level and low GSH level) in the group with PTZ-induced epilepsy. The ability to control ROS is thus critical in neurodegenerative diseases because neuronal damage occurs when the “oxidant–antioxidant” balance is disturbed in favour of excess oxidative stress [27, 39]. In our present study, the balance observed between oxidative and nitrosative metabolism in the NPY-treated group indicated that exogenous administration of NPY may exert neuroprotective effects in epilepsy. The discovery that limbic seizures increase the levels of mRNAs encoding nerve growth factor [40] led to the idea that seizure-induced expression of neurotrophic factors may contribute to the maintenance of structural and functional changes underlying epileptogenesis [41-44]. Recent in vitro and in vivo findings place BDNF in the cascade of electrophysiologic and behavioral changes that underlie the epileptic state [44]. Transgenic mice overexpressing BDNF exhibit hyperexcitability in the hippocampal CA3 area and entorhinal cortex and show increased status epilepticus severity in response to systemic injection of kainic acid [45]. Accordingly, chronic infusion of BDNF in rat hippocampus eventually results in motor seizures and the infused animals show increased sensitivity to induction of seizures by pilocarpine [46]. The long-term effects of BDNF on epileptogenesis were merely studied in a kindling model. Amygdala kindling was impaired in BDNF knockout mice [47, 48] or by intraventricular infusion of “TrkB receptor bodies” [49], and was abolished in conditional TrkB knockouts [48]. Furthermore, mice exhibiting decreased BDNF signaling developed fewer recurrent seizures after an initial kainic acid-induced status epilepticus [50]. Together, these data lead to the hypothesis of a positive feedback loop, in which seizures increase BDNF expression and in turn aggravate the severity of seizures [51]. For devising antiepileptic therapies, Binder et al. [44] emphasized the importance of understanding the association between BDNF and hyperexcitability in animal models of epilepsy. In this study, hippocampal BDNF was present in effective levels in epileptic rats treated with PTZ to induce seizures. In NPY-treated animals, the decrease was not statistically significant, although there was an apparent decrease in BDNF levels compared with those in the group with PTZ-induced seizures. Corvino et al. [12] investigated the correlation between exogenously administered NPY and BDNF and found that NPY caused an increase in BDNF mRNA levels. However, BDNF levels were evaluated only after 3–5 days to show the effects of NPY in trimethyl-induced hippocampal neurodegeneration and temporal lobe epilepsy. In our present study, to determine BDNF levels, we collected tissues 24 h after administering NPY. Gelfo et al. [52] found that NPY administered ip causes a decrease in BDNF levels in rat hypothalamus but not hippocampus. BDNF production will in turn induce NPY production, considering the current view that BDNF activates intracellular processes leading to transcriptional activation of NPY [46, 53-55]. Those results suggest that Y2 receptors play an important role in the cross-talk between BDNF and NPY and are responsible, at least in part, for the control of BDNF production[56]. In the present study, administration of NPY may have caused an apparent decrease in endogenous BDNF levels through feedback inhibition. Because epilepsy is a chronic disease leading to neurodegeneration, chronic administration of NPY is necessary to clearly demonstrate the effects of NPY on BDNF levels, and such experiments will be more informative. Further investigations are required to elucidate the link between BDNF and NPY in epileptic brains.
CONCLUSION
In conclusion, we demonstrated that single dose treatment with NPY decreased the MDA and NO levels and increased GSH levels in rats with PTZ-induced epileptic seizures. Consideration of the balance between oxidative and nitrosative metabolism strengthens the possibility that NPY exerts neuroprotective effects.
DECLARATION OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- [1].Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001;303(1-2):19–24. doi: 10.1016/s0009-8981(00)00337-5. [DOI] [PubMed] [Google Scholar]
- [2].Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. 2004;37(12):1951–62. doi: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- [3].Estevez AG, Spear N, Manuel SM, Radi R, Henderson CE, Barbeito L, et al. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18(3):923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cardile V, Pavone A, Gulino R, Renis M, Scifo C, Perciavalle V. Expression of brain-derived neurotrophic factor (BDNF) and inducible nitric oxide synthase (iNOS) in rat astrocyte cultures treated with Levetiracetam. Brain Res. 2003;976(2):227–233. doi: 10.1016/s0006-8993(03)02720-3. [DOI] [PubMed] [Google Scholar]
- [5].Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70(5):271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kuczewski N, Porcher C, Gaiarsa JL. Activity-dependent dendritic secretion of brain-derived neurotrophic factor modulates synaptic plasticity. Eur J Neurosci. 2010;32(8):1239–1244. doi: 10.1111/j.1460-9568.2010.07378.x. [DOI] [PubMed] [Google Scholar]
- [8].Zhu H, Chen MF, Yu WJ, Wang WJ, Li F, Liu WC, et al. Time-dependent changes in BDNF expression of pentylenetetrazole-induced hippocampal astrocytes in vitro. Brain Res. 2012;1439:1–6. doi: 10.1016/j.brainres.2011.12.035. [DOI] [PubMed] [Google Scholar]
- [9].Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22(1):25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- [10].Deller T, Leranth C. Synaptic connections of neuropeptide Y (NPY) immunoreactive neurons in the hilar area of the rat hippocampus. J Comp Neurol. 1990;300(3):433–447. doi: 10.1002/cne.903000312. [DOI] [PubMed] [Google Scholar]
- [11].Gray WP. Neuropeptide Y signalling on hippocampal stem cells in health and disease. Mol Cell Endocrinol. 2008;288(1-2):52–62. doi: 10.1016/j.mce.2008.02.021. [DOI] [PubMed] [Google Scholar]
- [12].Corvino V, Marchese E, Giannetti S, Lattanzi W, Bonvissuto D, Biamonte F, et al. The neuroprotective and neurogenic effects of neuropeptide Y administration in an animal model of hippocampal neurodegeneration and temporal lobe epilepsy induced by trimethyltin. J Neurochem. 2012;122(2):415–426. doi: 10.1111/j.1471-4159.2012.07770.x. [DOI] [PubMed] [Google Scholar]
- [13].Gelfo F, De Bartolo P, Tirassa P, Croce N, Caltagirone C, Petrosini L, et al. Intraperitoneal injection of neuropeptide Y (NPY) alters neurotrophin rat hypothalamic levels: Implications for NPY potential role in stress-related disorders. Peptides. 2011;32(6):1320–1323. doi: 10.1016/j.peptides.2011.03.023. [DOI] [PubMed] [Google Scholar]
- [14].Dillioglugil MO, Kir HM, Demir C, Ilbay G, Sahin D, Dillioglugil O, et al. Effect of pentylenetetrazole and sound stimulation induced single and repeated convulsive seizures on the MDA, GSH and NO levels, and SOD activities in rat liver and kidney tissues. Brain Res Bull. 2010;83(6):356–359. doi: 10.1016/j.brainresbull.2010.09.007. [DOI] [PubMed] [Google Scholar]
- [15].Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- [16].Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- [17].Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36(8 Pt 1):1440–1443. [PubMed] [Google Scholar]
- [18].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- [19].Swinyard EA, Kupferberg HJ. Antiepileptic drugs:detection, quantification, and evaluation. Fed Proc. 1985;44(10):2629–2633. [PubMed] [Google Scholar]
- [20].Löscher W, Hönack D, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res. 1991;8(3):171–189. doi: 10.1016/0920-1211(91)90062-k. [DOI] [PubMed] [Google Scholar]
- [21].Yahyavi-Firouz-Abadi N, Tahsili-Fahadan P, Riazi K, Ghahre-mani MH, Dehpour AR. Involvement of nitric oxide pathway in the acute anticonvulsant effect of melatonin in mice. Epilepsy Res. 2006;68(2):103–113. doi: 10.1016/j.eplepsyres.2005.09.057. [DOI] [PubMed] [Google Scholar]
- [22].Frantseva MV, Perez Velazquez JL, Tsoraklidis G, Mendonca AJ, Adamchik Y, Mills LR, Carlen PL, Burnham MW. Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience. 2000;97(3):431–435. doi: 10.1016/s0306-4522(00)00041-5. [DOI] [PubMed] [Google Scholar]
- [23].Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30(4):379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Silva-Adaya D, Pérez-De La Cruz V, Herrera-Mundo MN, Mendoza-Macedo K, Villeda-Hernández J, Binienda Z, et al. Excitotoxic damage, disrupted energy metabolism, and oxidative stress in the rat brain: antioxidant and neuroprotective effects of L-carnitine. J Neurochem. 2008;105(3):677–689. doi: 10.1111/j.1471-4159.2007.05174.x. [DOI] [PubMed] [Google Scholar]
- [25].Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- [26].Itoh K, Watanabe M. Paradoxical facilitation of pentylenetetrazole-induced convulsion susceptibility in mice lacking neuronal nitric oxide synthase. Neuroscience. 2009;59(2):735–743. doi: 10.1016/j.neuroscience.2008.12.040. [DOI] [PubMed] [Google Scholar]
- [27].Swamy M, Yusof WR, Sirajudeen KN, Mustapha Z, Govindasamy C. Decreased glutamine synthetase, increased citrulline-nitric oxide cycle activities, and oxidative stress in different regions of brain in epilepsy rat model. J Physiol Biochem. 2011;67(1):105–113. doi: 10.1007/s13105-010-0054-2. [DOI] [PubMed] [Google Scholar]
- [28].Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21(15):5804–5812. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sperk G, Drexel M, Pirker S. Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia. 2009;50(Suppl 12):29–31. doi: 10.1111/j.1528-1167.2009.02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lapouble E, Montécot C, Sevestre A, Pichon J. Phosphinothricin induces epileptic activity via nitric oxide production through NMDA receptor activation in adult mice. Brain Res. 2002;957(1):46–52. doi: 10.1016/s0006-8993(02)03597-7. [DOI] [PubMed] [Google Scholar]
- [31].Rundfeldt C, Koch R, Richter A, Mevissen M, Gerecke U, Löscher W. Dose-dependent anticonvulsant and proconvulsant effects of nitric oxide synthase inhibitors on seizure threshold in a cortical stimulation model in rats. Eur J Pharmacol. 1995;274(1-3):73–81. doi: 10.1016/0014-2999(94)00711-f. [DOI] [PubMed] [Google Scholar]
- [32].Radenovic L, Selakovic V. Differential effects of NMDA and AMPA/kainate receptor antagonists on nitric oxide production in rat brain following intrahippocampal injection. Brain Res Bull. 2005;67(1-2):133–141. doi: 10.1016/j.brainresbull.2005.06.019. [DOI] [PubMed] [Google Scholar]
- [33].Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21(17):6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brown GC. Nitric oxide and neuronal death. Nitric Oxide. 2010;23(3):153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- [35].Lancaster JR., Jr Nitroxidative, nitrosative, and nitrative stress: kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol. 2006;19(9):1160–1174. doi: 10.1021/tx060061w. [DOI] [PubMed] [Google Scholar]
- [36].Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, et al. Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci U S A. 2004;101(24):8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thomas DD, Ridnour LA, Espey MG, Donzelli S, Ambs S, Hussain SP, et al. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 2006;281(36):25984–25993. doi: 10.1074/jbc.M602242200. [DOI] [PubMed] [Google Scholar]
- [38].Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76(2):126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- [39].Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145(1):256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245(4919):758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- [41].Gall C, Lauterborn J, Bundman M, Murray K, Isackson P. Seizures and the regulation of neurotrophic factor and neuropeptide gene expression in brain. Epilepsy Res Suppl. 1991;4:225–245. [PubMed] [Google Scholar]
- [42].Gall CM, Lauterborn JC, Guthrie KM, Stinis CT. Seizures and the regulation of neurotrophic factor expression: associations with structural plasticity in epilepsy. Adv Neurol. 1997;72:9–24. [PubMed] [Google Scholar]
- [43].Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol. 2001;63(2):125–149. doi: 10.1016/s0301-0082(00)00022-8. [DOI] [PubMed] [Google Scholar]
- [44].Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Croll SD, Suri C, Compton DL, Simmons MV, Yancopoulos GD, Lindsay RM, et al. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93(4):1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Scharfman HE, Goodman JH, Sollas AL, Croll SD. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp Neurol. 2002;174(2):201–214. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- [47].Kokaia M, Ernfors P, Kokaia Z, Elmér E, Jaenisch R, Lindvall O. Suppressed epileptogenesis in BDNF mutant mice. Exp Neurol. 1995;133(2):215–224. doi: 10.1006/exnr.1995.1024. [DOI] [PubMed] [Google Scholar]
- [48].He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43(1):31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- [49].Binder DK, Routbort MJ, Ryan TE, Yancopoulos GD, McNamara JO. Selective inhibition of kindling development by intraventricular administration of TrkB receptor body. J Neurosci. 1999;19(4):1424–1436. doi: 10.1523/JNEUROSCI.19-04-01424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lähteinen S, Pitkänen A, Saarelainen T, Nissinen J, Koponen E, Castren E. Decreased BDNF signalling in transgenic mice reduces epileptogenesis. Eur J Neurosci. 2002;15(4):721–734. doi: 10.1046/j.1460-9568.2002.01897.x. [DOI] [PubMed] [Google Scholar]
- [51].Heinrich C, Lähteinen S, Suzuki F, Anne-Marie L, Huber S, Häussler U, et al. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol Dis. 2011;42(1):35–47. doi: 10.1016/j.nbd.2011.01.001. [DOI] [PubMed] [Google Scholar]
- [52].Gelfo F, Tirassa P, De Bartolo P, Croce N, Bernardini S, Caltagirone C, et al. NPY intraperitoneal injections produce antidepressant-like effects and downregulate BDNF in the rat hypothalamus. CNS Neurosci Ther. 2012;8(6):487–492. doi: 10.1111/j.1755-5949.2012.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Reibel S, Vivien-Roels B, Lê BT, Larmet Y, Carnahan J, Marescaux C, et al. Overexpression of neuropeptide Y induced by brain-derived neurotrophic factor in the rat hippocampus is long lasting. Eur J Neurosci. 2000;12(2):595–605. doi: 10.1046/j.1460-9568.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- [54].Barnea A, Roberts J, Croll SD. Continuous exposure to brain-derived neurotrophic factor is required for persistent activation of TrkB receptor, the ERK signaling pathway, and the induction of neuropeptide Y production in cortical cultures. Brain Res. 2004;1020(1-2):106–117. doi: 10.1016/j.brainres.2004.06.018. [DOI] [PubMed] [Google Scholar]
- [55].Wirth MJ, Patz S, Wahle P. Transcellular induction of neuropeptide Y expression by NT4 and BDNF. Proc Natl Acad Sci U S A. 2005;102(8):3064–3069. doi: 10.1073/pnas.0404712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xapelli S, Bernardino L, Ferreira R, Grade S, Silva AP, Salgado JR, et al. Interaction between neuropeptide Y (NPY) and brain-derived neurotrophic factor in NPY-mediated neuroprotection against excitotoxicity: a role for microglia. Eur J Neurosci. 2008;27(8):2089–2102. doi: 10.1111/j.1460-9568.2008.06172.x. [DOI] [PubMed] [Google Scholar]


