Abstract
Maintaining the quality of life by preserving ovarian function in premenopausal patients with cervical cancer undergoing radiation is crucial. This can be accomplished with a simple and safe laparoscopic ovarian transposition procedure. This procedure aims to move the ovary out of the irradiation field, protecting it from direct radiation and irreversible damage and preserving its function. However, this procedure is often forgotten and seldom offered to patients. This review aims to lay stress on and reconsider the importance of laparoscopic ovarian transposition as a simple, safe, and extremely useful procedure. The biological effects of radiation are described briefly and several studies are evaluated, which reveal that this procedure has more benefits than risks.
1. Introduction
Cervical cancer is one of the commonest malignancies in Indonesian women. The annual incidence is 9.25 per 100000 population. Due to advances in screening and treatment, patients with cervical cancer are being diagnosed at a younger age and earlier stage of the disease. Approximately half the patients are premenopausal and under 45 years old. Radiotherapy, which constitutes almost 80% of all cancer treatment modalities, causes irreversible ovarian damage and leads to premature menopause, which affects the quality of life. Young women with cervical cancer who are irradiated often have to suffer the long-term consequences of ovarian failure. In addition to curing those patients, maintaining their quality of life is important for a gynecologist. Improved quality of life is significantly associated with improved survival in patients with cervical cancer [1–8].
A simple procedure for preventing radiotherapy-induced ovarian damage is laparoscopic ovarian transposition. This procedure has not been implemented widely even though many studies have revealed its benefits and efficacy. This review assesses the use of ovarian transposition as an effective method for preserving ovarian function in young patients with cervical cancer undergoing radiotherapy and compares the benefits of ovarian transposition performed via laparoscopy and via laparotomy [9–13].
2. The Biological Effect of Radiotherapy and the Fate of Retained versus Transposed Ovaries
Radiotherapy aims to deliver a precisely measured dose of radiation to a defined tumor volume with minimum possible damage to the surrounding normal tissue. Ionizing radiotherapy interacts with DNA. The initial DNA damage leads to a cascade of biologic events that cause lethality when the cells attempt to divide (mitotic death) or programmed cell death (apoptosis), as well as sublethal damage that leads to aging, malformations, and malfunction (Figure 1) [14, 15].
Figure 1.
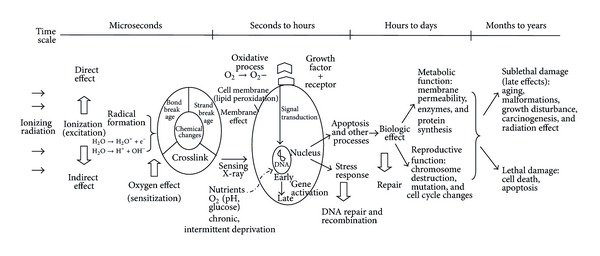
Fate of irradiated cell. Schematic representation of the various processes that take place after cell irradiation [14–16].
The human ovary contains a finite number of ovarian follicles, which are vulnerable to DNA damage from radiotherapy. The degree and persistence of ovarian damage and the suppression of its function are related to the patient's age and the dose of radiation delivered to the ovaries. After the ovaries are exposed to ionizing radiation, if the dose of radiation exceeds the lethal dose, most of the primordial follicles and granulose cells will die in microseconds. Some of those follicles and cells experience sublethal damage, leading to accelerated functional failure. Only a small number would escape the damage, undergo repair, and still have their function. Pyknotic granulose cells would be seen soon after irradiation, and with sufficient destruction of the granulose cells, the follicle would become atrophic. There would be a loss of the cortical stromal cells, and in time, the cortical volume would be replaced by collagen [6, 12, 13, 15, 17, 18].
The cutoff dose for radiation-induced ovarian failure is around 8–20 Gy. A dose >8 Gy causes permanent ovarian damage in almost all patients older than 40 years. A dose >20 Gy causes permanent sterility in patients of any age, with complete or near complete disappearance of the primordial follicles. Radiotherapy used in cervical cancer treatment consists of high-dose external radiotherapy and brachytherapy. The dose of radiotherapy for cervical cancer should be lethal to the cervical tumor tissue. It ranges from 45 Gy to as high as 90 Gy, exceeding the lethal dose for ovarian follicles, and results in permanent damage and loss of ovarian functions in all patients of any age, unless some interventions are applied. Transposing the ovaries is a method of minimizing ovarian follicle exposure to radiation [12, 13, 19].
3. Rationale, Benefits, Complications, and Indications of Ovarian Transposition
Transposing the ovary away from the radiation field can be done surgically at the time of radical hysterectomy or before radiotherapy. In patients with cervical cancer, the ovaries are transposed or moved to the lateral side of the abdomen above the pelvic inlet to place them far enough from external pelvic radiation exposure or brachytherapy. If the ovaries are transposed laterally, about 3 cm above the pelvic inlet, they will receive only 1%–10% of the total radiotherapy dose. If the total radiotherapy dose is 45 Gy, then the dose received by the transposed ovaries is only 0.45–4.5 Gy, whereas the retained ovaries could receive 50%–70% of the total dose, which is 20–32 Gy [13, 20–24] (Figure 2).
Figure 2.
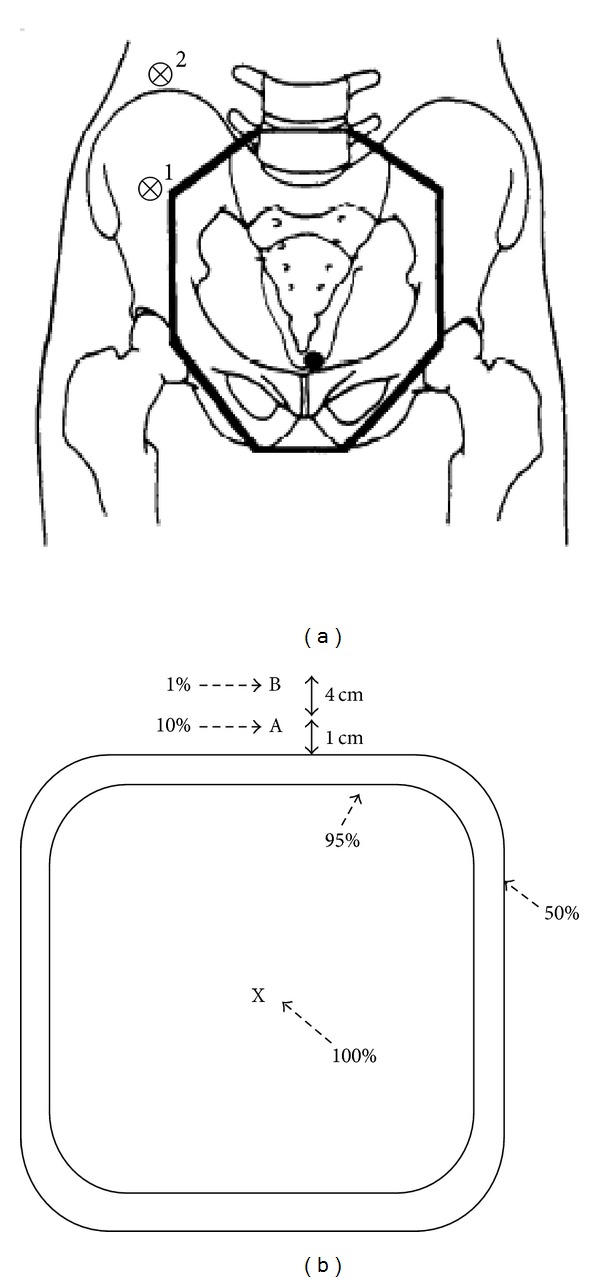
(a) Diagram showing the (AP-PA) whole pelvic radiation therapy field relative to the position of the transposed ovary. Position 1 represents the suboptimal placement of the ovary; position 2 represents the optimal placement of the ovary in terms of maintaining ovarian function. (b) Diagram of a dose distribution. The dose at point A is 10% of the dose at the center of the field (point X, 100%). The dose at point B is 1% of the dose at point X. Therefore, if the prescribed dose was 45 Gy, the dose is 4.5 Gy at point A and 0.45 Gy at point B [13, 25].
Successfully preserving ovarian function depends on the distance between the transposed ovaries and the edge of the radiation field. Therefore, the ovaries should be transposed as laterally and as cranially as possible from the pelvic brim. However, attention should be paid to avoid torsion and extension of the ovarian vessels, which may reduce blood supply to the ovaries [26, 27].
Lateral ovarian transposition can be performed towards the subcutaneous tissue of the flank or the paracolic gutter. However, attaching the ovary to the flank produces more pain complaints than lateral transposition of the ovaries into the paracolic gutter, which is more widely accepted and results in minimal complications (Table 1). Lateral ovarian transposition to the paracolic gutter lateral to the ascending or descending colon is considered a simple standard procedure and can be done laparoscopically [23, 25, 28–32].
Table 1.
Studies reporting the outcome of lateral ovarian transposition in patients with cervical cancer.
| Author | Procedure | Number of subjects | Position of transposed ovary/ovaries | Therapy | Outcome |
|---|---|---|---|---|---|
| Feeney et al. [29] | Lateral ovarian transposition following hysterectomy | 28 | To the paracolic gutter | RT/RH + RT | Ovarian preservation was achieved in 14/28 (50%) patients |
|
| |||||
| Fujiwara et al. [28] | Subcutaneous ovarian transposition following hysterectomy | 27 | To the fascia of the abdominal tissue | RT + RH | Only 12 patients (44%) had normal ovarian function |
|
| |||||
| Anderson et al. [23] | Ovarian transposition | 82 | Sutured to the posterior peritoneum, above the pelvic brim at the level of the lower pole of the kidney | RT | Ovarian preservation was achieved in 53% of subjects. Painful ovarian cyst occurred in 20% of cases. There was one case of ovarian metastasis (1.2%) |
|
| |||||
| Huang et al. [24] | Laparoscopic bilateral ovarian transposition | 14 (<45 years old) | To a high anterolateral position, 3-4 cm above the umbilical line | CCRT/RT/RH + RT/NCT + RH + RT | No intraoperative or postoperative complications occurred. No metastasis was observed. All patients tolerated the procedure. Seven of the 14 patients (50%) developed ovarian failure, shown by the elevation of FSH level |
|
| |||||
| Morice et al. [31] | Bilateral ovarian transposition | 107 (21–42 years old) | To the paracolic gutter (laparotomy, 102 cases; laparoscopy, 5 cases) | RT/RH + RT | One case (1%) with ovarian metastasis. No other postoperative complications occurred. Ovarian function preservation was achieved in 83% of patients |
|
| |||||
| Morice et al. [33] | Bilateral ovarian transposition | 24 | To the paracolic gutter (laparoscopy) | RT/RH + RT/NCT + RT + RH | Ovarian preservation was achieved in 79% patients; three pregnancies were obtained |
|
| |||||
| Chambers et al. [25] | Lateral ovarian transposition (by laparotomy) | 34 | Below and above the iliac crest | RT/RH + RT/CCRT | Ovarian preservation was achieved in 71%. Symptomatic ovarian cyst occurred in 18% of cases |
|
| |||||
| Clough et al. [30] | Laparoscopic unilateral (right) ovarian transposition | 20 | To the paracolic gutter | RT | There were (18/20; 85.3%) cases with normal ovarian function. No postoperative complication was observed |
|
| |||||
| van Eijkeren et al. [34] | Lateral ovarian transposition following hysterectomy | 18 | To the abdominal sidewall at the level of the lowest rib | RT | Ovarian preservation was achieved in 13/18 (72%) patients |
CCRT: concurrent chemotherapy radiotherapy (adding cisplatin as radio sensitizer with a dose of 50 mg/m2 weekly for 6 courses); RT: radiotherapy; RH: radical hysterectomy; NCT: neoadjuvant chemotherapy (combined cisplatin 50 mg/m2, vincristine 1 mg/m2, and bleomycin 25 mg/m2 in an interval of 10 days, 3 courses in total).
Studies on lateral ovarian transposition show that it is 44%–85% effective in preserving ovarian function and that complications such as symptomatic ovarian cyst formation range from 0% to 27%. Symptomatic cyst formation occurs more frequently after lateral ovarian transposition to the subcutaneous adipose tissue (20%–27%) than after lateral ovarian transposition to the paracolic gutter. Ovarian metastasis is rare (0%–1.2%) but is reported. A case of abdominal trocar insertion metastasis after laparoscopic lateral ovarian transposition in a patient with cervical cancer, adenocarcinoma stage IIB was reported. The incidence of trocar insertion metastasis is <1%. As lateral ovarian transposition to the paracolic gutter is a simple and safe procedure for preserving ovarian function, its benefits outweighs the risks of complications [23–25, 28–35] (Table 1).
Lateral ovarian transposition is indicated in young patients with cervical cancer after radical hysterectomy, after neoadjuvant chemotherapy, before radiotherapy, or before concurrent chemoradiotherapy. Some studies showed that the addition of cisplatin in a low dose of 50 mg/m2, used in neoadjuvant chemotherapy or concurrent chemoradiotherapy, did not significantly alter ovarian function. However, adding cisplatin concurrently as radiotherapy functioned as a radiosensitizer and was proven to have better results in some studies. The addition of vincristine and bleomycin in low doses of 1 mg/m2 and 25 mg/m2, respectively, as neoadjuvant chemotherapy did not alter ovarian function either. Lateral ovarian transposition is still used in young patients with cervical cancer after they receive chemotherapeutic agents such as cisplatin, vincristine, and bleomycin in low doses as used in neoadjuvant chemotherapy or concurrent chemoradiotherapy [24, 25, 33, 36–42].
4. Uterine Transplantation and Surrogacy
Besides maintaining ovarian function, ovarian transposition can be used in women who wish to maintain their fertility and reproductive function. For patients with cervical cancer who have undergone radiotherapy that extended to the uterus, and/or hysterectomy, uterine transplantation and surrogacy after ovarian transposition are alternatives. However, uterine transplantation has succeeded only in the animal research setting, and only one failed attempt of human uterus transplantation has been reported. Moreover, uterus transplantation and surrogacy are still under ethical debate concerning both the recipient and the donor's reproductive rights [43–46].
5. Laparoscopy versus Laparotomy Unilateral Ovarian Transposition
Lateral ovarian transposition is a simple procedure that can be performed laparoscopically. Laparoscopic ovarian transposition is superior to laparotomy. Many studies showed that laparoscopic ovarian transposition, like other minimally invasive procedures, produced less risk of adhesion, inflammation, and shortened length of hospital stay in addition to reduced recovery time, resulting in fewer delays in radiotherapy compared with laparotomy. Since laparotomy necessitates a longer postoperative recovery time, it significantly delays radiotherapy. In some cases, the transposed ovary has returned to the previous position due to the delay, resulting in failure to preserve the ovaries [9, 10, 33].
Laparoscopic ovarian transposition can be performed in a day and the patient is sent for radiotherapy. The minimal postoperative wound will not affect mobility and functional activity, as well as quality of life in cervical cancer patients. Studies showed that the risk of adhesion and cyst formation was less with laparoscopic ovarian transposition compared with laparotomy [9, 10, 28, 29, 33, 34].
Laparoscopic ovarian transposition can be performed on either ovary. Studies show that steroid hormone production from only one ovary is enough to prevent ovarian function failure. Clough et al. [30] and Giacalone et al. [47] showed that unilateral right ovarian transposition effectively preserves ovarian function in 85% of subjects. Additionally, unilateral ovarian transposition of the right ovary to the paracolic gutter, as high as the subhepatic region, is technically easier, resulting in fewer complications.
It is important to measure ovarian reserve in patients with cervical cancer aged 41–49 years and in those who have received neoadjuvant chemotherapy before laparoscopic ovarian transposition. To test the ovarian reserve, the menstruation cycle should be assessed and levels of the follicle stimulating hormone (FSH), estradiol, and anti-Müllerian hormone (AMH) should be measured. The AMH has an added value since its level is independent of the menstrual cycle, so its assessment can be used in patients who have undergone radical hysterectomy or experienced acute amenorrhea due to neoadjuvant chemotherapy. In some studies, the cutoff value of the AMH indicating good ovarian reserve varied, such as ≥0.3 ng/mL, ≥0.5 ng/mL, and ≥1.4 ng/mL [48–50].
6. Conclusion
Laparoscopic ovarian transposition is a simple, safe, effective, but often forgotten, procedure for young premenopausal patients with cervical cancer who are undergoing radiotherapy. We believe this procedure should be offered to all young premenopausal patients with cervical cancer undergoing radiotherapy to preserve their ovarian function. However, further studies to evaluate the efficacy of laparoscopic ovarian transposition in our center are still needed.
Conflict of Interests
No potential conflict of interests relevant to this paper was reported.
References
- 1.Randall ME, Michael H, Morken JV, Stehman F. Uterine cervix. In: Hoskins WJ, Perez C, Young RC, Barakat RR, Markman M, Randall ME, editors. Principles and Practice of Gynecologic Oncology. 4th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2005. pp. 743–822. [Google Scholar]
- 2.Castlellsague X, de Sanjose S, Aguado, et al. HPV and cervical cancer in the world 2007 report. Vaccine. 2007;25(supplement 3):C1–C26. [Google Scholar]
- 3.Aziz MF. Gynecological cancer in Indonesia. Journal of Gynecologic Oncology. 2009;20(1):8–10. doi: 10.3802/jgo.2009.20.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schellekens MC, Dijkman A, Aziz MF, et al. Prevalence of single and multiple HPV types in cervical carcinomas in Jakarta, Indonesia. Gynecologic Oncology. 2004;93(1):49–53. doi: 10.1016/j.ygyno.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Kesic V. Management of cervical cancer. European Journal of Surgical Oncology. 2006;32(8):832–837. doi: 10.1016/j.ejso.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Stroud JS, Mutch D, Rader J, Powell M, Thaker PH, Grigsby PW. Effects of cancer treatment on ovarian function. Fertility and Sterility. 2009;92(2):417–427. doi: 10.1016/j.fertnstert.2008.07.1714. [DOI] [PubMed] [Google Scholar]
- 7.Korfage IJ, Essink-Bot M, Mols F, van de Poll-Franse L, Kruitwagen R, van Ballegooijen M. Health-related quality of life in cervical cancer survivors: a population-based survey. International Journal of Radiation Oncology Biology Physics. 2009;73(5):1501–1509. doi: 10.1016/j.ijrobp.2008.06.1905. [DOI] [PubMed] [Google Scholar]
- 8.Wahidin M, Noviani R, Hermawan S, Andriani V, Ardian A, Djarir H. Population-based cancer registration in Indonesia. Asian Pacific Journal of Cancer Prevention. 2012;13(4):1709–1710. doi: 10.7314/apjcp.2012.13.4.1709. [DOI] [PubMed] [Google Scholar]
- 9.Chen MD, Teigen GA, Reynolds HT, Johnson PR, Fowler JM. Laparoscopy versus laparotomy: an evaluation of adhesion formation after pelvic and paraaortic lymphadenectomy in a porcine model. The American Journal of Obstetrics and Gynecology. 1998;178(3):499–503. doi: 10.1016/s0002-9378(98)70428-4. [DOI] [PubMed] [Google Scholar]
- 10.Bisharah M, Tulandi T. Laparoscopic preservation of ovarian function: an underused procedure. The American Journal of Obstetrics and Gynecology. 2003;188(2):367–370. doi: 10.1067/mob.2003.38. [DOI] [PubMed] [Google Scholar]
- 11.Ashing-Giwa KT, Lim JW, Tang J. Surviving cervical cancer: does health-related quality of life influence survival? Gynecologic Oncology. 2010;118(1):35–42. doi: 10.1016/j.ygyno.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Falcone T, Attaran M, Bedaiwy MA, Goldberg JM. Ovarian function preservation in the cancer patient. Fertility and Sterility. 2004;81(2):243–257. doi: 10.1016/j.fertnstert.2003.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Barbera L. Effects of pelvic radiation therapy on fertility. CME Journal of Gynecologic Oncology. 2003;8(2):101–106. [Google Scholar]
- 14.Falk S. Principles of cancer treatment by radiotherapy. Surgery. 2009;27(4):169–172. [Google Scholar]
- 15.Perez C, Purdy J, Li Z, Hall E. Biologic and physical aspects of radiation oncology. In: Hoskins W, Perez C, Young R, Barakat R, Markman M, Mandall M, editors. Principles and Practice of Gynecologic Oncology. 4th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2005. pp. 375–459. [Google Scholar]
- 16.Yarnold J. Molecular aspects of cellular responses to radiotherapy. Radiotherapy and Oncology. 1997;44(1):1–7. doi: 10.1016/s0167-8140(97)00049-2. [DOI] [PubMed] [Google Scholar]
- 17.Presti AL, Ruvolo G, Gancitano RA, Cittadini E. Ovarian function following radiation and chemotherapy for cancer. European Journal of Obstetrics Gynecology and Reproductive Biology. 2004;113:S33–S40. doi: 10.1016/j.ejogrb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Kim SS. Fertility preservation in female cancer patients: current developments and future directions. Fertility and Sterility. 2006;85(1):1–11. doi: 10.1016/j.fertnstert.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto K, Sakuragi N, Fujimoto S. Ovarian function and survival of patients with cervical carcinoma treated with radical hysterectomy and ovarian transposition. In: Leung P, Adashi E, editors. The Ovary. 2nd edition. New York, NY, USA: Elsevier; 2004. pp. 559–567. [Google Scholar]
- 20.Maltaris T, Seufert R, Fischl F, et al. The effect of cancer treatment on female fertility and strategies for preserving fertility. European Journal of Obstetrics Gynecology and Reproductive Biology. 2007;130(2):148–155. doi: 10.1016/j.ejogrb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JD, Hitchen C, Vlachaki MT. Role of ovarian transposition based on the dosimetric effects of craniospinal irradiation (CSI) on the ovaries: a case report. International Journal of Radiation Oncology Biology Physics. 2006;66(3, supplement):p. S708. doi: 10.1016/j.meddos.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Bidzinski M, Lemieszczuk B, Zielinski J. Evaluation of the hormonal function and features of the ultrasound picture of transposed ovary in cervical cancer patients after surgery and pelvic irradiation. European Journal of Gynaecological Oncology. 1993;14, supplement:77–80. [PubMed] [Google Scholar]
- 23.Anderson B, LaPolla J, Turner D, Chapman G, Buller R. Ovarian transposition in cervical cancer. Gynecologic Oncology. 1993;49(2):206–214. doi: 10.1006/gyno.1993.1109. [DOI] [PubMed] [Google Scholar]
- 24.Huang KG, Lee CL, Tsai CS, Han CM, Hwang LL. A new approach for laparoscopic ovarian transposition before pelvic irradiation. Gynecologic Oncology. 2007;105(1):234–237. doi: 10.1016/j.ygyno.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Chambers SK, Chambers JT, Kier R, Peschel RE. Sequelae of lateral ovarian transposition in irradiated cervical cancer patients. International Journal of Radiation Oncology Biology Physics. 1991;20(6):1305–1308. doi: 10.1016/0360-3016(91)90242-v. [DOI] [PubMed] [Google Scholar]
- 26.Kwik M, O’Neill A, Hamani Y, Chapman M, Chou D. Laparoscopic ovarian transposition with potential preservation of natural fertility. Journal of Minimally Invasive Gynecology. 2010;17(4):411–412. doi: 10.1016/j.jmig.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Tulandi T, Al-Took S. Laparoscopic ovarian suspension before irradiation. Fertility and Sterility. 1998;70(2):381–383. doi: 10.1016/s0015-0282(98)00155-1. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara K, Mohri H, Yoshida T, Yamauchi H, Kohno I. Subcutaneous transposition of the ovary following hysterectomy. International Journal of Gynecology and Obstetrics. 1997;58(2):223–228. doi: 10.1016/s0020-7292(97)00087-8. [DOI] [PubMed] [Google Scholar]
- 29.Feeney DD, Moore DH, Look KY, Stehman FB, Sutton GP. The fate of the ovaries after radical hysterectomy and ovarian transposition. Gynecologic Oncology. 1995;56(1):3–7. doi: 10.1006/gyno.1995.1002. [DOI] [PubMed] [Google Scholar]
- 30.Clough KB, Goffinet F, Labib A, et al. Laparoscopic unilateral ovarian transposition prior to irradiation: prospective study of 20 cases. Cancer. 1996;77(12):2638–2645. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2638::AID-CNCR30>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 31.Morice P, Juncker L, Rey A, El-Hassan J, Haie-Meder C, Castaigne D. Ovarian transposition for patients with cervical carcinoma treated by radiosurgical combination. Fertility and Sterility. 2000;74(4):743–748. doi: 10.1016/s0015-0282(00)01500-4. [DOI] [PubMed] [Google Scholar]
- 32.Morice P, Haie-Meder C, Pautier P, Lhomme C, Castaigne D. Ovarian metastasis on transposed ovary in patients treated for squamous cell carcinoma of the uterine cervix: report of two cases and surgical implications. Gynecologic Oncology. 2001;83(3):605–607. doi: 10.1006/gyno.2001.6447. [DOI] [PubMed] [Google Scholar]
- 33.Morice P, Castaigne D, Haie-Meder C, et al. Laparoscopic ovarian transposition for pelvic malignancies: indications and functional outcomes. Fertility and Sterility. 1998;70(5):956–960. doi: 10.1016/s0015-0282(98)00284-2. [DOI] [PubMed] [Google Scholar]
- 34.van Eijkeren MA, van der Wijk I, El Sharouni SY, Heintz APM. Benefits and side effects of lateral ovarian transposition (LOT) performed during radical hysterectomy and pelvic lymphadenectomy for early stage cervical cancer. International Journal of Gynecological Cancer. 1999;9(5):396–400. doi: 10.1046/j.1525-1438.1999.99051.x. [DOI] [PubMed] [Google Scholar]
- 35.Picone O, Aucouturier JS, Louboutin A, Coscas Y, Camus E. Abdominal wall metastasis of a cervical adenocarcinoma at the laparoscopic trocar insertion site after ovarian transposition: case report and review of the literature. Gynecologic Oncology. 2003;90(2):446–449. doi: 10.1016/s0090-8258(03)00271-3. [DOI] [PubMed] [Google Scholar]
- 36.Stehman FB, Ali S, Keys HM, et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a gynecologic oncology group trial. The American Journal of Obstetrics and Gynecology. 2007;197(5):503.e1–503.e6. doi: 10.1016/j.ajog.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Candelaria M, Garcia-Arias A, Cetina L, Dueñas-Gonzalez A. Radiosensitizers in cervical cancer. Cisplatin and beyond. Radiation Oncology. 2006;1(1, article 15) doi: 10.1186/1748-717X-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi Y, Akiyama F, Hasumi K. A case of successful pregnancy after treatment of invasive cervical cancer with systemic chemotherapy and conization. Gynecologic Oncology. 2006;100(1):213–215. doi: 10.1016/j.ygyno.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Maneo A, Chiari S, Bonazzi C, Mangioni C. Neoadjuvant chemotherapy and conservative surgery for stage IB1 cervical cancer. Gynecologic Oncology. 2008;111(3):438–443. doi: 10.1016/j.ygyno.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstetrics and Gynecology. 2003;101(2):251–257. doi: 10.1016/s0029-7844(02)02508-5. [DOI] [PubMed] [Google Scholar]
- 41.Seshadri T, Hourigan MJ, Wolf M, Mollee PN, Seymour JF. The effect of the Hyper-CVAD chemotherapy regimen on fertility and ovarian function. Leukemia Research. 2006;30(4):483–485. doi: 10.1016/j.leukres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Kang H, Kim TJ, Kim WY, et al. Outcome and reproductive function after cumulative high-dose combination chemotherapy with bleomycin, etoposide and cisplatin (BEP) for patients with ovarian endodermal sinus tumor. Gynecologic Oncology. 2008;111(1):106–110. doi: 10.1016/j.ygyno.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 43.Díaz-García C, Akhi SN, Wallin A, Pellicer A, Brännström M. First report on fertility after allogeneic uterus transplantation. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(11):1491–1494. doi: 10.3109/00016349.2010.520688. [DOI] [PubMed] [Google Scholar]
- 44.Hanafy A, Diaz-Garcia C, Olausson M, Brännström M. Uterine transplantation: one human case followed by a decade of experimental research in animal models. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2011;51(3):199–203. doi: 10.1111/j.1479-828X.2010.01283.x. [DOI] [PubMed] [Google Scholar]
- 45.Kisu I, Banno K, Mihara M, Iida T, Yoshimura Y. Current status of surrogacy in Japan and uterine transplantation research. European Journal of Obstetrics Gynecology and Reproductive Biology. 2011;158(2):135–140. doi: 10.1016/j.ejogrb.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 46.Lefkowitz A, Edwards M, Balayla J. The montreal criteria for the ethical feasibility of uterine transplantation. Transplant International. 2012;25(4):439–447. doi: 10.1111/j.1432-2277.2012.01438.x. [DOI] [PubMed] [Google Scholar]
- 47.Giacalone PL, Laffargue F, Bénos P, Dechaud H, Hédon B. Successful in vitro fertilization-surrogate pregnancy in a patient with ovarian transposition who had undergone chemotherapy and pelvic irradiation. Fertility and Sterility. 2001;76(2):388–389. doi: 10.1016/s0015-0282(01)01895-7. [DOI] [PubMed] [Google Scholar]
- 48.Barad DH, Weghofer A, Gleicher N. Comparing anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertility and Sterility. 2009;91(4):1553–1555. doi: 10.1016/j.fertnstert.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 49.Kwee J, Schats R, McDonnell J, Themmen A, de Jong F, Lambalk C. Evaluation of anti-Müllerian hormone as a test for the prediction of ovarian reserve. Fertility and Sterility. 2008;90(3):737–743. doi: 10.1016/j.fertnstert.2007.07.1293. [DOI] [PubMed] [Google Scholar]
- 50.van Rooij IAJ, Broekmans FJM, Velde ERT, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Human Reproduction. 2002;17(12):3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]


