Abstract
Increased peripheral blood-activated NK cell counts are associated with increased risk of miscarriage and failed in vitro fertilization treatment. However, assessment of activated peripheral NK cells in normal and pathological pregnancies beyond implantation and early miscarriage has not been described. Total CD69 expressing NK cells counts were measured by flow cytometry in healthy women with singleton pregnancies, including 45 at 11+6–13+6 weeks' gestation, 46 at 20+0–22+4 weeks, and 42 at 31+6–33+5 weeks. The number of peripheral blood NK cells decreased, whereas the percentage of activated CD69 expressing NK cells increased from the first to the third trimester of pregnancy. This study shows the course of peripheral blood NK cells and activated CD69 expressing NK cells in uncomplicated nulliparous singleton pregnancies. This is a first step in understanding their implication in pathological pregnancies.
1. Introduction
It is now accepted that for a normal pregnancy to occur, the maternal immune system must be rendered more tolerant towards the semiallogeneic fetus. The required changes must ultimately result in controlled modulation of the uterine natural killer (uNK) cells that represent the most abundant cell population at the fetomaternal interface. These cells play a critical role in implantation and particularly in vascular remodelling and trophoblast invasion [1, 2]. As such NK cells have a critical role in the healthy progression of the pregnancy by maintaining the balance between placental function and fetal requirements.
NK cells are also found in the peripheral blood and like uNK cells, these may be recognized by the expression of cell surface markers. They are usually CD3 negative, but express CD16 and CD56. NK cells are subdivided, by the intensity of expression of CD56, into a CD16+CD56bright and CD16+CD56dim. Peripheral blood NK (pNK) cells are predominantly CD16+CD56dim, whereas uNK cells are predominantly CD16+CD56bright [3, 4]. The exact relationship between these two sub-groups of NK cells is unclear but it has been suggested that uNK migrates from the systemic vascular system [5, 6]. Importantly, however, the factors that regulate uterine and peripheral blood NK cells are likely similar. Thus assessing the level of activation of the peripheral blood NK cells gives information of the state of the uterine cells [7].
CD69 is one of the earliest specific markers of NK cell activation [8–10]. Activated NK cells release cytokines that activate other NK cells and the cellular immune system generally [10]. Elevated NK cell CD69 expression is associated with increased cytotoxicity and target cell lysis [11, 12]. In normal pregnancy, compared to an anembryonic pregnancy, NK cell cytotoxicity is decreased suggesting that activated CD69 expressing NK cells play an important role in the control of trophoblast growth and placental development [13]. Supportive evidence is provided by in vitro models which demonstrated that activated CD69 positive NK cells are capable of lysing trophoblasts [14, 15]. There is evidence that increased pNK cells and activated NK cells are associated with increased risk of miscarriage and failed in vitro fertilization (IVF) treatment [16–19]. Moreover, immunomodulatory therapies that aim to reduce the numbers of these activated NK cells have shown benefit with improved outcomes [20]. However, the value of assessing activated pNK cells in the investigation of pregnancy complications beyond implantation and early miscarriage has not been described.
The aims of this pilot study were firstly, to examine if the number of activated NK cells changes with the progression of a normal pregnancy and in particular to observe variations between each trimester of pregnancy and secondly, to define a provisional normal range of peripheral blood total CD16/56 NK cells and their CD69 expressing activated subset as a first step for the investigation of these parameters in pathological pregnancies.
2. Material and Methods
This was a prospective cross-sectional observational study of consecutive nulliparous pregnant women attending for their routine antenatal visits in the first, second, and third trimesters of pregnancy between October 2011 and February 2012 at King's College Hospital, London. Written informed consent was obtained from the women agreeing to participate in the study, which was approved by King's College Hospital Ethics Committee.
Blood (6 mL) obtained from the antecubital vein was collected into heparinized tubes from a total of 133 healthy women with singleton pregnancies, including 45 at 11+6–13+6 weeks' gestation, 46 at 20+0–22+4 weeks, and 42 at 31+6–33+5 weeks. Analysis of the samples was carried out within 6 hours of collection.
2.1. Sample Analysis
This is the first study to assess the variation in numbers of activated NK cells across gestation in normal pregnancy. We have therefore estimated the sample size from our previous work on activated NK cells in recurrent failed IVF [18].
Total lymphocyte and total CD16/56 NK cell counts were measured in all the 133 women. Due to purchasing and shipping difficulties of the reagents, CD69 activation marker on the NK cells was only measured in the last 90 cases and there was no selection bias. CD69 was chosen to indicate NK cell activation as our previous unpublished work had shown it to be one of the earliest and probably most robust activation marker of NK cell activation. Additionally we found it to be altered little by several hours of sample storage. This was important as the patients in our study were seen at another hospital and the transit time was up to 5 hours.
A lyse no-wash protocol was used to stain the whole blood sample. 10 μL of anti-CD56PE (BD Pharmingen), 10 μL of anti-CD16 FITC (BD Pharmingen), 10 μL of anti-CD3 PE Cy5 (BD Pharmingen), and 10 μL of anti-CD69 APC (BD Pharmingen) were added into a Falcon (BD Biosciences) tube. An isotype control tube containing anti-CD3, CD16, and CD56 but replacing the anti-CD69 with an isotype control antibody (IgG1k, BD Pharmingen) was included for every patient. 50 μL of well-mixed whole blood was then added and tubes were incubated for 15 minutes in the dark. 1 mL of Easylyse (BD Biosciences) was added and tubes were incubated in the dark for a further 15 minutes. Finally 50 μL of Cytocount beads (Dako) was added and the tubes were gently vortexed. Tubes were analyzed on a Fascalibur (BD Biosciences) using Cellquest Pro software. 10,000 beads were counted which correlates with 20,000 to 30,000 lymphocytes depending on the absolute lymphocyte count. The isotype control was used to set the cutoff for the negative population (Figure 1).
Figure 1.
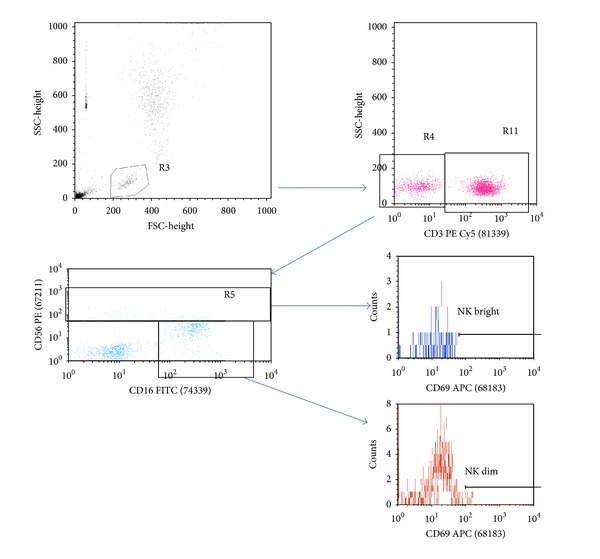
Gating strategy for NK CD69 enumeration: lymphocytes were identified using FSC/SSC and the CD3 negative population was identified. They were further classified into CD16 positive and either CD56 dim or CD56 bright. Each of these populations was then interrogated for CD69 expression.
2.2. Statistical Analysis
The characteristics of the study population are presented in median and range for continuous variables and in number (%) for categorical variables.
Normality of distribution was assessed using probability plots and the Kolmogorov-Smirnov test. Total lymphocyte, total CD16/56 NK cell and CD69 counts were normally distributed (P = 0.900; P = 0.120; P = 0.799). The NK cell count was expressed as a percentage of the total lymphocyte count (NK cell/lymphocyte). CD69 was expressed as a percentage of NK cell count (CD69/NK cell). The mean and 95% confidence interval of lymphocyte, NK cell, NK cell/lymphocyte, CD69, and CD69/NK cell in each trimester were presented in error bar plots. ANOVA with posthoc LSD test was used to compare the mean values between the first and the second trimester, between the second and third trimester, and between the first and the third trimester of pregnancy.
The statistical software package SPSS 20.0 (SPSS Inc., Chicago, IL) was used for data analysis.
3. Results and Discussion
3.1. Results
The maternal characteristics of the study population are presented in Table 1.
Table 1.
Characteristics of the study population (median and range).
| First trimester (n = 45) |
Second trimester (n = 46) |
Third trimester (n = 42) |
|
|---|---|---|---|
| Gestational age in wks, median (range) | 12+6 (11+6–13+6) | 22+0 (20+0–22+4) | 32+1 (31+6–33+5) |
| Maternal age in yrs, median (range) | 29 (17–36) | 28 (16–38) | 29 (17–39) |
| Maternal weight in Kg on date of sampling, median (range) | 68.4 (54.0–89.0) | 69.0 (42.9–104.0) | 76.0 (46.9–112.0) |
| BMI in Kg/m2 on date of sampling, median (range) | 24.2 (18.0–32.7) | 25.3 (17.1–36) | 29.0 (17.4–42.3) |
| BMI in Kg/m2 at booking, median (range) | 24.2 (18.0–32.7) | 24.1 (17–34.9) | 24.8 (13.5–40) |
| Racial origin | |||
| Caucasian, n (%) | 25 (58.1) | 25 (54.3) | 25 (56.8) |
| Afro-Caribbean, n (%) | 18 (41.9) | 21 (45.7) | 19 (43.2) |
The mean (SD) of total lymphocyte count was 1.93 × 109/L (0.57 × 109/L) in the first trimester, 1.68 × 109/L (0.41 × 109/L) in the second trimester, and 1.79 × 109/L (0.43 × 109/L) in the third trimester (Table 2). There was a significant decrease in the total lymphocyte count from the first to the second trimester (P = 0.015) and no significant change from the second to the third trimester (P = 0.286; Figure 2). There was no significant difference in the mean total lymphocyte count between Caucasian and Afro-Caribbean women in each trimester of pregnancy (P = 0.484, P = 0.295, P = 0.586). In both racial groups, the changes in the total lymphocyte count across each trimester were similar to the total study population. Maternal age had no impact on the total lymphocyte count.
Table 2.
Mean (SD) total lymphocyte, natural killer cell, and CD69 counts in the study population.
| N | First trimester | N | Second trimester | N | Third trimester | |
|---|---|---|---|---|---|---|
| Total lymphocyte count (×109/L) | ||||||
| Total, mean (SD) | 45 | 1.93 (0.57) | 46 | 1.68 (0.41) | 42 | 1.79 (0.43) |
| Caucasian, mean (SD) | 25 | 1.87 (0.44) | 25 | 1.74 (0.44) | 25 | 1.82 (0.47) |
| Afro-Caribbean, mean (SD) | 20 | 1.99 (0.71) | 21 | 1.60 (0.45) | 17 | 1.74 (0.37) |
| NK cell count (×106/L) | ||||||
| Total, mean (SD) | 45 | 232.6 (149.5) | 46 | 183.4 (108.1) | 42 | 166.9 (77.7) |
| Caucasian, mean (SD) | 25 | 230.0 (130.1) | 25 | 198.7 (126.1) | 25 | 171.3 (85.3) |
| Afro-Caribbean, mean (SD) | 20 | 236.0 (174.3) | 21 | 165.1 (81.0) | 17 | 160.4 (67.0) |
| NK cell/lymphocyte (%) | ||||||
| Total, mean (SD) | 45 | 11.6 (5.8) | 46 | 10.8 (4.6) | 42 | 9.3 (3.3) |
| Caucasian, mean (SD) | 25 | 12.0 (5.3) | 25 | 11.0 (4.8) | 25 | 9.3 (3.1) |
| Afro-Caribbean, mean (SD) | 20 | 11.3 (6.4) | 21 | 10.5 (4.6) | 17 | 9.2 (3.7) |
| CD69 count (×106/L) | ||||||
| Total, mean (SD) | 30 | 1.88 (1.08) | 30 | 2.14 (1.90) | 30 | 2.56 (1.42) |
| Caucasian, mean (SD) | 15 | 2.02 (1.25) | 15 | 2.69 (2.48) | 15 | 3.07 (1.50) |
| Afro-Caribbean, mean (SD) | 15 | 1.75 (0.89) | 15 | 1.59 (0.81) | 15 | 2.05 (1.16) |
| CD69/NK cell (%) | ||||||
| Total, mean (SD) | 30 | 1.14 (0.76) | 30 | 1.24 (0.72) | 30 | 1.82 (1.29) |
| Caucasian, mean (SD) | 15 | 1.03 (0.65) | 15 | 1.45 (0.91) | 15 | 1.98 (0.94) |
| Afro-Caribbean, mean (SD) | 15 | 1.25 (0.86) | 15 | 1.04 (0.39) | 15 | 1.67 (1.59) |
NK: natural killer.
Figure 2.
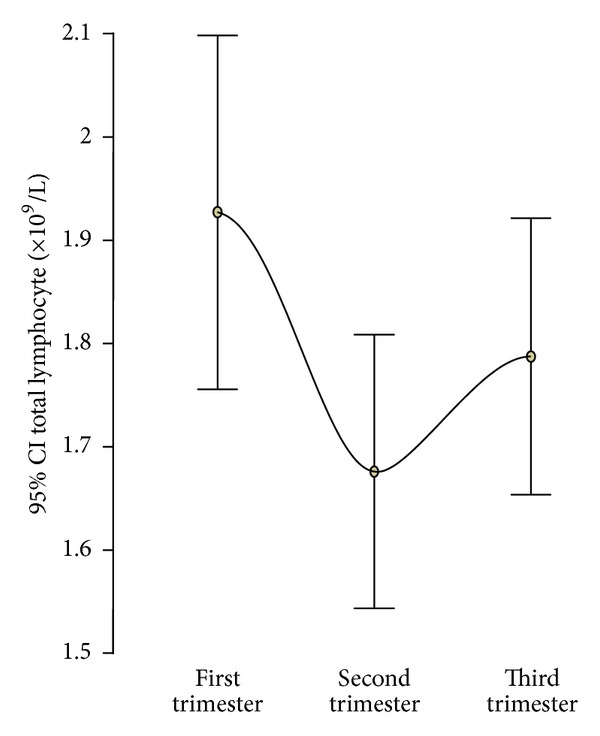
Error bar plot of the mean and 95% confidence interval of total lymphocyte count in each trimester of pregnancy.
The distributions of the total CD16/56 NK cells are illustrated in Figure 3. The mean (SD) of total CD16/56 NK cells was 232.6 × 106/L (149.5 × 106/L) in the first trimester, 183.4 × 106/L (108.1 × 106/L) in the second trimester, and 166.9 × 106/L (77.7 × 106/L) in the third trimester (Table 2). There was a significant decrease in the total NK cell count from the first to the second trimester (P = 0.045) and no significant change from the second to the third trimester (P = 0.508). Overall there was a significant decrease in both the total NK cell count (P = 0.009) and NK cell/lymphocyte (P = 0.020) from the first to the third trimester of pregnancy (Figure 3). There was no significant difference in the mean total NK cell count between Caucasian and Afro-Caribbean women in each trimester of pregnancy (P = 0.895, P = 0.300, P = 0.661).
Figure 3.
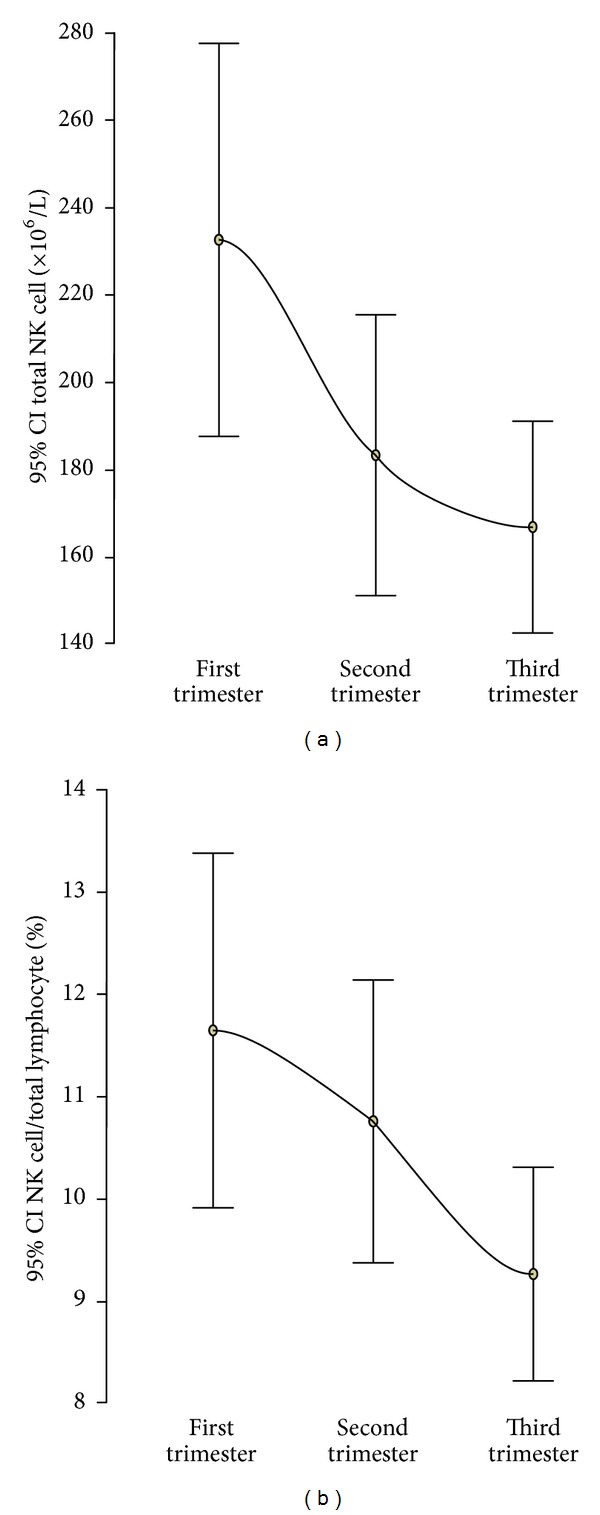
Error bar plots of the mean and 95% confidence interval of total natural killer (NK) cell count (a) and NK cell/total lymphocyte count (%) (b) in each trimester of pregnancy.
The mean (SD) of CD69 activation marker on NK cells was 1.88 × 106/L (1.08 × 106/L) in the first trimester, 2.14 × 106/L (1.90 × 106/L) in the second trimester, and 2.56 × 106/L (1.42 × 106/L) in the third trimester (Table 2). The mean CD69 showed no significant change from the first to the third trimester (P = 0.086; Figure 3). However, the CD69/NK cell (%) showed a significant increase from the first to the third trimester (P = 0.007; Figure 4). There was no significant difference in the mean CD69 between Caucasian and Afro-Caribbean women in each trimester of pregnancy (P = 0.498, P = 0.114, P = 0.051).
Figure 4.
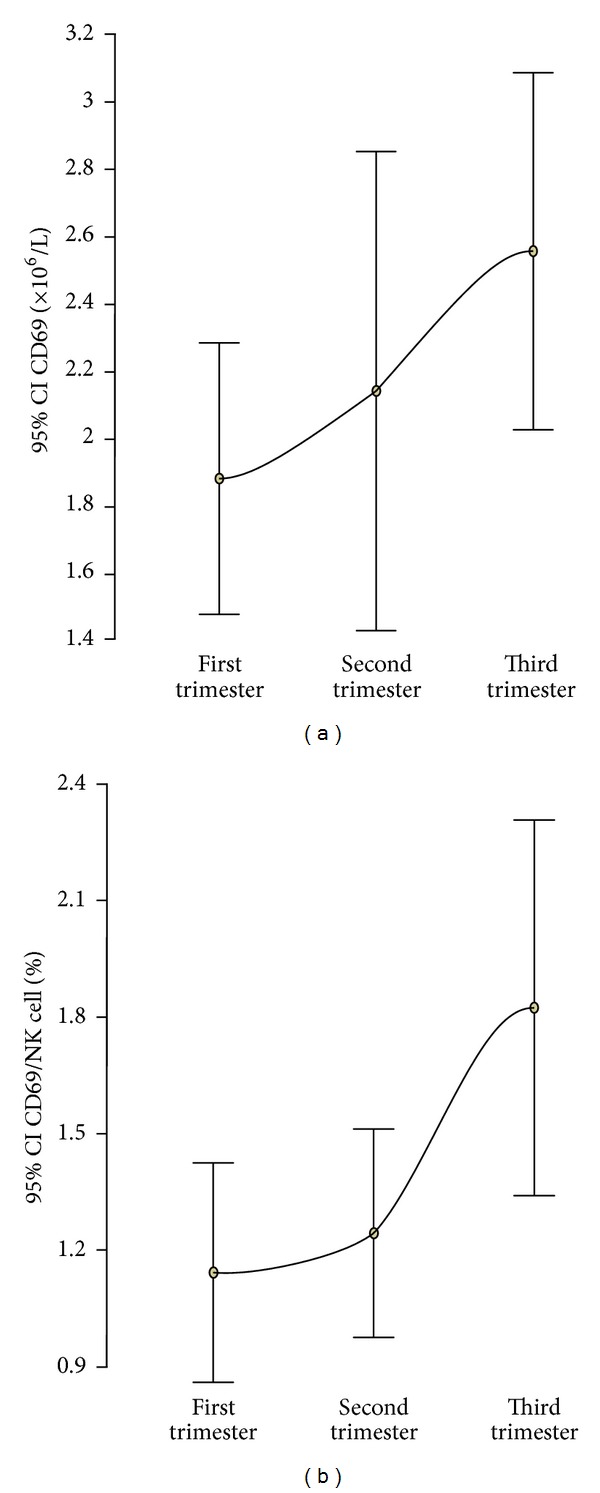
Error bar plots of the mean and 95% confidence interval of CD69 count (a) and CD69/natural killer cell count (%) (b) in each trimester of pregnancy.
Both subsets of CD16+CD56bright and CD16+CD56dim express the activation marker CD69. There was a significant reduction in the CD16+CD56dim from the first to the third trimester (205.2 × 106/L, [SD 119 × 106/L] versus 149.2 × 106/L, [SD 84.64 × 106/L]; P = 0.039) but not in the CD16+CD56bright (14.4 × 106/L [SD 7.1 × 106/L] versus 14.9 × 106/L [SD 8.9 × 106/L]; P = 0.786), and therefore, the overall reduction in NK cells from the first to the third trimester is from the CD16+CD56dim. There was a significant increase in the CD16+CD56bright NK cells of CD69 activation marker from the first to the third trimester (0.37 × 106/L [SD 0.29 × 106/L] versus 0.69 × 106/L [SD 0.53 × 106/L]; P = 0.002), but not in the CD16+CD56dim NK cells (1.55 × 106/L [SD 1.04 × 106/L] versus 1.85 × 106/L [SD 1.11 × 106/L]; P = 0.424), and therefore, the overall increase in CD69/NK cell across gestation is from CD69 on CD16+CD56bright NK cells.
3.2. Discussion
This study of normal singleton nulliparous pregnancies without a history of miscarriages has demonstrated that the total lymphocyte count decreased between the first and second trimesters of pregnancy with a non-significant increase from the second to the third trimester. The number of peripheral blood NK cells decreases whereas the percentage of activated CD69 expressing NK cells increases from the first to the third trimester of pregnancy. Monitoring these variables in the same women over their individual pregnancies proved logistically difficult as the samples needed to be transported to the laboratory and analysed within 6 hours. While this would have provided more robust data, we nevertheless feel that our results are sufficiently sound to indicate genuine changes. Comparing these results with women suffering preterm labour, preeclampsia, and other pregnancy-related problems would be an interesting area of research.
Our findings on the changes in total lymphocyte count with gestation are comparable with those of previous studies. Valdimarsson et al. [21] examined 77 women longitudinally throughout pregnancy and reported that the total lymphocyte count decreased with gestational age to reach a nadir at 25–28 weeks and increasing thereafter until term. Similarly, Lurie et al. [22] examined 726 women longitudinally between the 5th and 41st week of gestation and reported that the total lymphocyte count decreased between the first and second trimesters and then increased in the third trimester. Our finding of a decrease in the number of peripheral blood NK cells with gestational age is contradictory to that of a previous longitudinal study in 23 pregnant women which reported no significant change with gestation [23]. There are also two studies which compared peripheral blood NK cell counts in women during the third trimester of pregnancy to nonpregnant controls and reported that the counts were significantly reduced during pregnancy [24, 25]. There are no previous reports on the percentage of activated CD69 expressing NK cells with gestational age.
Uterine NK cells play an important role in trophoblast invasion and spiral artery remodelling [4, 26]. Peripheral blood NK cells on the other hand are cytotoxic and an increase in number and activity has been associated with miscarriages and implantation failure [18, 27]. In our study, the pNK cells of pregnant women in the first trimester are reduced compared to nonpregnant women [28]. This was also observed by Kühnert et al. [23] and may be viewed as a logical adaption of the immune system to a healthy pregnancy. The precise role of uNK and pNK cells and their interrelationship in the later stages of pregnancy is unclear. As the pNK cells are regulated in the same way as the uNK cells, the increase in activated pNK cells though slight in terms of actual NK cell numbers is likely still significant and may reflect slightly raised antifetal maternal immune activity possibly in preparation for parturition. Although the CD56bright pNK cells contribute less than 10% to the total pNK count, our study has demonstrated that it is mainly this type of pNK cells that become activated during pregnancy. However, while uNK cells show phenotypic and functional differences from pNK cells, changes in the numbers of the two subsets have been shown to be correlated by Park et al. [29] in women with recurrent miscarriage. Interestingly, CD56+ NK cells, bearing the natural cytotoxicity NKp46 receptor in the uterus and in the peripheral blood, have a similar cytokine profile [30]. The production of interferon-γ by uterine and peripheral blood NK cells after stimulation with soluble HLA G has also been shown to be similar [31]. Moreover, an NK1 type shift, the NK counterpart to Th1 cells, has been observed in the peripheral blood and uterine NKp46 cells in women with recurrent miscarriage [32]. Thus peripheral blood and uterine NK cells share certain important characteristics. As such an assessment of the state of activity of the peripheral blood NK cells provides a snap shot of uterine NK cell activity.
Pregnancy is associated with a systemic inflammatory response [33–35] and with a significant involvement of interleukin 6 (IL-6) [36] and IL-33 [37]. The latter is gaining increasing importance in pregnancy and infertility as its receptor (ST2) is expressed by Th2 and NK cells [38]. Pregnancy is accompanied by an increase in the total number of leukocytes, mostly related to an increased neutrophil count [21, 22]. Additionally, there is a rise in the erythrocyte sedimentation rate and C-reactive protein [39, 40]. Such changes are observed in inflammation and sepsis and therefore, it is thought that the increase in the number of activated NK cells is in part related to the systemic inflammatory response that is evident in pregnancy. The growing mass of HLA mismatched tissue and circulating trophoblast tissue/debris are the additional components that contribute to the immunological response in pregnancy. Superimposed on the aforementioned processes are the decreasing circulating levels of tolerance promoting factors such as human chorionic gonadotropin that has very significant immunomodulating properties [41–44]. It is possible that the combined effect of these various factors may explain the increase in the activation of NK cells in pregnancy and possible maternal immune preparation for parturition.
4. Conclusion
This study demonstrates the changes of peripheral blood NK cells and activated CD69 expressing NK cells in normal pregnancy, which may be used in the investigation of pathological singleton pregnancies in nulliparous women. The underlying cause of the changes in activated NK cells levels in the different stages of pregnancy remains unclear.
Acknowledgment
This study was supported by a Grant from the Fetal Medicine Foundation (Charity no. 1037116).
Conflict of Interests
This is to state that in this paper none of the authors has any conflict of interests regarding the trademarks mentioned in the paper: Cellquest Pro software, SPSS Inc, Cytocount beads (Dako), and Fascalibur (BD Biosciences).
References
- 1.Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Molecular Immunology. 2005;42(4):511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Moffett-King A. Natural killer cells and pregnancy. Nature Reviews Immunology. 2002;2(9):656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 3.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends in Immunology. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 4.Yagel S. The developmental role of natural killer cells at the fetal-maternal interface. American Journal of Obstetrics and Gynecology. 2009;201(4):344–350. doi: 10.1016/j.ajog.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocrine Reviews. 2005;26(1):44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- 6.Van den Heuvel MJ, Hatta K, Peralta CG, Han VK, Clark DA. CD56+ cells are recruited to the uterus in two waves: at ovulation and during the first 2 weeks after missed menses. American Journal of Reproductive Immunology. 2008;59(2):90–98. doi: 10.1111/j.1600-0897.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 7.Bansal AS. Joining the immunological dots in recurrent miscarriage. American Journal of Reproductive Immunology. 2010;64(5):307–315. doi: 10.1111/j.1600-0897.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- 8.Craston R, Koh M, McDermott A, Ray N, Prentice HG, Lowdell MW. Temporal dynamics of CD69 expression on lymphoid cells. Journal of Immunological Methods. 1997;209(1):37–45. doi: 10.1016/s0022-1759(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 9.Llera AS, Viedma F, Sánchez-Madrid F, Tormo J. Crystal structure of the C-type lectin-like domain from the human hematopoietic cell receptor CD69. Journal of Biological Chemistry. 2001;276(10):7312–7319. doi: 10.1074/jbc.M008573200. [DOI] [PubMed] [Google Scholar]
- 10.Marzio R, Mauël J, Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacology and Immunotoxicology. 1999;21(3):565–582. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 11.De Maria R, Cifone MG, Trotta R, et al. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. Journal of Experimental Medicine. 1994;180(5):1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanier LL, Buck DW, Rhodes L, et al. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. Journal of Experimental Medicine. 1988;167(5):1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho HN, Chao KH, Chen CK, Yang YS, Huang SC. Activation status of T and NK cells in the endometrium throughout menstrual cycle and normal and abnormal early pregnancy. Human Immunology. 1996;49(2):130–136. doi: 10.1016/0198-8859(96)00120-6. [DOI] [PubMed] [Google Scholar]
- 14.Avril T, Iochmann S, Brand D, Bardos P, Watier H, Thibault G. Human choriocarcinoma cell resistance to natural killer lysis due to defective triggering of natural killer cells. Biology of Reproduction. 2003;69(2):627–633. doi: 10.1095/biolreprod.102.009290. [DOI] [PubMed] [Google Scholar]
- 15.Helige C, Hagendorfer G, Smolle J, Dohr G. Uterine natural killer cells in a three-dimensional tissue culture model to study trophoblast invasion. Laboratory Investigation. 2001;81(8):1153–1162. doi: 10.1038/labinvest.3780327. [DOI] [PubMed] [Google Scholar]
- 16.Coulam CB, Roussev RG. Correlation of NK cell activation and inhibition markers with NK cytoxicity among women experiencing immunologic implantation failure after in vitro fertilization and embryo transfer. Journal of Assisted Reproduction and Genetics. 2003;20(2):58–62. doi: 10.1023/A:1021736007376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacks G, Yang Y, Gowen E, Smith S, Fay L, Chapman M. Detailed analysis of peripheral blood natural killer cells in women with repeated IVF failure. Journal of Reproductive Immunology. 2012;67:434–442. doi: 10.1111/j.1600-0897.2012.01105.x. [DOI] [PubMed] [Google Scholar]
- 18.Thum MY, Bhaskaran S, Abdalla HI, et al. An increase in the absolute count of CD56dimCD16+CD69+ NK cells in the peripheral blood is associated with a poorer IVF treatment and pregnancy outcome. Human Reproduction. 2004;19(10):2395–2400. doi: 10.1093/humrep/deh378. [DOI] [PubMed] [Google Scholar]
- 19.Yoo JH, Kwak-Kim J, Han AR, et al. Peripheral blood NK cell cytotoxicities are negatively correlated with CD8+ T cells in fertile women but not in women with a history of recurrent pregnancy loss. American Journal of Reproductive Immunology. 2012;68(1):38–46. doi: 10.1111/j.1600-0897.2012.01133.x. [DOI] [PubMed] [Google Scholar]
- 20.Winger EE, Reed JL, Ashoush S, El-Toukhy T, Ahuja S, Taranissi M. Elevated preconception CD56+ 16+ and/or Th1:Th2 levels predict benefit from IVIG therapy in subfertile women undergoing IVF. American Journal of Reproductive Immunology. 2011;66(5):394–403. doi: 10.1111/j.1600-0897.2011.01018.x. [DOI] [PubMed] [Google Scholar]
- 21.Valdimarsson H, Mulholland C, Fridriksdottir V, Coleman DV. A longitudinal study of leucocyte blood counts and lymphocyte responses in pregnancy: a marked early increase of monocyte-lymphocyte ratio. Clinical and Experimental Immunology. 1983;53(2):437–443. [PMC free article] [PubMed] [Google Scholar]
- 22.Lurie S, Rahamim E, Piper I, Golan A, Sadan O. Total and differential leukocyte counts percentiles in normal pregnancy. European Journal of Obstetrics Gynecology and Reproductive Biology. 2008;136(1):16–19. doi: 10.1016/j.ejogrb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Kühnert M, Strohmeier R, Stegmüller M, Halberstadt E. Changes in lymphocyte subsets during normal pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1998;76:147–151. doi: 10.1016/s0301-2115(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud F, Abul H, Omu A, Al-Rayes S, Haines D, Whaley K. Pregnancy-associated changes in peripheral blood lymphocyte subpopulations in normal Kuwaiti women. Gynecologic and Obstetric Investigation. 2001;52(4):232–236. doi: 10.1159/000052981. [DOI] [PubMed] [Google Scholar]
- 25.Veenstra van Nieuwenhoven AL, Bouman A, Moes H, et al. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertility and Sterility. 2002;77(5):1032–1037. doi: 10.1016/s0015-0282(02)02976-x. [DOI] [PubMed] [Google Scholar]
- 26.Lash GE, Bulmer JN. Do uterine natural killer (uNK) cells contribute to female reproductive disorders? Journal of Reproductive Immunology. 2011;88(2):156–164. doi: 10.1016/j.jri.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Beer AE, Kwak JYH, Ruiz JE. Immunophenotypic profiles of peripheral blood lymphocytes in women with recurrent pregnancy losses and in infertile women with multiple failed in vitro fertilization cycles. American Journal of Reproductive Immunology. 1996;35(4):376–382. doi: 10.1111/j.1600-0897.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 28.Thum MY, Bhaskaran S, Bansal AS, et al. Simple enumerations of peripheral blood natural killer (CD56+ NK) cells, B cells and T cells have no predictive value in IVF treatment outcome. Human Reproduction. 2005;20(5):1272–1276. doi: 10.1093/humrep/deh774. [DOI] [PubMed] [Google Scholar]
- 29.Park DW, Lee HJ, Park CW, Hong SR, Kwak-Kim J, Yang KM. Peripheral blood NK cells reflect changes in decidual NK cells in women with recurrent miscarriages. American Journal of Reproductive Immunology. 2010;63(2):173–180. doi: 10.1111/j.1600-0897.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 30.Yokota M, Fukui A, Funamizu A, et al. Role of NKp46 expression in cytokine production by CD56-positive NK cells in the peripheral blood and the uterine endometrium. American Journal of Reproductive Immunology. 2013;69(3):202–211. doi: 10.1111/aji.12062. [DOI] [PubMed] [Google Scholar]
- 31.Van der Meer A, Lukassen HGM, van Cranenbroek B, et al. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Molecular Human Reproduction. 2007;13(2):123–133. doi: 10.1093/molehr/gal100. [DOI] [PubMed] [Google Scholar]
- 32.Fukui A, Funamizu A, Yokota M, et al. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. Journal of Reproductive Immunology. 2011;90(1):105–110. doi: 10.1016/j.jri.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Sacks GP, Studena K, Sargent IL, Redman CWG. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. American Journal of Obstetrics and Gynecology. 1998;179(1):80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 34.Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CWG. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. Journal of Reproductive Immunology. 2003;59(2):153–160. doi: 10.1016/s0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 35.Sargent IL, Borzychowski AM, Redman CWG. NK cells and human pregnancy—an inflammatory view. Trends in Immunology. 2006;27(9):399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. Journal of Reproductive Immunology. 2012;95(1-2):1–14. doi: 10.1016/j.jri.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Granne I, Southcombe JH, Snider JV, et al. ST2 and IL-33 in pregnancy and pre-eclampsia. PLoS ONE. 2011;6(9):1 pages. doi: 10.1371/journal.pone.0024463.e24463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N. Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunologic Research. 2012;52(1-2):89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 39.Ozanne P, Linderkamp O, Miller FC, Meiselman HJ. Erythrocyte aggregation during normal pregnancy. American Journal of Obstetrics and Gynecology. 1983;147(5):576–583. doi: 10.1016/0002-9378(83)90021-2. [DOI] [PubMed] [Google Scholar]
- 40.Picklesimer AH, Jared HL, Moss K, Offenbacher S, Beck JD, Boggess KA. Racial differences in C-reactive protein levels during normal pregnancy. American Journal of Obstetrics and Gynecology. 2008;199(5):523.e1–523.e6. doi: 10.1016/j.ajog.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bansal AS, Bora S, Saso S, Smith JR, Johnson MR, Thum MY. The mechanism of human chorionic gonadotrophin mediated immunomodulation in pregnancy. Expert Review of Clinical Immunology. 2012;8(8):747–753. doi: 10.1586/eci.12.77. [DOI] [PubMed] [Google Scholar]
- 42.Cole LA. Biological functions of hCG and hCG-related molecules. Reproductive Biology and Endocrinology. 2010;8, article 102 doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norris W, Nevers T, Sharma S, Kalkunte S. Review: HCG, preeclampsia and regulatory T cells. Placenta. 2011;32(2):S182–S185. doi: 10.1016/j.placenta.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, d'Hauterive SP. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. Journal of Reproductive Immunology. 2010;85(1):93–98. doi: 10.1016/j.jri.2009.11.008. [DOI] [PubMed] [Google Scholar]


