Abstract
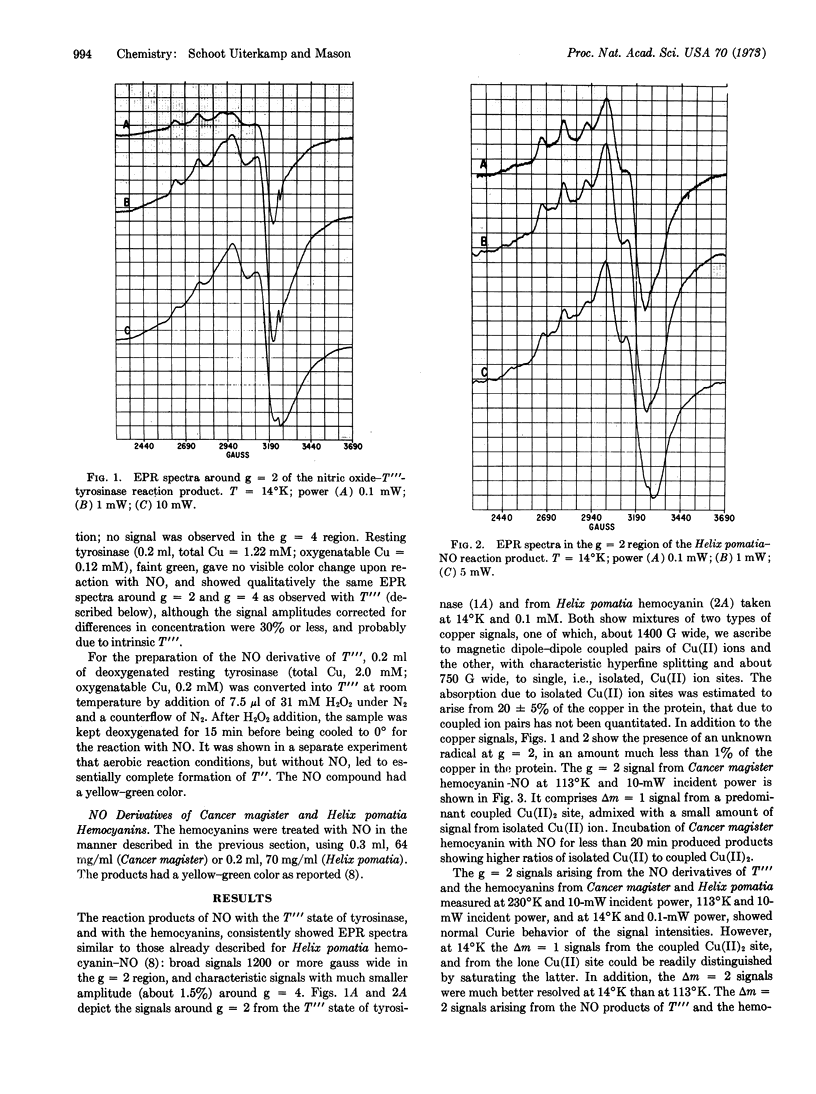
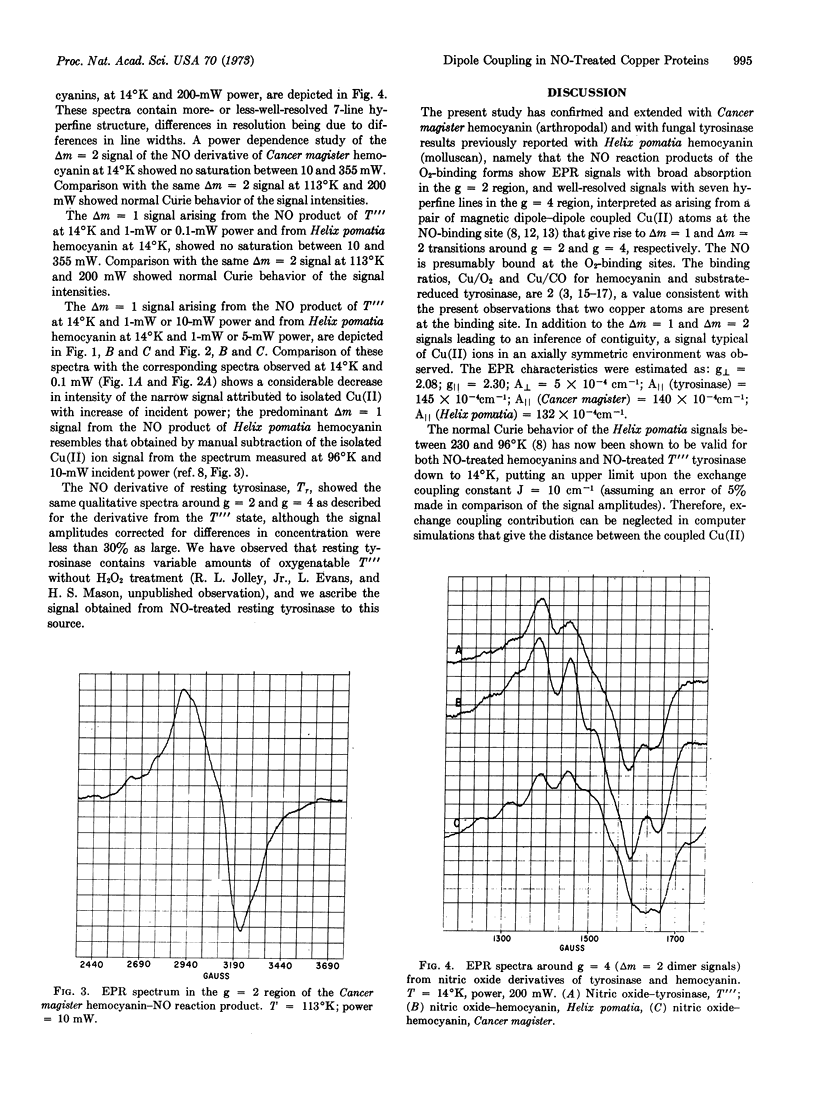
The Tr and T[unk] states of tyrosinase were treated with NO. EPR spectra of the products observed at 14°K and at 113°K showed mixtures of two signals. One had components in the region of g = 2, about 1200 G wide, and in the region of g = 4, showing hyperfine splitting. The other signal was similar to that arising from isolated Cu(II) ions in an axially symmetric environment. The first signal was indicative of Δm = 1 and Δm = 2 transitions arising from magnetic dipole-dipole coupled Cu(II) ion pairs. It closely resembled previously reported EPR spectra obtained from NO-treated hemocyanin, which were confirmed in this study. The normal Curie behavior of the signals between 230°K and 14°K ruled out significant exchange coupling between the ion pairs. The Δm = 2 signals were not saturable up to 350 mW at 14°K. The broad Δm = 1 signals could be separated from accompanying signals by the saturation characteristics of the latter at about 10 mW at 14°K. The results establish the presence of a pair of copper ions at the active site of tyrosinase, and a clsoe structural relationship between this active site and that of hemocyanin.
Keywords: O2 binding, Cu proteins, electron paramagnetic resonance
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOUCHILLOUX S., McMAHILL P., MASON H. S. The multiple forms of mushroom tyrosinase. Purification and molecular properties of the enzymes. J Biol Chem. 1963 May;238:1699–1707. [PubMed] [Google Scholar]
- Jolley R. L., Evans L. H., Mason H. S. Reversible oxygenation of tyrosinase. Biochem Biophys Res Commun. 1972 Jan 31;46(2):878–884. doi: 10.1016/s0006-291x(72)80223-7. [DOI] [PubMed] [Google Scholar]
- Konings W. N., van Driel R., van Bruggen E. F., Gruber M. Structure and properties of hemocyanins. V. Binding of oxygen and copper in Helix pomatia hemocyanin. Biochim Biophys Acta. 1969 Nov 11;194(1):55–66. doi: 10.1016/0005-2795(69)90179-2. [DOI] [PubMed] [Google Scholar]
- MASON H. S. OXIDASES. Annu Rev Biochem. 1965;34:595–634. doi: 10.1146/annurev.bi.34.070165.003115. [DOI] [PubMed] [Google Scholar]
- THOMSON L. C., HINES M., MASON H. S. On the binding of copper by hemocyanin. Arch Biochem Biophys. 1959 Jul;83(1):88–95. doi: 10.1016/0003-9861(59)90013-x. [DOI] [PubMed] [Google Scholar]
- Uiterkamp A. J.M.S. Monomer and magnetic dipole-coupled Cu(2+) EPR signals in nitrosylhemocyanin. FEBS Lett. 1972 Jan 15;20(1):93–96. doi: 10.1016/0014-5793(72)80025-5. [DOI] [PubMed] [Google Scholar]



