Abstract
We assessed the feasibility of developing a suitable international reference standard for determination of in vitro biological activity of human sequence recombinant PEG-G-CSF products with a 20 kD linear PEG linked to the N-terminal methionyl residue of G-CSF (INN Filgrastim), produced using a conjugation process and coupling chemistry similar to that employed for the lead PEGfilgrastim product. Based on initial data which showed that the current WHO 2nd international standard, IS for G-CSF (09/136) or alternatively, a PEG-G-CSF standard with a unitage traceable to the G-CSF IS may potentially serve as the IS for PEG-G-CSF products, two candidate preparations of PEG-G-CSF were formulated and lyophilized at NIBSC. These preparations were tested by 23 laboratories using in vitro bioassays in a multi-centre collaborative study. Results indicated that on the basis of parallelism, the current WHO 2nd IS for G-CSF or any of the PEG-G-CSF samples could be used as the international standard for PEG-G-CSF preparations. However, because of the variability in potency estimates seen when PEG-G-CSF preparations were compared with the current WHO 2nd IS for G-CSF, a candidate PEG-G-CSF was suitable as the WHO IS. The preparation 12/188 was judged suitable to serve as the WHO IS based on in vitro biological activity data. Therefore, the preparation coded 12/188 was established by the WHO Expert Committee on Biological Standardization (ECBS) in 2013 as the WHO 1st IS for human PEGylated G-CSF with an assigned in vitro bioactivity of 10,000 IU per ampoule.
Keywords: Biosimilar, Biological activity, International standard, Modified G-CSF
1. Introduction
Granulocyte-colony stimulating factor (G-CSF) is used therapeutically for several indications relating to neutropenia and increasingly for stem cell mobilization. As a result, there are several recombinant human G-CSF approved products (INN Filgrastim — Escherichia. coli expressed; INN Lenograstim — CHO cell expressed) in clinical use. Due to the short half-life of G-CSF which requires repeated administration (Möhle and Kanz, 2007), several long-acting recombinant G-CSF forms produced using different technologies are in development worldwide. These include polyethylene glycol (PEG) conjugated G-CSF, G-CSF fused to proteins with long half-lives such as albumin, the Fc fragment of IgGs or to polymers (Zhai et al., 2009; Huang et al., 2010; Cox et al., 2014; Volovat et al., 2014).
Two PEG conjugated G-CSF products which have different INNs (INN PEGfilgrastim, brand name — Neulasta; INN — lipegfilgrastim, brand name — Lonquex) are already approved; their use is limited to neutropenia and related indications (but not stem cell-mobilization). While the lipegfilgrastim product is produced by a site specific enzyme mediated covalent attachment of a 20 kD PEG molecule via a glycolinker to the natural O-glycosylation site at the threonine residue (Thr134) of Filgrastim (Lonquex EPAR assessment report, EMA), the PEGfilgrastim has a 20 kD monomethoxy PEG at the N-terminal methionyl residue of Filgrastim (Neulasta EPAR assessment report, EMA; Molineux, 2004; Kinstler et al., 1996).
As patent expiry is imminent for the PEGfilgrastim product, several ‘copy’ PEGylated G-CSF products have entered the market in poorly regulated countries while biosimilar and novel molecules are in clinical development worldwide. While the copy and biosimilar versions are likely to employ PEG molecules of similar size and form and target the same site and use the same coupling chemistry as the reference product (Neulasta), novel products are likely to use PEG molecules of different sizes, forms, and potentially target different site(s) and employ different chemistries (Veronese et al., 2007; Zhai et al., 2009; Maullu et al., 2009; Huang et al., 2010). Depending on the size and shape of PEG chains attached to G-CSF and the amino acid ligation sites in the product, the biological properties of PEGylated products may differ significantly from the parental or unmodified G-CSF (Veronese et al., 2007; Zhai et al., 2009; Maullu et al., 2009; Huang et al., 2010).
While it is assumed that manufacturers have measured the potency of their PEG-CSF products in bioassays calibrated using the standard available for the parent molecule i.e., current WHO 2nd IS for G-CSF (09/136), the suitability of reporting potencies in the respective IU has not been formally established. As practices are likely to vary between manufacturers, this may result in availability of products (particularly copy products including biosimilars as patent expiry is imminent) with discrepant bioactivities. A reference standard is, therefore required for determination of biological activity of these products.
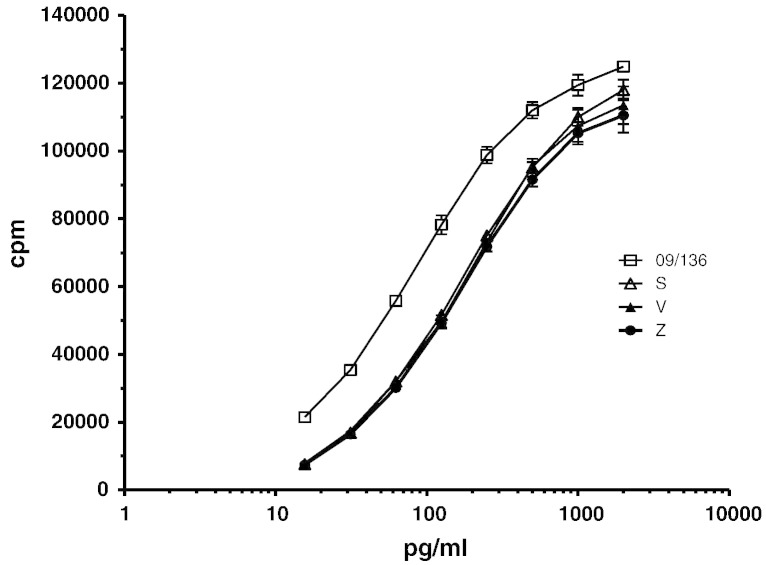
Since there is intense activity in the development of copy/biosimilar versions of the PEGfilgrastim product (lead marketed product), we concentrated our efforts on assessing the feasibility of developing a suitable reference standard for determination of biological activity of PEGylated G-CSF products which have a 20 kD linear PEG linked to the N-terminal methionyl residue of G-CSF, INN Filgrastim and are produced using a conjugation process and coupling chemistry similar to that employed for the lead PEGfilgrastim product. Therefore, we initially evaluated the biological activity of several PEG-G-CSF products (20 kD linear PEG linked to the N-terminal methionyl residue of G-CSF) relative to the current 2nd IS for G-CSF (code 09/136), which is available (Wadhwa et al., 2011), in an in vitro cell based bioassay using the G-CSF responsive cell-line, G-NFS-60. Our results indicated that the PEGylated products were less potent than the current WHO 2nd IS for G-CSF (representing the unmodified parent molecule) in the in vitro assay, however, preliminary data derived from dose response curves comparing the current WHO 2nd IS for G-CSF and PEG-G-CSF products suggested that the current IS for G-CSF (Fig. 1) or alternatively, a PEG-G-CSF standard with a unitage traceable to the current IS for G-CSF may potentially serve as the IS for PEG-G-CSF products.
Fig. 1.
Comparison of PEG-G-CSF preparations (S, V and Z) relative to the current IS for G-CSF (09/136) using the GNFS-60 cell-line.
Based on the above premise, we further evaluated in an international collaborative study, two candidate PEG-G-CSF preparations relative to the current WHO 2nd IS for G-CSF with the aim of selecting and characterising a suitable WHO IS for in vitro bioactivity and for assigning a unitage for in vitro biological activity of PEG-G-CSF. To achieve this, the study sought a) to assess the suitability of the current WHO 2nd IS for G-CSF (as a reference standard for the biological activity of PEG-G-CSF) or a candidate preparation of human PEG-G-CSF to serve as the 1st IS for the bioassay of human PEG-G-CSF by assaying their biological activity in a range of routine, ‘in-house’ bioassays, b) to assess the activity of the ampouled PEG-G-CSF preparations in different bioassays in current use for these materials and to calibrate the candidate PEG-G-CSF IS (if possible) against the current WHO 2nd IS for G-CSF (09/136) if the WHO 2nd IS for G-CSF is not suitable as a reference standard for PEG-G-CSF preparations and c) to compare the ampouled PEG-G-CSF preparations with characterised ‘in-house’ laboratory standards of PEG-G-CSF where these are available.
2. Materials and methods
Two preparations of PEG-conjugated human sequence recombinant G-CSF, both pure and expressed in E. coli and in large amounts were kindly donated to WHO for development of a reference standard (see Acknowledgements). Limited amounts of conjugated protein were provided to NIBSC by another manufacturer for evaluation in bioassays. Trial fills were conducted from the materials (supplied for reference standard) and the biological activity of the lyophilized preparations was compared with the bulk materials in a bioassay based on G-CSF induced proliferation of a murine myeloid cell-line, G-NFS-60 which is a G-CSF responsive variant of the parent NFS-60 cell-line. This bioassay was also used in the collaborative study for the current WHO 2nd IS for G-CSF (Wadhwa et al., 2011). As the trial lyophilizations of PEG-G-CSF were performed appropriately in the bioassay, final lyophilization of different PEG-G-CSF preparations into ampoules was carried out at NIBSC as per the procedures used for International Biological Standards (ECBS guidelines — WHO Expert Committee on Biological Standardization, 2006).
Buffers with excipients as shown in Table 1, were prepared using nonpyrogenic water and depyrogenated glassware. Buffer solutions were filtered using sterile nonpyrogenic filters (0.22 μM Stericup filter system, Millipore, USA) where appropriate.
Table 1.
Materials used in study.
| Ampoule code | Fill date | Study code | No. of ampoules in stock | Protein | Protein (predicted Mass – μg) |
Expression system | Excipients |
|---|---|---|---|---|---|---|---|
| 12/222 | 1/11/12 | B, C | 4700 | PEG-G-CSF | 1 | E. coli | Trehalose, Tween 20, Phenylalanin,e Arginine, Human serum albumin |
| 12/188 | 13/9/12 | A | 4700 ~ | PEG-G-CSF | 1 | E. coli | |
| 09/136 | 2/07/09 | 2nd IS G-CSF | 3400 ~ | G-CSF | 1 | E. coli |
For the study, the preparation 12/188 was coded A while B and C are duplicates of the same preparation 12/222 (Table 1). The mass content of the preparations was determined by the manufacturers. As the protein content of the ampoules cannot be verified by direct measurement of absolute mass, the content is assumed to be the theoretical mass, calculated from the dilution of the bulk material of known protein mass content, and the volume of formulated solution delivered to the ampoule. This mass value is given as “predicted μg”.
For both preparations, a solution at a concentration predicted as 1 μg/ml PEG-G-CSF was distributed in 1.0 ml aliquots, giving the theoretical protein content per ampoule shown in Table 1.
The precision of filling of ampoules had a CV in the range of 0.163–0.305% as assessed by determination of mean fill weights of all preparations. Each solution was lyophilized, and the ampoules were sealed under dry nitrogen by heat fusion of the glass and stored at − 20 °C in the dark. The mean residual moisture of each preparation, measured by the coulometric Karl–Fischer method (Mitsubishi CA100, A1-Envirosciences Ltd, Luton, UK), varied between 0.205 and 0.279% for all preparations. Mean headspace oxygen content determined by frequency modulated spectroscopy using the Lighthouse FMS-760 Instrument (Lighthouse Instruments, LLC) varied from 0.13 to 0.19 for all preparations. Testing for microbial contamination using the total viable count method did not show any evidence of microbial contamination.
2.1. Participants
Twenty-three participants from eleven countries contributed bioassay data used for the study (Table 2). The participants included 3 control, 2 pharmacopoeial, 16 manufacturers' and 2 contract research organisation laboratories.
Table 2.
List of participants.
| The following participants contributed data to the study. In this report, each laboratory has been identified by a number from 1 to 24 that is not related to this order of listing. |
| Xinchang Shi and Rao Chunming, Division of Biopharmaceuticals, National Institutes for Food and Drug Control (NIFDC), 2 Tiantan Xili, Beijing 100050, P.R. China |
| Till Koenig, Novartis Pharma AG,WKL-681.3.05, 4002 Basel, Switzerland |
| Beate Hartung and Sonja Klingelhoefer, Biological Assays, Richter-Helm-Biologics, Suhrenkamp 59, D-22335 Hamburg, Germany |
| Taina Cruz, Amgen Manufacturing Limited, Road 31 Km 24.6, Juncos, PR 00777-4060, Puerto Rico |
| Chris Bird, Cytokines and Growth Factors Section, Biotherapeutics Group, NIBSC, South Mimms, Herts, EN6 3QG, UK |
| Meihua Yang and Zeng Yan, Xiamen Amoytop Biotech Co. Ltd, No. 330, Wengjiao Road, Haicang, Xiamen, Fujian, P.R. China, 361022 |
| Andrea López, Federico Parnizari, Control calidad biológico, Laboratorios Clausen S.A., Bv. Artigas 3896, Montevideo CP 11700, Uruguay |
| Cecilia Medrano, Bioch., Head of Quality Control, Gema Biotech S.A., Fray Justo Sarmiento 2350 edificio 2B 5 piso B1636AXK, Olivos, Buenos Aires, Argentina |
| Dong-Yeon Kim, Chankyu Lee, Bio Engineering Lab., Chong Kun Dang Pharm., 464-3, Jung-dong, Yongin Si Giheung-gu, Gyeonggi-Do, Seoul 446-916, Rep. of Korea |
| MN Dixit, Manjunath Patil, Bioanalytical Laboratory, 3rd Floor Clinigene International Limited, Clinigene House, Electronics City, Phase 2, Bangalore 560100, India |
| Zeljka Antolvic, Ela Kosor Krnic, Hospira Zagreb d.o.o., Prudnicka cesta 60,10291 Prigorje Brdovecko, Croatia |
| Subba Raju BV, Sahana S, Shridhar Bagal, Amit Inchal, Quality control-QC-Q8, B1 block, Biocon Limited, Biocon Park, Jigani Link Road, Plot 2, 3 & 4 Bommasandra IV Phase, Bangalore 560 099, India |
| Himanshu Gadgil, Intas Biopharmaceuticals Ltd., Plot No. 423/P/A, Sarkhej-Bavla Highway, Village-Moraiya, Taluka – Sanand, Ahmedabad 382213, Gujarat, India |
| Susobhan Das, Biologics & Biotechnology Division, United States Pharmacopeia-India (P) Ltd, Plot No. D6 & D8, IKP Knowledge Park, Genome Valley, Shameerpet, Hyderabad 500078, RR District, Andhra Pradesh, India |
| Veena Raiker and Alok Sharma, Research and Development, Lupin Ltd, Biotech Division, Gat #1156, Ghotawade Village, Mulshi Taluka, Pune 412 115, Maharashtra, India |
| Renu Jain and Shalini Tewari, Recombinant Product Laboratory, National Institute of Biologicals, A-32, Sector 62 (Institutional Area), NOIDA 201 307, Uttar Pradesh, India |
| Sridevi Khambhampaty, Manish Reddy, Biologics Development Centre, Dr Reddy's Laboratories, Survey No: 47, Bachupally, Qutubullapur, RR Dist 500090, Andhra Pradesh, India |
| Sanjay Bandyopadhyay, Zydus Research Centre, Biotech Division, Cadila Healthcare Ltd., |
| Sarkhej-Bavla N. H. 8A, Moraiya. Tal: Sanand, Ahmedabad 380015, Gujarat, India |
| Kwanyub Kang, Mogam biotechnology research institute, Greencross Corp., 341 Bojeong-dong, Giheung-gu, Yongin, 446-799, Rep. of Korea |
| Michael Ambrose, US Pharmacopeia, 12601 Twinbrook Parkway, Rockville, MD 20852, USA |
| Swarnendu Kaviraj, Analytical Development, Vaccine Formulation and Research Center, |
| Gennova Biopharmaceuticals Ltd, BTS 2 Building Chrysalis Enclave Block 2, International Biotech Park, I.T.B.T. Park Phase II Hinjewadi MIDC, Pune, Maharashtra 411057, India |
| Zhang Xuan, Tianjin PEGylatt Biotechnology Co.,Ltd., Lab Buiding N1801, International Joint Academy Of Biotechnology & Medicine, 220 Dongting Road, TEDA, Tianjin, P.R. China, 300457 |
| Mr Yanzhuo Wu, Technology Center, Beijing SL Pharmaceutical Co., Ltd, No.69, Fushi Road, Haidian District, Beijing, P.R. China |
2.2. Study design and assay methods
Participants were asked to bioassay all samples including the current WHO 2nd IS for G-CSF (09/136) concurrently on a minimum of three separate occasions using their own routine bioassay methods within a specified layout which allocated the samples across 3 plates and allowed testing of replicates as per the specified study protocol. It was requested that participants perform eight dilutions of each preparation using freshly reconstituted ampoules for each bioassay and include 09/136 and their own in-house standard where available on each plate.
A summary of the methods used in the study is given in Table 3. A majority of participants used cell-lines and read-outs that are commonly used for G-CSF bioassays (Wadhwa et al., 2011).
Table 3.
Brief details of bioassays contributed to the study.
| laboratory Code | Bioassay cell line** | Assay type | Assay duration (h) | Assay readout |
|---|---|---|---|---|
| 1 | MNFS-60 | Proliferation | 24 | Luminescence (cell-titer Glo) |
| 2 | NFS-60 | Proliferation | 34–38 | Colorimetric (MTS) |
| 3 | G- CSFRLuc | Reporter-gene | 3–4 | Luminescence (luciferase) |
| 4 | NFS-60 | Proliferation | 26–30 | Fluorescence (Alamar Blue) |
| 5 | NFS-60 | Proliferation | 40–48 | Colorimetric (MTT) |
| 6 | GNFS-60 | Proliferation | 48 | 3H Thymidine |
| 7 | NFS-60 | Proliferation | 48 | Colorimetric (MTS) |
| 8 | NFS-60 | Proliferation | 48 | Colorimetric (Cell Titer96 Aqueous One, MTS) |
| 9 | MNFS-60 | Proliferation | 44 | Colorimetric (WST-1) |
| 10 | MNFS-60 | Proliferation | 44–48 | Colorimetric (Cell Titer96 Aqueous One, MTS) |
| 11 | MNFS-60 | Proliferation | 40–44 | Fluorescence (Alamar Blue) |
| 12 | MNFS-60 | Proliferation | 44 | Colorimetric (MTS) |
| 13 | MNFS-60 | Proliferation | 42–44 | Fluorescence (Alamar Blue) |
| 14 | MNFS-60 | Proliferation | 48 | Colorimetric (MTS) |
| 15 | MNFS-60 | Proliferation | 44 | Luminescence (Cell-titer Glo) |
| 16 | NFS-60 | Proliferation | 20–22 | Colorimetric (MTT) |
| 18 | NFS-60 | Proliferation | 48 | Colorimetric (MTS) |
| 19 | MNFS-60 | Proliferation | 44 | Colorimetric (MTS) |
| 20 | MNFS-60 | Proliferation | 28–32 | Luminescence (Cell-titer Glo) |
| 21 | NFS-60 | Proliferation | 48 | Colorimetric (MTT) |
| 22 | NFS-60 | Proliferation | 48 | Colorimetric (MTT) |
| 23 | MNFS-60 | Proliferation | 44 | Colorimetric (MTS) |
| 24 | MNFS-60 | Proliferation | 48 | Colorimetric (Cell Titer96 Aqueous One, MTS) |
Participating laboratories were sent five sets of three study samples coded A–C along with the current WHO 2nd IS for G-CSF (09/136) as detailed in Table 1. Samples B and C were coded duplicate samples of the same material (candidate standard 12/222).
Participants were requested to return their raw bioassay data, using spreadsheet templates provided, and also their own calculations of potency of the study samples relative to the 2nd IS for G-CSF.
2.3. Statistical analysis
Relative potencies of the study samples were calculated by analysis of the raw data at NIBSC using the EDQM CombiStats software (Combistats V5.0. EDQM). All bioassays were analysed using a simple parallel-line model based on a linear section of the dose response range (Finney, 1978). In the majority of laboratories, no transformation of the bioassay response was applied. For the laboratories 5 and 14, a log transformation of the bioassay response was used. Validity of the bioassay was assessed by calculation of the ratio of slopes for the two test samples under consideration. The samples were concluded to be non-parallel when the slope ratio was outside of the range 0.80–1.25 and no potency estimates were calculated.
Potency estimates from all valid bioassays were combined to generate an unweighted geometric mean (GM) for each laboratory and these laboratory means were used to calculate an overall unweighted geometric mean for each sample. Variability between bioassays within laboratories and between laboratories has been expressed using geometric coefficients of variation (GCV = {10s − 1} × 100% where s is the standard deviation of the log10 transformed estimates). Analysis of variance with Duncan's multiple range test (Duncan, 1975) using the log transformed potency estimates was used to compare laboratories and samples (p < 0.05 used to conclude significance).
The agreement between duplicate samples was assessed by calculating the difference in log potency estimates (relative to sample A) of samples B and C for each assay, calculating the mean of the squared difference for each laboratory, taking the square root to give a root mean square (RMS) value, and expressing this as an average percentage difference.
2.4. Stability studies
Samples of the candidate standard 12/188 were stored at elevated temperatures (20 °C, 37 °C and 45 °C) for seven months and tested concurrently with those stored at the recommended storage temperature of − 20 °C, and baseline samples stored at − 70 °C. A total of six independent bioassays were performed, with three plates per assay. The potencies of all samples were expressed relative to the appropriate − 70 °C baseline samples. In addition, the stability of the samples at 4 °C and 20 °C after a period of 24 h and 1 week following reconstitution and after a series of freeze–thaw cycles (1 up to 4) was assessed. Three independent bioassays were performed for 12/188, with each bioassay consisting of three plates. All studies were conducted at NIBSC using the GNFS-60 assay.
3. Results
23 laboratories submitted data. Each participating laboratory has been assigned a code number allocated at random, and not necessarily representing the order of listing in Table 2 to retain confidentiality in the report.
The majority of the laboratories returned data from three bioassays as requested, using three plates per assay. Laboratories 4, 6, 11 and 20 performed four assays, using three plates per assay. Laboratory 2 returned data from three assays, using four plates per assay. Laboratory 13 returned data from six assays, using three plates per assay. Laboratory 21 performed two assays, using four plates per assay. Laboratories 16 and 22 each performed one bioassay using four plates. For laboratory 3, responses in plate columns 2 and 11 were excluded from the analysis as these showed a clear plate effect giving a lower level of response.
In general, acceptable parallelism was achieved between all study samples as indicated by the slope ratios obtained in a majority of laboratories using the bioassays employed in the study. However, there was a greater tendency towards non-parallelism when the candidate preparations coded A, B and C were compared with the current WHO 2nd IS for G-CSF, 09/136 (16.0%, 20.1% and 19.2% of cases respectively), as opposed to comparisons of B or C relative to A. This is partly due to greater variability in the slope ratios when comparing the candidate PEG-G-CSF preparations against the current WHO 2nd IS for G-CSF rather than among themselves and steeper slopes observed for samples A, B and C compared to the IS in some laboratories. For example, slope ratios for sample B relative to the WHO 2nd IS for G-CSF (09/136) had a GCV of 20.2% while slope ratios for B relative to A had a GCV of 12.7%. Laboratory 5 was noted as obtaining steeper slopes for samples A, B and C when compared with 09/136 in all assays. A similar pattern of generally steeper slopes for samples A, B and C was observed in laboratories 7 and 20. However, no overall trend in slope ratios across all laboratories was observed.
3.1. Potencies of samples A, B and C relative to IS 09/136
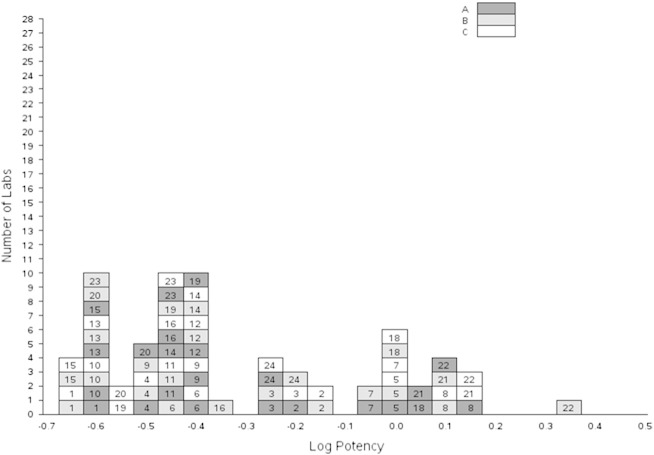
Relative potency estimates for samples A–C relative to 09/136 are summarised in Table 4 and Fig. 2.
Table 4.
Potencies of samples A, B and C relative to IS 09/136.
| Lab | Sample A |
Sample B |
Sample C |
||||||
|---|---|---|---|---|---|---|---|---|---|
| GM | GCV | n | GM | GCV | n | GM | GCV | n | |
| 1 | 0.24 | 10.0 | 8 | 0.23 | 5.0 | 7 | 0.23 | 17.4 | 8 |
| 2 | 0.66 | 14.3 | 12 | 0.71 | 15.9 | 11 | 0.71 | 10.4 | 12 |
| 3 | 0.55 | 21.7 | 9 | 0.53 | 16.3 | 9 | 0.61 | 13.7 | 9 |
| 4 | 0.33 | 8.0 | 12 | 0.32 | 9.9 | 12 | 0.32 | 8.4 | 16 |
| 5 | 1.00 | 14.4 | 6 | 0.96 | 10.4 | 6 | 0.95 | 7.0 | 4 |
| 6 | 0.39 | 13.2 | 14 | 0.36 | 17.8 | 14 | 0.38 | 17.5 | 15 |
| 7 | 0.85 | 22.9 | 7 | 0.90 | 22.9 | 4 | 0.95 | . | 2 |
| 8 | 1.39 | 19.4 | 8 | 1.28 | 9.1 | 6 | 1.27 | 20.9 | 8 |
| 9 | 0.38 | 27.5 | 5 | 0.31 | 27.0 | 3 | 0.39 | 20.4 | 6 |
| 10 | 0.26 | 14.0 | 8 | 0.25 | 9.8 | 9 | 0.24 | 13.6 | 8 |
| 11 | 0.36 | 14.4 | 11 | 0.34 | 18.6 | 11 | 0.35 | 11.9 | 11 |
| 12 | 0.40 | 10.5 | 4 | 0.38 | 7.8 | 6 | 0.38 | 12.8 | 9 |
| 13 | 0.27 | 11.2 | 17 | 0.25 | 17.2 | 16 | 0.26 | 13.1 | 17 |
| 14 | 0.37 | 16.6 | 9 | 0.40 | 21.9 | 8 | 0.39 | 13.8 | 11 |
| 15 | 0.24 | 7.3 | 9 | 0.22 | 9.6 | 9 | 0.23 | 7.9 | 12 |
| 16 | 0.36 | 40.9 | 3 | 0.45 | 36.0 | 3 | 0.36 | 44.5 | 4 |
| 18 | 1.09 | 25.2 | 8 | 1.02 | 37.8 | 8 | 1.00 | 33.8 | 8 |
| 19 | 0.41 | 12.3 | 6 | 0.34 | 35.4 | 7 | 0.29 | 3.0 | 3 |
| 20 | 0.30 | 18.7 | 8 | 0.26 | 23.9 | 9 | 0.28 | 23.3 | 9 |
| 21 | 1.08 | 63.4 | 4 | 1.30 | 67.6 | 5 | 1.40 | 44.3 | 8 |
| 22 | 1.33 | . | 1 | 2.15 | . | 1 | 1.44 | . | 1 |
| 23 | 0.35 | 44.6 | 7 | 0.26 | 24.9 | 5 | 0.36 | 46.3 | 6 |
| 24 | 0.58 | 19.0 | 8 | 0.61 | 18.9 | 6 | 0.59 | 15.5 | 6 |
| GM (95% CI) |
0.49 (0.38–0.62) |
0.48 (0.36–0.63) |
0.48 (0.37–0.63) |
||||||
| Between-lab GCV | 76.0 | 92.6 | 83.3 | ||||||
| GMa | 0.44 | 0.42 | 0.43 | ||||||
| GMb (95% CI) |
0.35 (0.30–0.40) |
0.32 (0.27–0.38) |
0.34 (0.29–0.40) |
||||||
| Between-lab GCVb | 30.2 | 34.8 | 34.8 | ||||||
| GMa,b | 0.34 | 0.32 | 0.33 | ||||||
GM — geometric mean.
CI — confidence interval.
GCV — geometric coefficient of variation (%).
n — number of estimates used in calculation.
Calculated as geometric mean of all individual assay estimates.
Excludes laboratories 2, 5, 7, 8, 16, 18, 21 and 22.
Fig. 2.
Laboratory mean potencies of samples A, B and C relative to the current IS for G-CSF (09/136).
Intra-laboratory variability, as measured by the within-laboratory GCVs shown in Table 4, ranged from 3.0% (laboratory 19, sample C) to 67.6% (laboratory 21, sample B). In the majority of cases, GCVs were less than 30%, with seventeen laboratories achieving this for all test samples.
Inter-laboratory variability, as measured by the between-laboratory GCVs shown in Table 4, indicated a high level of variability between laboratories (76.0%, 92.6% and 83.3% for samples A, B and C respectively). For all samples, laboratories 5, 7, 8, 18, 21 and 22 gave significantly higher estimates than all the other laboratories (p < 0.05). As these laboratories used the same bioassay method, the NFS60 cell-line based bioassay with a colorimetric readout, overall means were also calculated excluding these laboratories, together with laboratories 2 and 16 who also used this method. Inter-laboratory variability was reduced, giving GCVs of 30.2%, 34.8% and 34.8% for samples A, B and C respectively. Overall mean relative potencies were reduced by around 30% following the exclusion of these laboratories.
3.2. Potencies of samples B and C relative to A
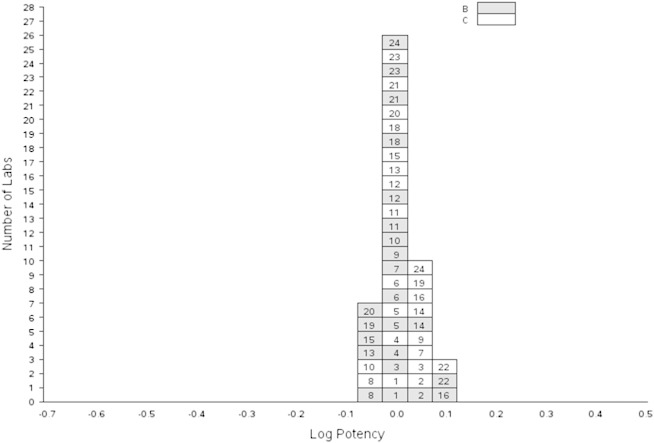
Relative potency estimates for samples B and C (which are coded duplicates of the same preparation) relative to A are summarised in Table 5 and Fig. 3. The potency estimates of these samples showed high concordance (0.99 and 1.02 for B and C respectively) relative to A.
Table 5.
Relative potencies of samples A, B and C.
| B relative to A |
C relative to A |
C relative to B (coded duplicates) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lab | GM | GCV | n | GM | GCV | n | GM | GCV | n |
| 1 | 0.99 | 8.1 | 9 | 0.98 | 16.7 | 9 | 0.99 | 14.7 | 9 |
| 2 | 1.08 | 11.7 | 11 | 1.07 | 14.9 | 11 | 1.00 | 10.8 | 11 |
| 3 | 0.97 | 8.6 | 9 | 1.10 | 24.1 | 9 | 1.14 | 16.5 | 9 |
| 4 | 0.97 | 8.6 | 12 | 0.97 | 7.3 | 12 | 1.00 | 8.2 | 12 |
| 5 | 0.97 | 6.1 | 9 | 0.99 | 7.4 | 9 | 1.03 | 5.5 | 9 |
| 6 | 0.96 | 13.6 | 20 | 1.00 | 14.5 | 20 | 1.01 | 12.9 | 12 |
| 7 | 0.99 | 25.4 | 8 | 1.08 | 28.7 | 7 | 1.14 | 38.6 | 9 |
| 8 | 0.92 | 31.8 | 8 | 0.86 | 21.8 | 8 | 1.09 | 11.9 | 8 |
| 9 | 0.99 | 26.9 | 6 | 1.07 | 17.9 | 6 | 0.96 | 35.8 | 4 |
| 10 | 0.98 | 8.6 | 9 | 0.94 | 12.7 | 9 | 0.96 | 11.0 | 9 |
| 11 | 1.00 | 9.9 | 11 | 0.99 | 12.1 | 12 | 0.99 | 12.3 | 11 |
| 12 | 1.00 | 11.1 | 8 | 1.00 | 8.7 | 8 | 1.04 | 8.7 | 9 |
| 13 | 0.92 | 16.0 | 18 | 0.96 | 8.7 | 18 | 1.05 | 12.8 | 18 |
| 14 | 1.06 | 13.4 | 9 | 1.10 | 8.0 | 9 | 1.04 | 17.0 | 9 |
| 15 | 0.89 | 5.6 | 9 | 0.96 | 3.2 | 9 | 1.08 | 4.2 | 9 |
| 16 | 1.24 | 55.4 | 3 | 1.17 | 13.3 | 3 | 0.94 | 39.5 | 3 |
| 18 | 0.95 | 22.6 | 9 | 0.96 | 14.0 | 7 | 1.05 | 15.9 | 8 |
| 19 | 0.90 | 37.8 | 9 | 1.07 | 42.4 | 5 | 1.04 | 48.8 | 6 |
| 20 | 0.87 | 27.0 | 12 | 0.95 | 22.8 | 11 | 1.08 | 27.4 | 12 |
| 21 | 0.97 | 28.4 | 6 | 0.98 | 16.9 | 5 | 0.94 | 20.4 | 4 |
| 22 | 1.33 | 33.3 | 3 | 1.27 | 23.2 | 2 | 1.16 | . | 1 |
| 23 | 1.03 | 9.4 | 5 | 1.00 | 6.7 | 6 | 1.00 | 11.2 | 8 |
| 24 | 0.99 | 30.9 | 7 | 1.08 | 14.8 | 9 | 1.09 | 26.7 | 7 |
| GM (95% CI) |
0.99 (0.95–1.04) |
1.02 (0.99–1.06) |
|||||||
| Between-lab | |||||||||
| GCV | 10.1 | 8.7 | |||||||
| GMa | 0.97 | 1.01 | |||||||
| GMb (95% CI) |
0.97 (0.94–1.00) |
1.01 (0.98–1.04) |
|||||||
| Between-lab | |||||||||
| GCVb | 5.7 | 5.7 | |||||||
| GMab | 0.96 | 1.00 | |||||||
GM — geometric mean.
CI — confidence interval.
GCV — geometric coefficient of variation (%).
n — number of estimates used in calculation.
Calculated as geometric mean of all individual assay estimates.
Excludes laboratories 2, 5, 7, 8, 16, 18, 21 and 22.
Fig. 3.
Laboratory mean potencies of samples B and C relative to A.
Intra-laboratory variability, as measured by the within-laboratory GCVs shown in Table 5, ranged from 3.2% (laboratory 15, sample C) to 55.4% (laboratory 16, sample B). In the majority of cases, GCVs were less than 30%, with eighteen laboratories achieving this for both test samples.
Inter-laboratory variability, as measured by the between-laboratory GCVs shown in Table 5, indicated excellent agreement between laboratories (10.1% and 8.7% for samples B and C respectively). Exclusion of laboratories 2, 5, 7, 8, 18, 16, 21 and 22 as noted above gave a between-laboratory GCV of 5.7% for both samples B and C.
3.3. Agreement between duplicates, B and C
Samples B and C were coded duplicates of the same material. The overall potency estimates for these samples relative to sample A were in very close agreement (0.99 and 1.02 respectively with a mean value of 1.01).
The agreement between the potency estimates of B and C within bioassays can be assessed in two ways. Firstly, the intra-laboratory GCVs for the potencies of sample C relative to sample B, shown in Table 5, represent the variability between bioassays of direct comparisons of C to B. They range from 4.2% (laboratory 15), representing excellent agreement between assays, to 48.8% (laboratory 19), which represents a higher level of variability. Eighteen laboratories had GCVs less than 30%. Secondly, as described in the Statistical analysis section, the average difference in potency estimates of samples B and C was calculated (root mean square difference in log potency) for each laboratory, and these differences, expressed as a percentage ranged from 5.0% (laboratory 5) to 41.1% (laboratory 19), with 16 laboratories having a value less than 20%.
3.4. Comparison of study samples with in-house standards
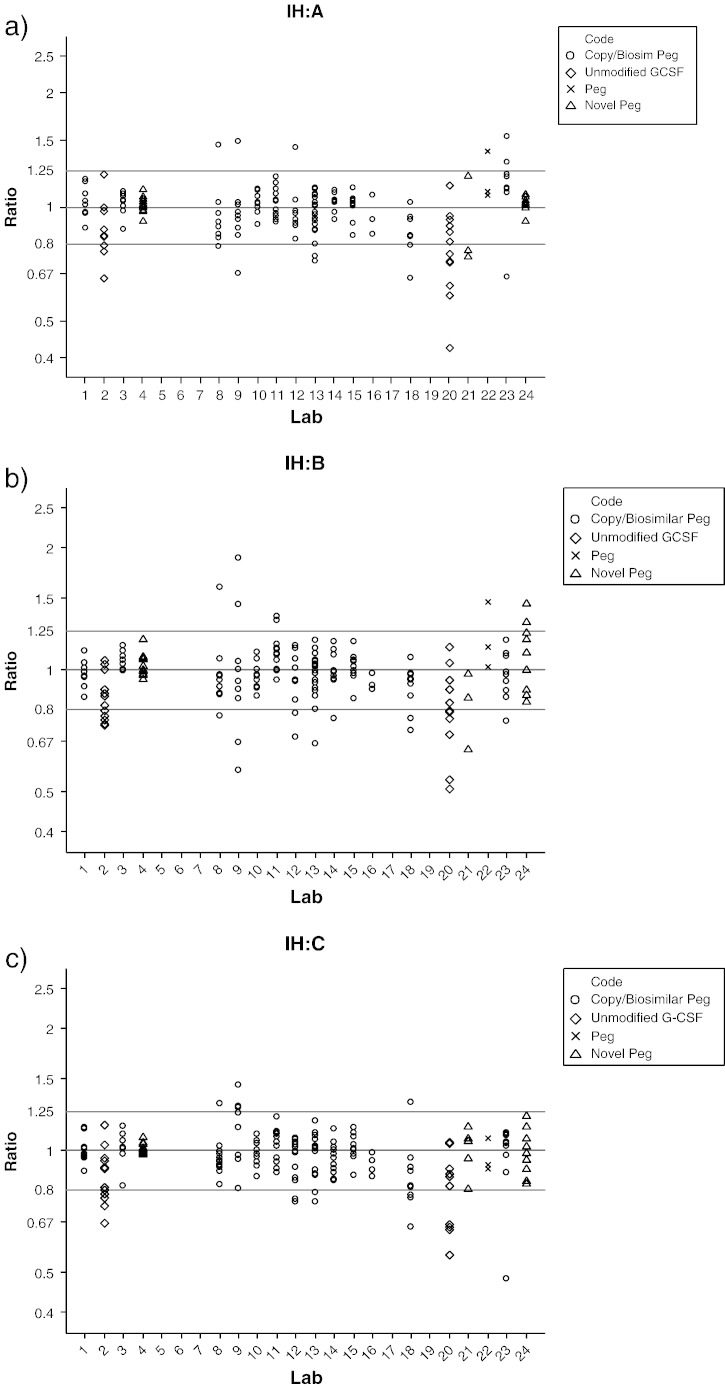
Intra-laboratory variability for in-house standards relative to samples A, B and C is summarised in Table 6. Slope ratios from individual plates are shown in Fig. 4. Bioassays performed on the first day by laboratory 21 are not shown as the slope ratios were < 0.30 in all bioassays on this day.
Table 6.
Intra-laboratory variability for in-house standards (IH) relative to samples A, B and C.
| IH relative to A |
IH relative to B |
IH relative to C |
||||
|---|---|---|---|---|---|---|
| Lab | GCV | n | GCV | n | GCV | n |
| 1 | 7.9 | 9 | 12.4 | 9 | 18.5 | 9 |
| 2 | 15.2 | 9 | 23.3 | 7 | 21.9 | 7 |
| 3 | 27.1 | 9 | 30.3 | 9 | 39.7 | 9 |
| 4 | 5.8 | 12 | 9.8 | 12 | 7.8 | 16 |
| 8 | 12.9 | 7 | 20.9 | 7 | 24.0 | 11 |
| 9 | 10.6 | 7 | 38.0 | 5 | 23.9 | 6 |
| 10 | 10.1 | 9 | 10.3 | 9 | 10.6 | 9 |
| 11 | 17.3 | 12 | 15.0 | 10 | 23.1 | 12 |
| 12 | 10.7 | 8 | 6.2 | 7 | 8.8 | 10 |
| 13 | 9.1 | 15 | 11.6 | 16 | 10.7 | 16 |
| 14 | 13.1 | 9 | 11.0 | 8 | 16.8 | 12 |
| 15 | 5.7 | 9 | 9.6 | 9 | 7.8 | 12 |
| 16 | 37.0 | 3 | 21.6 | 3 | 18.6 | 4 |
| 18 | 187.8 | 6 | 168.9 | 7 | 138.7 | 5 |
| 20 | 24.9 | 6 | 29.6 | 5 | 24.9 | 8 |
| 21 | 1 | 24.4 | 2 | 18.7 | 5 | |
| 22 | 1 | 1 | 35.6 | 3 | ||
| 23 | 13.2 | 6 | 10.5 | 8 | 11.6 | 8 |
| 24 | 22.3 | 9 | 36.7 | 7 | 29.4 | 9 |
GCV — geometric coefficient of variation (%).
n — number of estimates used in calculation.
Fig. 4.
Slope ratios for in-house standards (IH) relative to samples A, B and C as shown in panels a, b and c.
Slope ratios showed non-parallelism in 16.2% of cases. Exclusion of laboratories 2 and 20 which used the unmodified GCSF protein as an in-house standard gave non-parallelism in only 14.7% of cases. In general, acceptable parallelism was observed for the comparison of in-house standards and samples A, B and C.
Excluding laboratory 18, intra-laboratory variability as measured by the within-laboratory GCVs shown in Table 6, ranged from 5.7% (laboratory 15, sample A) to 39.7% (laboratory 3, sample C). In the majority of cases, GCVs were less than 30%, indicating a comparable level of variability to that observed for the common samples tested by all laboratories in the study.
3.5. Stability studies
Geometric mean potency estimates of samples of the candidate standard 12/188 stored at elevated temperatures for over 7 months (expressed relative to those stored at − 70 °C) are shown in Table 7. No detectable loss of potency is detected, even at 45 °C. Therefore, it is not possible to predict a yearly loss for this preparation.
Table 7.
Potency estimates for candidate standard 12/188 stored at elevated temperatures for 7 months relative to ampoules stored at − 70 °C.
| Storage temperature | Potency relative to − 70 °C |
|||
|---|---|---|---|---|
| GM | 95% CI | GCV | n | |
| − 20 °C | 1.03 | 0.98–1.09 | 11.0 | 18 |
| + 20 °C | 1.03 | 0.98–1.09 | 10.9 | 18 |
| + 37 °C | 0.99 | 0.95–1.03 | 8.9 | 18 |
| + 45 °C | 1.05 | 1.01–1.09 | 8.3 | 18 |
GM — geometric mean.
CI — confidence interval.
GCV — geometric coefficient of variation (%).
n — number of estimates used in calculation.
Further studies showed that the potency of 12/188 is not diminished after a week of storage at either 4 °C or 20 °C following reconstitution or after a limited number of freeze–thaw cycles (up to 4 cycles) suggesting that the preparation is sufficiently stable to serve as an international standard (data not shown).
4. Discussion
It is well recognised that PEGylation can variably reduce potency in vitro while increasing half-life in vivo and, therefore, assessment of these products in practice would require in addition to potency evaluation by in vitro bioassays, determination of the pharmacokinetic activity of the PEG-G-CSF product. Here, we have focussed on the development of a reference standard for determination of in vitro biological activity of PEG-G-CSF following a demand from manufacturers worldwide for a bioactivity standard for PEG-G-CSF products.
Although the approved PEGylated G-CSF product is dosed in mass units and the label does not provide any information relating to its biological activity (i.e., international unit or specific activity of protein), it is a regulatory requirement to determine the bioactivity in vitro for lot release and stability assessment using an appropriate reference standard. Guidance on declaration of the quantitative composition/potency labelling of biological medicinal products that contain modified proteins is available (EMA/CHMP/BWP/85290/2012; EMEA/CPMP/BWP/3068/03).
Some manufacturers have measured the potency of their PEGylated G-CSF products in bioassays calibrated using the current WHO 2nd IS for G-CSF (09/136) but the suitability of reporting potencies in the respective IU has not been formally established. Since many PEGylated G-CSF products currently in development have a 20 kD linear PEG attached to the N-terminal methionyl residue of G-CSF (INN Filgrastim) and use a conjugation process and coupling chemistry similar to that employed for the licenced innovator product (INN PEG-Filgrastim), two candidate preparations specifically representing these types of PEGylated G-CSF products were assessed in this collaborative study.
The candidate PEG-G-CSF preparations were evaluated relative to the current WHO 2nd IS for G-CSF using in vitro biological activity assays for G-CSF with the aim of a) determining the suitability of the current WHO 2nd IS for G-CSF (the standard for the parent molecule) or alternatively, a PEG-G-CSF candidate preparation to serve as the reference standard for biological activity of PEG-G-CSF products and b) assigning a unitage to the reference PEG-G-CSF standard should the WHO 2nd IS for G-CSF not be suitable. A strategy involving three options was formulated (Table 8) at the outset as the basis for assigning a unitage to the PEG-G-CSF standard.
Table 8.
Assigning a unitage to the PEG-G-CSF standard.
| Option | Question | Answer | Pros | Cons |
|---|---|---|---|---|
| 1 | Should the unitage be traceable to the IS for G-CSF? | Possible — Study includes 2nd IS for G-CSF but the traceability issue to be determined by statistical analysis of data. If bioassays valid relative to the IS, units can be traceable to G-CSF IS. | Traceable to G-CSF IS Align with other products if this approach has been used Likely to encourage developers of novel PEG-G-CSF products to consider calibration of in-house reference standards using the WHO 2nd IS for G-CSF, if possible Provides objectivity for independent testing |
Difficult to ensure similar relationship between the two standards and between PEG-G-CSF products and G-CSF IS Risk of discontinuity when G-CSF IS is replaced |
| 2 | Should the standard be assigned independent units? | Will be determined by statistical analysis of study data as described above. If data relative to G-CSF IS is inappropriate and gives statistically invalid estimates, independent units likely to be assigned. | Usual and easy approach No impact in case of replacement of current G-CSF IS |
Risk of disconnection with novel PEG-G-CSF & other modified G-CSF products Potential for confusion for users |
| 3 | Assign independent units and indicate relationship with G-CSF IS | Possible — Study includes 2nd IS for G-CSF. Will be determined by statistical analysis of study data as described above. | Ideal approach — provides an independent unitage as well as a relationship with G-CSF IS (and consequently a link with the parent molecule). May be suitable for novel PEGylated G-CSF products Provides a basis for linking novel PEG-G-CSF and other modified G-CSF products to the parent molecule |
With the exception of a single laboratory which used a luciferase reporter gene assay, most laboratories performed bioassays based on G-CSF-induced proliferation of the NFS-60 cell-line or its variants, M-NFS-60 or G-NFS-60. These bioassays were used previously in the study for the WHO 2nd IS for G-CSF and employ different readouts for detection, for example, a radioactive label (3H-thymidine) or a colorimetric/fluorescence dye (Wadhwa et al., 2011).
Results from this study showed that acceptable parallelism was achieved between all study samples as indicated by the slope ratios obtained in a majority of laboratories using the bioassays employed in the study. However, there was a greater tendency towards non-parallelism when the candidate preparations coded A, B and C were compared with the 2nd IS for G-CSF, 09/136 (containing unmodified G-CSF), as opposed to B or C relative to A. While this is partly attributed to the higher variability in the slope ratios when comparing the candidate samples against the WHO 2nd IS for G-CSF rather than among themselves and steeper slopes for samples A, B and C compared to the IS in some laboratories, the low potency of PEG-G-CSF relative to the WHO 2nd IS for G-CSF is also a likely contributory factor to the non-parallelism seen in some laboratories. Nevertheless, no overall trend in slope ratios across all laboratories was observed.
In most laboratories, the PEGylated candidate preparations had a reduced potency in comparison with the WHO 2nd IS for G-CSF. While the bioassays from many laboratories showed very similar results for potencies of samples A–C relative to 09/136 (GM potency of 0.49 for A or 0.48 for B and C) as shown in Table 4 and Fig. 2, a high variability (GCV ranging from 76 to 93%) depending on the sample being compared was observed. This was because data from some laboratories, in particular those using the NFS-60 cell-line and the colorimetric readout, MTT/MTS showed significantly higher potency estimates relative to other laboratories. Such high estimates were not evident for laboratory 4 which used the NFS-60 and a fluorescence dye, Alamar Blue for detection. Although the reason is not clear, it is possible that differences in sourcing and maintenance of the NFS-60 cells result in insensitivity and inability of the NFS-60 cell-line to discriminate between the modified and unmodified G-CSF in some laboratories. In contrast, the variant cell-lines, G-NFS-60 or M-NFS-60 are highly sensitive to G-CSF and capable of distinguishing between the two G-CSF forms. It is possible that the use of the colorimetric dye is also a likely contributory factor as bioassays using MTT which forms an insoluble precipitate requiring solubilisation as opposed to bioassays using the soluble MTS formazan product (Buttke et al., 1993) were generally associated with a high variability. If data from the laboratories using NFS-60 and the colorimetric dye (n = 8) are excluded, the between-laboratory variability in the potency estimates is diminished to 30–35% (from GCV of 76–93%).
If expressing unitage for sample A (candidate PEG-G-CSF preparation) in terms of the current WHO 2nd IS for G-CSF, a relationship is evident as shown in Table 4. Based on the high variability and the bias in potency estimates if NFS-60 bioassays are considered, a mean potency of 0.35 can be derived by excluding these assays. Since the current WHO 2nd IS for G-CSF (09/136) has an assigned unitage of 95,000 IU per ampoule, the mean potency estimate for sample A is equivalent to 33,250 IU of G-CSF per ampoule. However, it should be noted that there is no formal relationship or conversion factor between the units for PEG-G-CSF and the units for the current WHO 2nd IS for G-CSF (coded 09/136).
In contrast to using the WHO 2nd IS for G-CSF, if sample A is used as a standard for comparison purposes, the variability in potency estimates of samples B and C is markedly reduced and there is excellent inter-laboratory agreement between potencies for B and C (coded duplicates of the same preparation) relative to A (Table 5). This reduction in variability when using A as a comparator is not unexpected as sample A is PEGylated in a similar fashion as samples B and C and is, therefore, highly similar to B and C in terms of its molecular species and structural entity as opposed to the parent protein, G-CSF. In this instance, no data were excluded as the potency estimates were not significantly different between laboratories.
The calibration of procedures is highly dependent on the quality and characteristics of the standard preparation used. The principle of comparing ‘like-with-like’ is well established for the bioassay of biological materials and such comparisons give better agreement as seen when sample A is used for calculating relative potencies of the different preparations B and C.
Data derived from coded duplicates, samples B and C were also highly consistent. The overall potency estimates relative to A were in very close agreement (0.99 and 1.02 respectively with a mean value of 1.01). There is also good agreement between the laboratory mean estimates of samples B and C (Table 5) for most laboratories.
Several participants (n = 18) assayed their in-house standards in the bioassays which provided an ideal opportunity to evaluate the behaviour of these in-house preparations relative to the candidate preparations. Although two laboratories used G-CSF preparations, a majority of participants (n = 16) included PEG-G-CSF preparations (manufactured in-house in many cases) as an in-house standard in their bioassays and provided brief information about these preparations (n = 15). While a major proportion of these preparations (n = 12) were representative of the candidate materials, three preparations were different in terms of the size of PEG or conjugation site. Slope ratios as shown in Fig. 4 indicate that, in general, acceptable parallelism was evident for the comparison of in-house standards and candidate standards. Levels of intra-laboratory variability were comparable to those observed for the common samples tested by all laboratories in the study.
Stability studies have shown that the potency of the preparation, coded 12/188 is not diminished after 1 week of storage at either 4 °C or 20 °C following reconstitution or after repeated freeze–thaw cycles. Results from stability studies at 7 months suggest that 12/188 is likely to be highly stable under long term storage conditions at − 20 °C. However, it is noted that because of the short duration of this study and the lack of detectable degradation of PEG-G-CSF, it is impossible to predict the degradation rate of the proposed standard. Therefore, it will be a future requirement to assess the stability of PEG-G-CSF in the residual ampoules that have remained in storage at elevated temperatures.
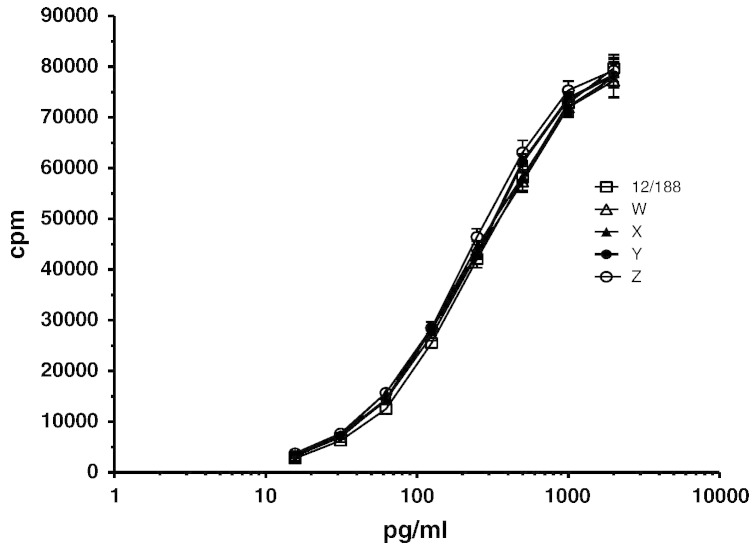
These results clearly indicate that the candidate PEG-G-CSF preparation A, coded 12/188 can be used as a reference standard for in vitro bioactivity of PEGylated G-CSF preparations (that are manufactured to be representative of the approved product, INN PEG-Filgrastim). Dose response curves of different PEG-G-CSF products relative to the PEG-G-CSF reference standard are illustrated in Fig. 5. It was proposed to the WHO ECBS that the candidate preparation (sample A, coded 12/188) be established as the WHO 1st IS for in vitro bioactivity of PEG-G-CSF with an assigned value of 10,000 IU/ampoule for biological activity. This unitage is arbitrary and unrelated to the unitage for the current WHO 2nd IS for G-CSF.
Fig. 5.
Comparison of PEG-G-CSF preparations (W–Z) relative to the IS for PEG-G-CSF (coded 12/188) using the GNFS-60 cell-line.
Of note, since 12/188 has only been evaluated for use in in vitro bioassays, it cannot be assumed to be suitable for evaluation in vivo or for pharmacokinetic studies without suitable validation.
Since both candidate preparations behaved similarly in the bioassays, 12/222 would serve as a suitable replacement standard when stock of the IS, coded 12/188 is exhausted. Taking the potency of 12/188 to be 10,000 IU/ampoule gives an estimated potency for 12/222 of 10,100 IU/ampoule.
In principle, the approach used here for defining the unitage of PEG-G-CSF standard may also be applicable to other modified proteins, however, this will need to be established on a case-by-case basis.
5. Conclusions
Based on the results of this study, the PEG-G-CSF preparation (sample A, coded 12/188) was judged suitable to serve as the WHO 1st IS for in vitro bioactivity of PEG-G-CSF products (that are representative of the approved product, INN PEG-Filgrastim). It was established by the WHO ECBS at its meeting on October 13, 2013 as the WHO 1st IS for PEG-G-CSF with an assigned value for in vitro biological activity of 10,000 IU/ampoule.
Acknowledgements
We are very grateful to the manufacturers (Sandoz, Austria; Amgen, USA; Biocon, India) for the supply of PEG-G-CSF preparations for use as candidate materials or for evaluation and to the participating laboratories for performing the laboratory tests. We are grateful to Paul Matejtschuk and Kiran Malik for assistance with pilot fills of PEG-G-CSF preparations and the staff of SPD for lyophilizing and despatching the candidate materials of the study.
References
- Buttke T.M., McCubrey J.A., Owen T.C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J. Immunol. Methods. 1993;157(1–2):233. doi: 10.1016/0022-1759(93)90092-l. [DOI] [PubMed] [Google Scholar]
- CombiStats v5.0, EDQM — Council of Europe, www.combistats.eu.
- Cox G.N., Chlipala E.A., Smith D.J., Carlson S.J., Bell S.J., Doherty D.H. Hematopoietic properties of granulocyte colony-stimulating factor/immunoglobulin (G-CSF/IgG-Fc) fusion proteins in normal and neutropenic rodents. PLoS One. 2014;9(3):e91990. doi: 10.1371/journal.pone.0091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan D.B. T-tests and intervals for comparisons suggested by the data. Biometrics. 1975;31:339. [Google Scholar]
- EMA Guidance on the description of composition of pegylated (conjugated) proteins in the SPC. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003661.pdf Available at:
- EMA Guideline on the declaration of the quantitative composition / potency labelling of biological medicinal products that contain modified proteins as active, substance. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/03/WC500163595.pdf Available at:
- Finney D.J. 3rd ed. Charles Griffin; London: 1978. Statistical Methods in Biological Assay. [Google Scholar]
- Huang Y.S., Wen X.F., Wu Y.L., Wang Y.F., Fan M., Yang Z.Y., Liu W., Zhou L.F. Engineering a pharmacologically superior form of granulocyte-colony-stimulating factor by fusion with gelatin-like-protein polymer. Eur. J. Pharm. Biopharm. 2010;74(3):435. doi: 10.1016/j.ejpb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Kinstler O.B., Brems D.N., Lauren S.L. Characterization and stability of N terminally PEGylated rhG-CSF. Pharm. Res. 1996:996. doi: 10.1023/a:1016042220817. [DOI] [PubMed] [Google Scholar]
- Lonquex EPAR (Available at 2013) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002556/WC500148382.pdf.
- Maullu C., Raimondo D., Caboi F., Giorgetti A., Sergi M., Valentini M., Tonon G., Tramontano A. Site-directed enzymatic PEGylation of the human granulocyte colony-stimulating factor. FEBS J. 2009;276(22):6741. doi: 10.1111/j.1742-4658.2009.07387.x. [DOI] [PubMed] [Google Scholar]
- Möhle R., Kanz L. Hematopoietic growth factors for hematopoietic stem cell mobilization and expansion. Semin. Hematol. 2007;44:193. doi: 10.1053/j.seminhematol.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Molineux G. The design and development of Pegfligrastim (PEG-rmetHuG-CSF, Neulasta) Curr. Pharm. Des. 2004;10:1235. doi: 10.2174/1381612043452613. [DOI] [PubMed] [Google Scholar]
- Neulasta E.P.A.R. 2004. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000420/WC500025945.pdf
- Veronese F.M., Mero A., Caboi F., Sergi M., Marongiu C., Pasut G. Site-specific pegylation of G-CSF by reversible denaturation. Bioconjug. Chem. 2007;18(6):1824. doi: 10.1021/bc070123+. [DOI] [PubMed] [Google Scholar]
- Volovat C., Gladkov O.A., Bondarenko I.M., Barash S., Buchner A., Bias P., Adar L., Avisar N. Efficacy and safety of balugrastim compared with pegfilgrastim in patients with breast cancer receiving chemotherapy. Clin. Breast Cancer. 2014;14(2):101. doi: 10.1016/j.clbc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Wadhwa M., Bird C., Hamill M., Heath A.B., Matejtschuk P., Thorpe R., Participants of the Collaborative Study The 2nd International Standard for human granulocyte colony stimulating factor. J. Immunol. Methods. 2011;367(1–2):63. doi: 10.1016/j.jim.2011.02.005. [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee on Biological Standardization . Vol. 932. 2006. Fifty-fifth report; p. 73. (Recommendations for the preparation, characterization and establishment of international and other biological reference standards: WHO Technical Report Series). [Google Scholar]
- Zhai Y., Zhao Y., Lei J., Su Z., Ma G. Enhanced circulation half-life of site-specific PEGylated rhG-CSF: optimization of PEG molecular weight. J. Biotechnol. 2009;142(3–4):259. doi: 10.1016/j.jbiotec.2009.05.012. [DOI] [PubMed] [Google Scholar]