Abstract
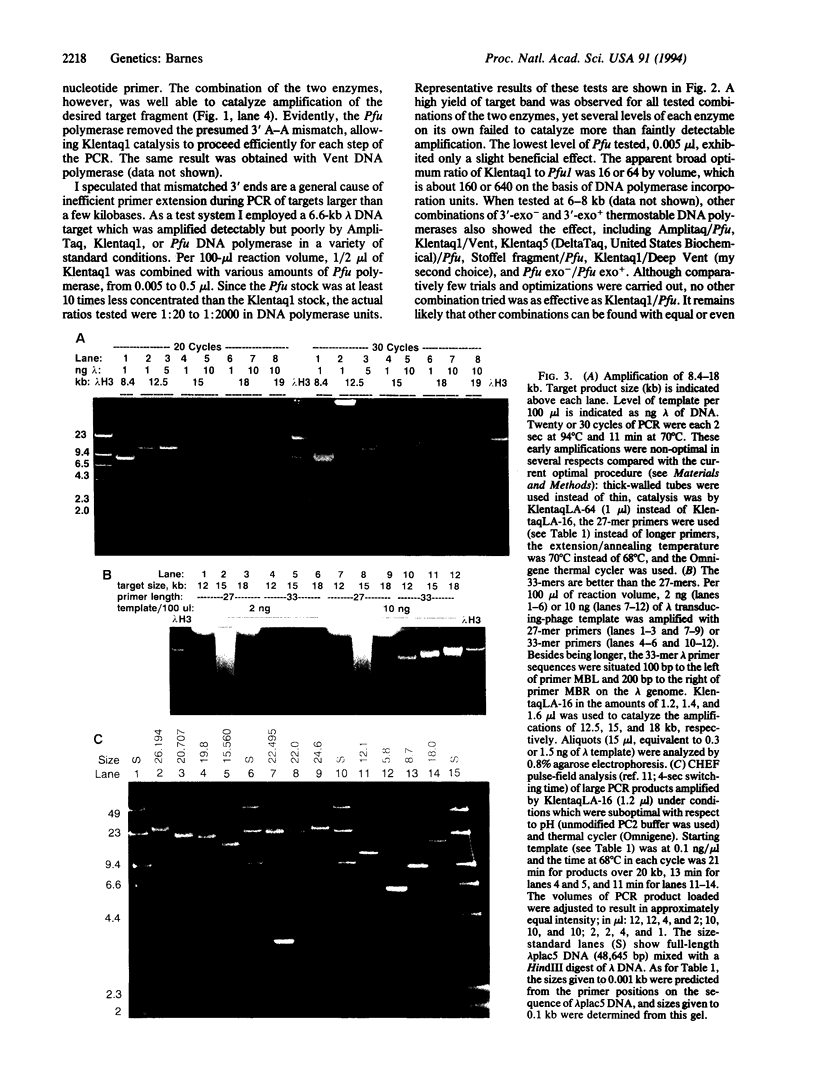
A target length limitation to PCR amplification of DNA has been identified and addressed. Concomitantly, the base-pair fidelity, the ability to use PCR products as primers, and the maximum yield of target fragment were increased. These improvements were achieved by the combination of a high level of an exonuclease-free, N-terminal deletion mutant of Taq DNA polymerase, Klentaq1, with a very low level of a thermostable DNA polymerase exhibiting a 3'-exonuclease activity (Pfu, Vent, or Deep Vent). At least 35 kb can be amplified to high yields from 1 ng of lambda DNA template.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. The fidelity of Taq polymerase catalyzing PCR is improved by an N-terminal deletion. Gene. 1992 Mar 1;112(1):29–35. doi: 10.1016/0378-1119(92)90299-5. [DOI] [PubMed] [Google Scholar]
- Brewer A. C., Marsh P. J., Patient R. K. A simplified method for in vivo footprinting using DMS. Nucleic Acids Res. 1990 Sep 25;18(18):5574–5574. doi: 10.1093/nar/18.18.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Clark J. M. Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 1988 Oct 25;16(20):9677–9686. doi: 10.1093/nar/16.20.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen K., Shapiro J. A., Beckwith J. R. Transposition of the lac region to the gal region of the Escherichia coli chromosome: isolation of lambda-lac transducing bacteriophages. J Bacteriol. 1971 Oct;108(1):5–9. doi: 10.1128/jb.108.1.5-9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainz P., Schmiedlechner A., Strack H. B. In vitro amplification of DNA fragments greater than 10 kb. Anal Biochem. 1992 Apr;202(1):46–49. doi: 10.1016/0003-2697(92)90203-j. [DOI] [PubMed] [Google Scholar]
- Krishnan B. R., Kersulyte D., Brikun I., Berg C. M., Berg D. E. Direct and crossover PCR amplification to facilitate Tn5supF-based sequencing of lambda phage clones. Nucleic Acids Res. 1991 Nov 25;19(22):6177–6182. doi: 10.1093/nar/19.22.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawyer F. C., Stoffel S., Saiki R. K., Chang S. Y., Landre P. A., Abramson R. D., Gelfand D. H. High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase and a truncated form deficient in 5' to 3' exonuclease activity. PCR Methods Appl. 1993 May;2(4):275–287. doi: 10.1101/gr.2.4.275. [DOI] [PubMed] [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993 Apr 22;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Lundberg K. S., Shoemaker D. D., Adams M. W., Short J. M., Sorge J. A., Mathur E. J. High-fidelity amplification using a thermostable DNA polymerase isolated from Pyrococcus furiosus. Gene. 1991 Dec 1;108(1):1–6. doi: 10.1016/0378-1119(91)90480-y. [DOI] [PubMed] [Google Scholar]
- Maga E. A., Richardson T. Amplification of a 9.0-kb fragment using PCR. Biotechniques. 1991 Aug;11(2):185–186. [PubMed] [Google Scholar]
- Ohler L. D., Rose E. A. Optimization of long-distance PCR using a transposon-based model system. PCR Methods Appl. 1992 Aug;2(1):51–59. doi: 10.1101/gr.2.1.51. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Spencer W. J., Rhoads R. E. Optimization of the annealing temperature for DNA amplification in vitro. Nucleic Acids Res. 1990 Nov 11;18(21):6409–6412. doi: 10.1093/nar/18.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shpakovski G. V., Akhrem A. A., Berlin YuA Structural bases of a long-stretched deletion: completing the lambda plac5 DNA primary structure. Nucleic Acids Res. 1988 Nov 11;16(21):10199–10212. doi: 10.1093/nar/16.21.10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailor R., Tippett J., Gibb G., Pells S., Pike D., Jordan L., Ely S. Identification and characterization of a novel Bacillus thuringiensis delta-endotoxin entomocidal to coleopteran and lepidopteran larvae. Mol Microbiol. 1992 May;6(9):1211–1217. doi: 10.1111/j.1365-2958.1992.tb01560.x. [DOI] [PubMed] [Google Scholar]