Abstract
Because of the limitations of current surgical methods in the treatment of degenerative disc disease, tissue-engineered intervertebral discs (TE-IVDs) have become an important target. This study investigated the biochemical and mechanical responses of composite TE-IVDs to dynamic unconfined compression. TE-IVDs were manufactured by floating an injection molded alginate nucleus pulposus (NP) in a type I collagen annulus fibrosus (AF) that was allowed to contract for 2 weeks before loading. The discs were mechanically stimulated at a range of strain amplitude (1–10%) for 2 weeks with a duty cycle of 1 h on–1 h off–1 h on before being evaluated for their biochemical and mechanical properties. Mechanical loading increased all properties in a dose-dependent manner. Glycosaminoglycans (GAGs) increased between 2.8 and 2.2 fold in the AF and NP regions, respectively, whereas the hydroxyproline content increased between 1.2 and 1.8 fold. The discs also experienced a 2-fold increase in the equilibrium modulus and a 4.3-fold increase in the instantaneous modulus. Full effects for all properties were seen by 5% strain amplitude. These data suggest that dynamic loading increases the functionality of our TE-IVDs with region-dependent responses using a method that may be scaled up to larger disc models to expedite maturation for implantation.
Introduction
Ninety billion dollars are spent annually treating lower back pain, the primary cause of which is intervertebral disc (IVD) degeneration.1 Degenerative disc disease has been linked with aging and is thought to be caused by a number of factors, including high mechanical loads, enzyme activity, smoking, and changes in cell health, because of decreasing diffusion of nutrients through the endplates.1 The native IVD is composed of two regions with different structures and mechanical properties. The central portion of the disc is the nucleus pulposus (NP), which is rich in glycosaminoglycans (GAGs) and has a jelly-like consistency. Surrounding the NP is the annulus fibrosus (AF), composed mainly of type I collagen and highly organized.2 During degeneration, decreases in proteoglycans, particularly in the inner NP, cause loss of hydration and inhibit the ability of the IVD to pressurize and bear loads. In the IVD, there are several failure modes, including bulging and herniated discs and loss of disc height and water content, all of which lead to destabilization of the segment.3 Current treatment options are limited, favoring either physical therapy and pain management or invasive surgical methods. Fusion of the segment, in particular, decreases the patient's range of motion and may cause further degradation of the adjacent discs.4 For this reason, biological approaches to IVD repair or regeneration have become of increasing interest.5–8
Several studies have investigated the generation of tissue-engineered composites for IVD replacement.5–10 Specifically, our laboratory has previously developed a tissue-engineered intervertebral disc (TE-IVD) made from primary ovine NP and AF cells suspended in alginate and type I collagen, respectively.11 These discs, when implanted in the caudal spines of rats, are viable for up to 8 months, integrating effectively into the spine and achieving mechanical and biochemical properties similar to native discs.12 Whereas these results are encouraging, the mechanical properties of our TE-IVDs are still well below the native IVD before implantation. Increasing the mechanical and biochemical properties before implantation will be critical when moving to larger animal models.
It is well known that dynamic mechanical stimulation of cartilaginous explants and tissue-engineered constructs can increase their biochemical and mechanical properties.13–20 This may be caused by direct cell deformations,21 but significant increases are due to the transport of growth factors and other nutrients through the scaffold materials.14,22,23 In the native IVD, mechanical loading is important for maintaining the correct balance of biochemical signals that maintain extracellular matrix (ECM) composition and function.24 Responses to dynamic compression in vivo and in organ culture have been shown to depend on a number of factors, including the duration of loading,25,26 the frequency of the cycles,27–30 and the magnitude of compression.27,29,31,32 Intermittent and short (∼2 h) sessions of dynamic compression have been shown previously to increase the mechanical and biochemical properties more than longer periods of stimulation, which may actually trigger catabolic pathways.33–36
Mechanical loading offers many advantages over other forms of stimulation such as growth factors. These include the ability to target specific regions of the complex tissues and the longer lasting effects on ECM component production. Different regions of complex tissues such as the IVD may experience different local loading regimens under simple dynamic compression with higher hydrostatic pressure in the center portion of the disc and higher fluid velocities in the outer portion.37,38 Additionally, growth factor treatment works over a narrow window of time and properties do not continue to improve once stimulation has been removed. In one study, up to 4 weeks after mechanical stimulation has ceased, improvements in the ECM content and construct stiffness were seen compared to static culture.39
A few previous studies have investigated the response of IVD cells in tissue-engineered scaffolds under either dynamic compression18,40 or hydrostatic pressure.41 No studies, however, looked at the effect of dynamic unconfined compression on both cell types in a composite implant. As such, our objective was to characterize the effect of dynamic compressive loading in the composition and mechanical properties of TE-IVD implants. We hypothesize that dynamic unconfined compression will increase the biochemical and mechanical properties of TE-IVD implants in a dose-dependent manner.
Materials and Methods
Cell isolation and culture
Cell isolation and culture techniques were based on previously reported protocols.42 The lumbar spines of three skeletally mature Finn/Dorset cross male sheep (∼14 months old) (Cornell University Sheep Program) were harvested for each group (15 total animals). Individual IVDs were removed from the spines and the AF and NP regions were separated. The tissues were digested in 125 mL of 0.3% wt/vol collagenase type II (Cappel Worthington Biochemicals, Malvern, PA) for 6 h at 37°C. The cell solutions were resuspended in Ham's 12 media (MediaTech, Manassas, VA), 10% fetal bovine serum (Gemini Bio Products, Sacramento, CA), 1% antibiotic–antimycotic solution (100 μg/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin B) (MediaTech), and ascorbic acid (25 μg/mL) (Sigma-Aldrich, St. Louis, MO). The two different cell types were cultured separately for 2 weeks at 37°C, 5% CO2, with media changed every 3 days. The cells maintained their cell morphologies throughout culture. The AF cells were elongated as fibrochondrocytes, and the NP cells were smaller and rounded as chondrocytes. Cells were removed from the flasks using 0.05% trypsin (MediaTech) and counted before being seeded in the TE-IVDs.
Engineered IVD construction
Alginate (3% wt/vol) seeded with ovine NP cells (25×106 cells/mL)5,9,11,12,42 was injected into a predesigned mold.11 The individual TE-NPs were cut from the mold and one each was placed in the center of a well of a sterile 24-well plate. A 2 mg/mL collagen gel solution43 was seeded with ovine AF cells (1×106 cells/mL)11,12,42 and 410 μL was pipetted around each TE-NP. The collagen was allowed to gel at 37°C for 45 min before 1 mL of media was added to each well. The collagen in the TE-IVDs was allowed to contract for 2 weeks, whereas media were changed every 3–4 days before being crosslinked further with riboflavin. One milliliter of 0.25 mM riboflavin (Sigma-Aldrich) was added to each well of the 24-well plate and incubated at 37°C for 2 h before being exposed to 458 nm light for 40 s. Media were changed 24 h after the riboflavin crosslinking.
Stimulation using dynamic compression
The TE-IVDs were loaded into a custom bioreactor. The loading platen was shaped to fit over a standard 24-well plate and to apply controlled displacement profiles to the constructs with the assumption that all TE-IVDs were 1 mm in height (Fig. 1). The bioreactor is controlled through a custom LabVIEW program into which the magnitude, duration, and frequency of dynamic strain can be entered. The constructs were compressively loaded under sinusoidal displacement control at 1 Hz with a 10, 25, 50, or 100 μm offset and 10, 25, 50, or 100 μm amplitude representing a 1%, 2.5%, 5%, or 10% displacement at 37°C, 5% CO2. Simulation occurred Monday, Wednesday, and Friday for 2 weeks with a duty cycle of 1 h on, 1 h off, and 1 h on each day of loading for a total of 6 h of stimulation per week. This specific duty cycle was chosen based on previous dynamic loading studies. While the TE-IVDs were not in the bioreactor, they were cultured under free-swell conditions. Free-swell TE-IVDs were cultured for 2 weeks without stimulation as a control.
FIG. 1.
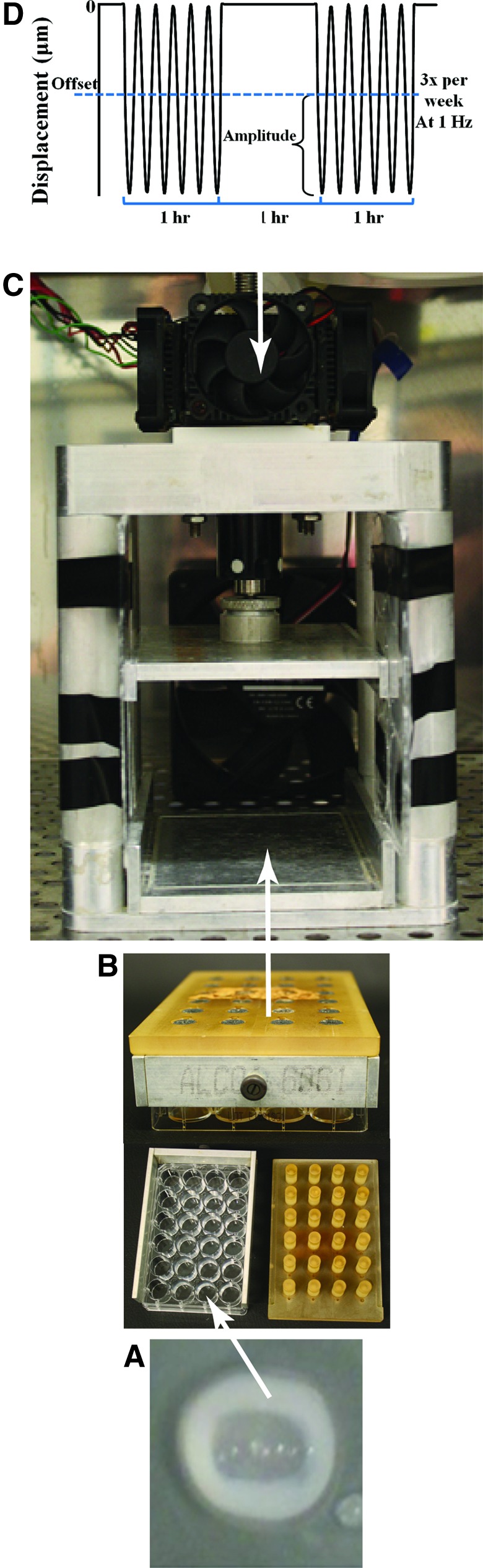
(A) Tissue-engineered intervertebral discs (TE-IVDs) are cultured in 24-well plates. (B) The loading platen fits over the plate before being loaded into a (C) custom bioreactor that applies a predefined displacement profile. (D) The loading duty cycle and waveform. Color images available online at www.liebertpub.com/tea
Construct analysis
Twelve discs were analyzed from each strain group for a total of 60 discs. Five discs from each group were taken for biochemical analysis, five were taken for mechanical testing, and two were fixed for histological evaluation. Photographs were taken each day of the TE-IVDs before loading to track contraction of the constructs. The images were uploaded to ImageJ (National Institutes of Health, Bethesda, MD), where the total disc area was calculated. The average percent of original area after the initial 2-week contraction period was determined for each group over time. Confocal images of the collagenous AF region were taken using a Zeiss 710 microscope with a LCI Plan-Apochromat 25×/0.8 water immersion objective (Carl Zeiss MicroImaging, Jena, Germany) to determine any differences in the collagen structure between the groups, using previously described methods.44 The TE-IVDs were fixed in 10% phosphate-buffered formalin for 2 weeks at room temperature. They were then removed to 70% ethanol for another 2 weeks before they were sectioned. Transverse, histological 5-μm-thick sections were taken of the TE-IVDs. The sections were stained with Picrosirius red for collagen and Alcian blue for proteoglycans.
Biochemical analysis was performed to determine the DNA, GAG, and collagen content in each of the distinct regions of the TE-IVDs (AF and NP). Before analysis, the TE-NP was separated from the TE-AF. Both the TE-AF and the TE-NP were digested using a papain digest (Sigma-Aldrich) with the alginate lyase buffer solution (Sigma-Aldrich) added to the alginate TE-NPs. The GAG content was measured using a spectrophotometric dimethylmethylene blue (DMMB) dye with absorbance measured at 525 nm at pH 1.5.45 The total collagen content was measured using a hydroxyproline assay46 with absorbance measured at 540 nm. All data were normalized to dry weight.
The effective composite mechanical properties of the TE-IVDs were determined as described previously.12 Individual samples were placed in a small cup, which was mounted in an ELF 3200 mechanical testing frame (EnduraTech, Mount Airy, NC) with a small impermeable platen mounted above. Each construct was surrounded in a protease inhibitor (Roche Diagnostics, Indianapolis, IN) to prevent degradation of the disc during testing. Unconfined stress relaxation tests were performed with steps of 5% strain to a total of 70% strain. Instantaneous modulus, equilibrium modulus, and effective hydraulic permeability were calculated using the resulting stresses.12
Statistics
Data analysis was performed using MATLAB and the Microsoft Excel software. Based on the idea that the effects of stimulation may be related to the activity of nutrients or growth factors, data were fit to a four-parameter sigmoidal dose–response model.47 Data are shown as the mean±standard deviation. All data were analyzed in SigmaPlot 11.0 using one-way analysis of variance (ANOVA) and using a Tukey t-test for post hoc analysis to test for pairwise differences among different levels of mechanical stimulation. A p-value of <0.05 was considered statistically significant.
Results
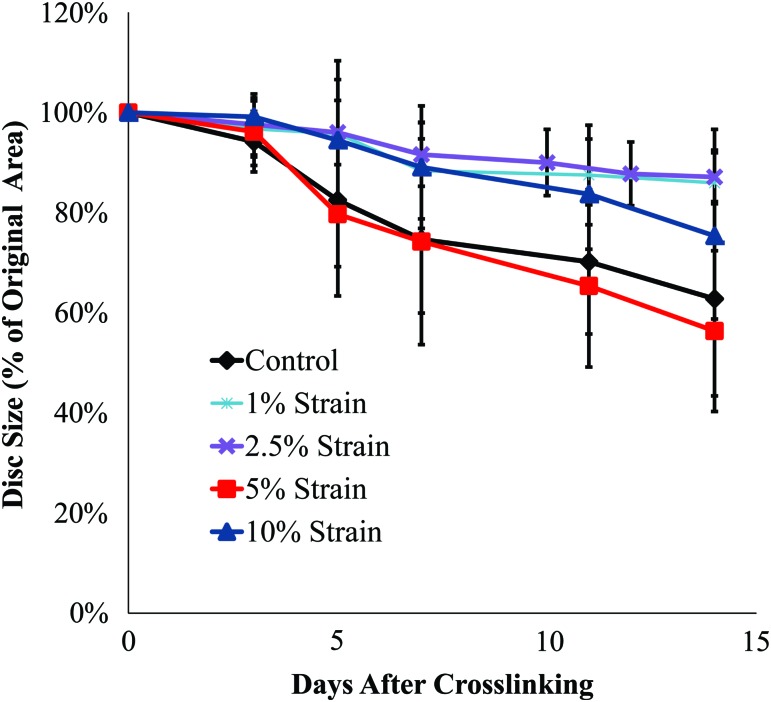
Over the 2-week loading period, the discs contracted to between 56% and 87% of their original area, although there were no significant differences in contraction between the various loading groups (Fig. 2). After the initial 2 weeks of contraction, the TE-IVDs were crosslinked using riboflavin. This crosslinking served to prevent further contraction of the collagen TE-AF during loading.
FIG. 2.
Contraction of the TE-IVDs over the 2-week loading period; values are the percent of the original area±standard deviation (SD). Color images available online at www.liebertpub.com/tea
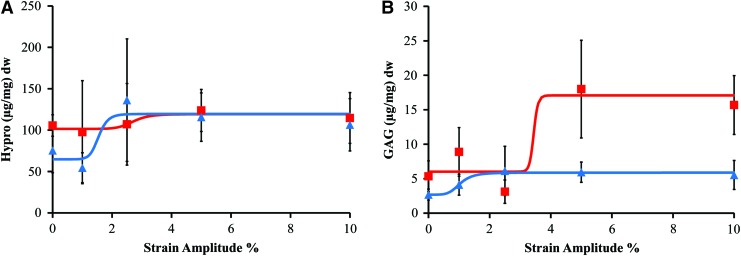
After 2 weeks of dynamic unconfined compression stimulation, the hydroxyproline content increased in a dose-dependent manner, with the increase in collagen content in both regions fitting well to a four-parameter sigmoidal dose–response curve (R2 values between 0.81 and 0.83) (Table 1). The total collagen content of the TE-AF region was relatively unaffected by the strain amplitude with only a 1.2-fold increase between the control and 10% strain (Fig. 3A). The EC50 (i.e., the strain amplitude that produced a half-maximal response) was calculated to be 2.7% with the full effect seen at 5%. In contrast, the collagen content in the TE-NP region increased 1.8 fold. The EC50 in the TE-NP was 1.5% strain with the full effect seen at 2.5% strain, half that of the TE-AF region. The GAG content in both the TE-AF and TE-NP regions exhibited more sensitivity to strain amplitude than collagen. Again, production in both regions was well represented by dose–response curves with R2 values between 0.88 and 0.98. A 2.2-fold increase in GAGs was seen in the TE-NP with as little as 2.5% strain amplitude (Fig. 3B). The EC50 of GAGs in the TE-NP region was low, at only 1% strain. The TE-AF responded at higher strain amplitudes with as much as a 2.8-fold increase (p<0.001 by one-way ANOVA). The EC50 of GAGs in the TE-AF region was much higher at 3.4% strain. The full biochemical effect of mechanical stimulation was seen at 5% strain with no changes between the 5% and 10% strain groups.
Table 1.
Biochemical Properties' Dose–Response Curve Fit Values, Including EC50, Fold Increase, and R2 Values
| EC50 (%) | Fold increase | R2 | |
|---|---|---|---|
| AF: Hypro | 2.7 | 1.2 | 0.81 |
| NP: Hypro | 1.5 | 1.8 | 0.83 |
| AF: GAG | 3.4 | 2.8 | 0.88 |
| NP: GAG | 1.0 | 2.2 | 0.98 |
AF, annulus fibrosus; GAG, glycosaminoglycan; NP, nucleus pulposus.
FIG. 3.
(A) Hydroxyproline content dry weight (dw)±SD (n=5) (squares represent annulus fibrosus [AF], triangles nucleus pulposus [NP]) versus the strain amplitude percent and (B) glycosaminoglycan (GAG) content dw±standard deviation (SD) (n=5) versus the average dynamic strain amplitude percent. Color images available online at www.liebertpub.com/tea
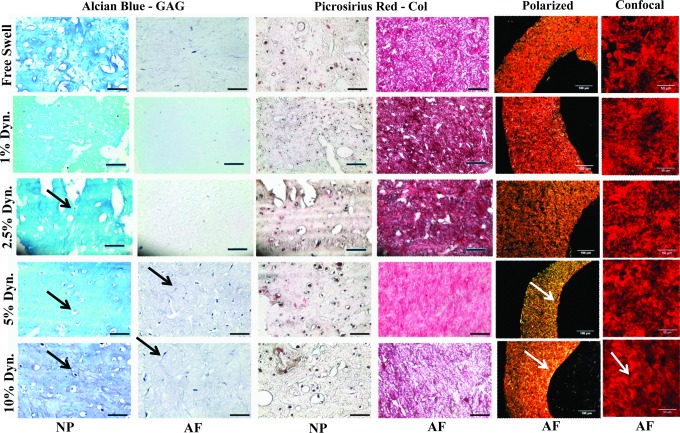
Histologically, the NP region of all the groups stained intensely for GAGs, whereas the AF region stained intensely for collagen. The morphology of the cells was similar to that of native cells with cells in the NP region maintaining a rounded chondrocyte-like shape (Fig. 4) and cells in the AF region displaying an elongated fibroblast-like profile. Polarized light images were taken of the Picrosirius red-stained slides to determine the extent of collagen organization. Minimal collagen alignment was seen in the control, and 1% and 2.5% strain groups, whereas the same alignment was seen in the 5% and 10% strain groups, as indicated by increased brightness along the interior rim of the AF region. Confocal images of the AF region show the same patterns with a low-level alignment seen in the 10% strain group.
FIG. 4.
Histological staining of the TE-IVD regions, Alcian blue stained for GAG, while Picrosirius red stained for collagen. Black arrows show larger cells in lacunae. Polarized light and confocal images of the AF region show collagen organization. White arrows indicate circumferential organization. Color images available online at www.liebertpub.com/tea
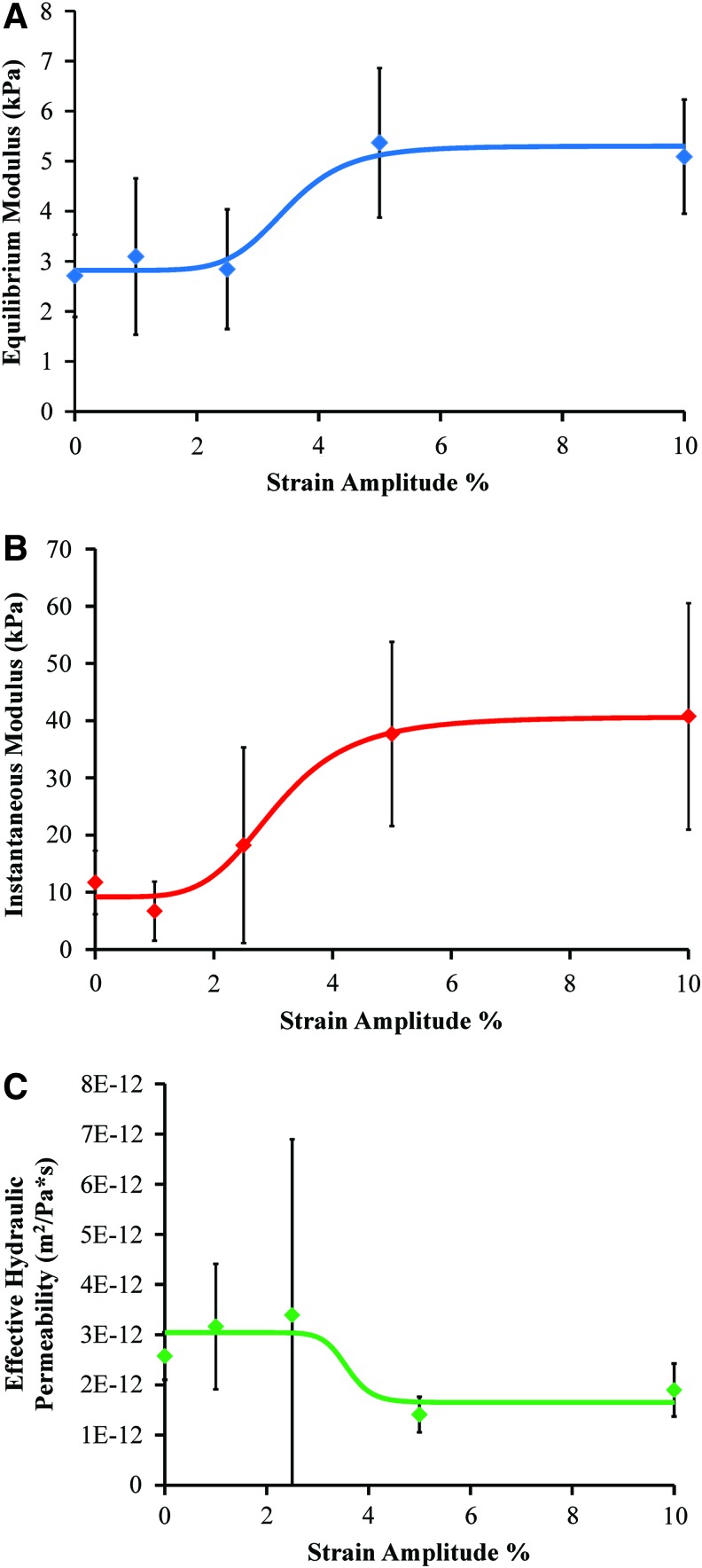
Dynamic stimulation improved the mechanical properties of the TE-IVDs in a dose-dependent manner with increases in the equilibrium and instantaneous moduli and a decrease in the effective hydraulic permeability. All data were fit to four-parameter sigmoidal dose–response curves with R2 values between 0.83 and 0.98 (Table 2). The mean equilibrium modulus of the discs increased twofold with 5% (p<0.05 by one-way ANOVA) and 10% (p=0.06 by one-way ANOVA) strain (Fig. 5A). The EC50 was calculated to be 3.7% strain and full effects seen at 5% strain. A more dramatic increase in the instantaneous modulus was seen with a 4.3-fold increase between the control and 10% (p<0.05 by one-way ANOVA) strain group (Fig. 5B). The EC50 of the dose–response curve was 2.3% strain with full effects in the 5% strain group. Finally, the effective hydraulic permeability of the discs was determined from the equilibrium modulus, diameter, and relaxation time constant of each disc. A decrease in the hydraulic permeability indicates more resistance to fluid flow out of the disc, which is important for NP pressurization. Increases in strain amplitude caused a 1.8-fold decrease in hydraulic permeability (Fig. 5C). The EC50 was calculated to be 3.7% strain with full effects seen again at 5% strain amplitude.
Table 2.
Mechanical Properties' Dose–Response Curve Fit Values, Including EC50, Fold Increase, and R2 Values
| EC50 (%) | Fold increase | R2 | |
|---|---|---|---|
| Equilibrium modulus | 3.7 | 2.0 | 0.93 |
| Instantaneous modulus | 2.3 | 4.3 | 0.98 |
| Effective hydraulic permeability | 3.6 | −1.8 | 0.83 |
FIG. 5.
(A) Equilibrium modulus±SD (n=5) versus the average dynamic strain amplitude percent, (B) instantaneous modulus±SD (n=5), and (C) effective hydraulic permeability±SD (n=5). ⋄ indicate average values at each strain amplitude %. Color images available online at www.liebertpub.com/tea
Discussion
This study investigated the hypothesis that increases in applied strain amplitude under dynamic unconfined compression to composite TE-IVDs will enhance their biochemical and mechanical properties of composite TE-IVDs in a dose-dependent manner. We found that increasing the dynamic strain from 0% to 10% increased all properties with responses that fit well to a four-parameter sigmoidal dose–response curve. Although EC50 values varied, maximum effects were consistently seen at 5% strain. Furthermore, regional differences in response were seen between the alginate TE-NP and the collagenous TE-AF. Whereas the TE-AF saw only a minimal increase in collagen content, the TE-NP was more sensitive to dynamic strain amplitude. Both regions saw significant increases in the GAG content with the TE-NP being slightly less sensitive than the TE-AF. The mechanical properties of the whole TE-IVDs improved with percent dynamic strain with both the equilibrium and instantaneous moduli increasing significantly. The hydraulic permeability decreased, indicating more pressurization and less fluid flow out of the discs. Histology also showed differences in regional response with the cells maintaining their native cell shape. The AF region also showed the beginning of collagen matrix alignment at higher strain amplitudes.
This is the first study that examined the response of both AF and NP cells to dynamic unconfined compression together and in a tissue-engineered environment that mimics the native disc structure. The IVD cells were separated into their respective regions and were stimulated simultaneously, but experienced different loading locally. Mechanical stimulation has been used for many years to improve the properties and function of cartilage explants14,37,38,48 and tissue-engineered scaffolds15,19,49 seeded with chondrocytes. Even small magnitudes of dynamic unconfined compression have consistently been shown to stimulate biosynthesis of GAGs and collagen. Sah et al. showed that higher frequency oscillatory compression amplitudes between 1% and 5% increased biochemical contents by 20–40%.48 Increases in oscillatory strain increased incorporation of 35S-sulfate (GAGs) and 3H-proline (collagen) in a similar manner to our study. These increases may be related to enhanced nutrient transport through the scaffolds during culture.14,22,50 Although the diffusion of smaller solutes does not seem to increase with cyclic compression,22 diffusion of larger solutes such as growth factors is increased.14 Convective transport in cartilage and tissue-engineered scaffolds has been modeled under dynamic compression, showing improved diffusion of larger solutes.23 The high degree of correlation of a classic dose–response model to the level of strain in our current study thus suggests that biochemical factors may play a key role in this stimulation.
It has been suggested that at smaller compression magnitudes (1–10%), hydrostatic pressure and fluid flow play an important role in the observed responses.48 This may partially account for the different regional responses in biochemical properties especially the increases in GAGs seen in the TE-AF region (Fig. 3B). It has been shown that porous tissues in unconfined compression experience higher hydrostatic pressures at the center of the disc, while fluid velocity increases on the outer edges of the disc.37,38 These studies also found greater proteoglycan synthesis on the outer edge of cartilage explants.
Although direct measurements of hydrostatic pressure were not done in this study, the theoretical maximum hydrostatic pressure is the difference between the equilibrium and instantaneous moduli. At 10% dynamic compression, the theoretical maximum hydrostatic pressure was calculated to be 36 kPa. Based on previous work identifying thresholds of stimulation by hydrostatic pressure of between 100 and 300 kPa,51,52 the metabolic influence is likely low compared to fluid flow. In our TE-IVDs, the inner TE-NP is constrained on all sides by either the platen and well plate or the surrounding TE-AF and, therefore, would be expected to experience a higher hydrostatic pressure than the TE-AF. In the same way, the TE-AF is not constrained on its outer rim, leading to a higher fluid flow during loading than in the TE-NP. This loading mechanism more closely mimics loading in the native disc than previous schemes, suggesting that differences in cell type and loading contribute to the divergent biochemical responses of the two regions, including the large increases in the GAG content seen in the outer TE-AF region of the TE-IVDs.
Another contributing factor may be the collagen in which the AF cells are seeded. Furthermore, greater GAG retention was seen in tissue-engineered menisci made with high-density collagen gels as opposed to those made with alginate.44 Similar increases in biosynthesis were seen in tissue-engineered cartilage with mechanical property increases as well.15,49 Mauck et al. demonstrated that 20% dynamic unconfined compression significantly increased the mechanical properties of agarose or alginate gels seeded with chondrocytes over a period of 4 weeks.19 All these studies show that dynamic unconfined compression enhances the biochemical and mechanical properties of tissue-engineered connective tissue.
While many studies have investigated the effects of mechanical stimulation on whole IVDs either in vitro or in vivo, these studies draw few conclusions about what schemes might maintain or increase the biochemical or mechanical properties although it is agreed that there are regional differences in response.25,27,29,31,32 Fewer studies have looked at the response of IVD cells in tissue-engineered scaffolds to dynamic compression. Salvatierra et al. found that IVD cells seeded in agarose and subjected to 10% compressive loading had increased metabolism, including increases in ATP release in both regions.18 Increasing the metabolism of the cells may increase the production of ECM components such as proteoglycans and collagen. Another study found that the age of the cell donor and the frequency of loading had an influence on the response of NP and AF cells.30 Mature cells respond to dynamic loading with increases in Col II and Col I expression, whereas young cells exhibited decreases in expression. Osmolarity of the ECM may also influence on how IVD cells respond to mechanical stimulation. Under iso-osmotic conditions, AF cells displayed no changes in Col I or Col II expression, whereas NP cells increase the expression of both Col I and Col II at 4% cyclic strain.53 Preliminary stimulation data indicate that both AF and NP cells seeded in agarose produce more proteoglycans and collagen when exposed to 10% dynamic unconfined compression.54 These studies support both the data presented here and the conclusions drawn when stimulating chondrocytes. No study has looked at the response of cells across multiple magnitudes of compression or of the two different cell types stimulated together in a scheme that mimics the anatomy of the native disc.
Although the properties of our TE-IVDs are still far below those of the native IVD,1,12 achieving native values before implantation may not be necessary. Our previous in vivo studies showed that implanted discs that started with low mechanical and biochemical property values achieved properties similar to native tissues11,12 after 8 months. Nevertheless, increasing the mechanical and biochemical properties before implantation will be critical when moving to larger animal models. For example, the caudal disc segments of beagles experience peak stresses two to three orders of magnitude greater than those of rats.55,56 To develop discs that are sufficiently robust to perform in larger animal models, conditioning using dynamic compression may be necessary. Currently, the two regions (AF and NP) cannot be separated while maintaining mechanical integrity. As such, we reported the effective mechanical properties for our composite construct rather than the mechanical properties of the two materials. Although we speculate on the influence of hydrostatic pressure and fluid flow on TE-IVD development during loading, we have not yet measured these conditions directly. Computational modeling of the compression of these composite implants may enable both the estimation of material properties of the AF and NP as well as predictions of spatial profiles of stresses, strain, pressure, and flow.
This study presents dynamic unconfined compression as a method for increasing both the biochemical and mechanical properties of TE-IVDs seeded with primary ovine IVD cells. Furthermore, we demonstrated that these responses are dose dependent with increases in percent strain causing corresponding increases in GAG and collagen accumulation and the equilibrium and instantaneous moduli and decreases in the hydraulic permeability. All these data suggest that dynamic loading increases the functionality of our TE-IVDs using a method that may be scaled up to larger disc models, including human discs, to expedite maturation for implantation. Future work may focus on determining the effects of duty cycle and frequency on the development of our TE-IVD.
Acknowledgments
The authors would like to thank the following funding sources: HHMI Med into Grad Fellowship (HHMI 56006761), NIH (T35 EB006732), AO Foundation (F-08-10B), AO Spine (104654-HJWA2010), and NSF Graduate Research Fellowship.
Disclosure Statement
No competing financial interests exist.
References
- 1.Whatley B.R., and Wen X.Intervertebral disc (IVD): structure, degeneration, repair and regeneration. Mater Sci Eng C 32,61, 2012 [Google Scholar]
- 2.Antoniou J., Steffen T., Nelson F., Winterbottom N., Hollander A.P., Poole R.A., et al. The human lumbar intervertebral disc. J Clin Invest 98,996, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raj P.P.Intervertebral disc : anatomy-physiology-pathophysiology-treatment. Pain Pract 8,18, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Cowan J.A., Dimick J.B., Wainess R., Upchurch G.R., Chandler W.F., and Marca F.La. Changes in utilization of spinal fusion in the United States. Neurosurgery 58,15, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mizuno H., Roy A.K., Zaporojan V., Vacanti C.A., Ueda M., and Bonassar LJ. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials 27,362, 2006; [DOI] [PubMed] [Google Scholar]
- 6.Nesti L.J., Li W.-J., Shanti R.M., Jiang Y.J., Jackson W., Freedman B.A., et al. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng Part A 14,1527, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S.-H, Gil E.S., Cho H., Mandal B., Tien L.W., Min B.-H., et al. Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng Part A 18,447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nerurkar N.L., Sen S., Huang A.H., Elliott D.M., and Mauck R.L.Engineered disc-like angle-ply structures for intervertebral disc replacement. Spine (Phila Pa 1976) 35,867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizuno H., Roy A.K., Vacanti C A., Kojima K., Ueda M., and Bonassar LJ.Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine (Phila Pa 1976) 29,1290, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Nerurkar N.L., Baker B.M., Sen S., Wible E.E., Elliott D.M., and Mauck R.L.Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater 8,986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowles R.D., Gebhard H.H., Dyke J.P., Ballon D.J., Tomasino A., Cunningham M.E., et al. Image-based tissue engineering of a total intervertebral disc implant for restoration of function to the rat lumbar spine. NMR Biomed 25,443, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Bowles R.D., Gebhard H.H., Härtl R., and Bonassar LJ.Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc Natl Acad Sci U S A 108,13106, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter C.J., Imler S.M., Malaviya P., Nerem R.M., and Levenston M.E.Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials 23,1249, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Bonassar L.J., Grodzinsky A.J., Frank E.H., Davila S.G., Bhaktav N.R., and Trippel S.B.The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. Orthop Res 19,11, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Buschmann M.D., Gluzband Y.A., Grodzinsky. A.J., and Hunziker E.B.Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci 108,1497, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Waldman S.D., Couto D.C., Grynpas M.D., Pilliar R.M., and Kandel R.A.A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis Cartilage 14,323, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Davisson T., Kunig S., Chen A., Sah R., and Ratcliffe A.Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res 20,842, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Salvatierra J.C., Yuan T.Y., Fernando H., Castillo A., Gu W.Y., Cheung H.S., et al. Difference in energy metabolism of annulus fibrosus and nucleus pulposus cells of the intervertebral disc. Cell Mol Bioeng 4,302, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauck R.L., Soltz M.A., Wang C.C.B., Wong D.D., Chao P.-H.G., and Ateshian G.A.Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomed Eng 122,252, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kisiday J.D., Jin M., DiMicco M.A., Kurz B., and Grodzinsky A.J.Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech 37,595, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Huang H., Kamm R.D., and Lee R.T. Cell mechanics and mechanotransduction: pathways, probes, and physiology Am J Physiol 287,C1, 2004 [DOI] [PubMed] [Google Scholar]
- 22.O'Hara B.P., Urban J.P.G., and Maroudas A. Influence of cyclic loading on nutrition of articular cartilage Ann Rheum Dis 49,536, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauck R.L., Hung C.T., and Ateshian G.A.Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng 125,602, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan S.C.W., Ferguson S.J., and Gantenbein-Ritter B.The effects of dynamic loading on the intervertebral disc. Eur Spine J 20,1796, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuertz K., Godburn K., MacLean J.J., Barbir A., Donnelly J.S., Roughley P.J., et al. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res 27,1235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLean J.J., Lee C.R., Alini M., and Iatridis J.C.The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthpaedic Res 23,1120, 2005 [DOI] [PubMed] [Google Scholar]
- 27.MacLean J.J., Lee C.R., Alini M., and Iatridis J.C.Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res 22,1193, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Illien-Jünger S., Gantenbein-Ritter B., Grad S., Lezuo P., Ing D., Ferguson S.J., et al. The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine (Phila Pa 1976) 35,1744, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Walsh A.J.L., and Lotz J.C.Biological response of the intervertebral disc to dynamic loading. J Biomech 37,329, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Korecki C.L., Kuo C.K., Tuan R.S., and Iatridis J.C.Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res 27,800, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuoka K., Michalek A.J., MacLean J.J., Stokes I.A.F., and Iatridis J.C.Different effects of static versus cyclic compressive loading on rat intervertebral disc height and water loss in vitro. Spine (Phila Pa 1976) 32,1974, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korecki C.L., MacLean J.J., and Iatridis J.C.Dynamic compression effects on intervertebral disc mechanics and biology. Spine (Phila Pa 1976) 33,1403, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kisiday J.D., Lee J.H., Siparsky P.N., Frisbie D.D., Flannery C.R., Sandy J.D., et al. Catabolic responses of chondrocyte-seeded peptide hydrogel to dynamic compression. Ann Biomed Eng 37,1368, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Kock L.M., Schulz R.M., van Donkelaar C.C., Thümmler C.B., Bader A., and Ito K.RGD-dependent integrins are mechanotransducers in dynamically compressed tissue-engineered cartilage constructs. J Biomech 42,2177, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Villanueva I., Hauschulz D.S., Mejic D., and Bryant S.J.Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis Cartilage 16,909, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauck R.L., Nicoll S.B., Seyhan S.L., Ateshian G.A., and Hung C.T.Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng 9,597, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Kim Y.-J., Bonassar L.J., Grodzinsky A.J., and Bonassar J.The role of cartilage streaming potential, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J Biomech 28,1055, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Kim Y.-J., Sah R.L., Grodzinsky A.J., Plaas A.H., and Sandy J.D.Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys 311,1, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Puetzer J.L., Ballyns J.J., and Bonassar L.J.The effect of the duration of mechanical stimulation and post-stimulation culture on the structure and properties of dynamically compressed tissue-engineered menisci. Tissue Eng Part A 18,1365, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Wang P., Yang L., and Hsieh A.H.Nucleus pulposus cell response to confined and unconfined compression implicates mechanoregulation by fluid shear stress. Ann Biomed Eng 39,1101, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Reza A.T., and Nicoll S.B.Hydrostatic pressure differentially regulates outer and inner annulus fibrosus cell matrix production in 3D scaffolds. Ann Biomed Eng 36,204, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Bowles R.D., Williams R.M., Zipfel W.R., and Bonassar L.J.Self-assembly of aligned tissue-engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng Part A 16,1339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elsdale T., and Bard J.Collagen substrata for studies on cell behavior. J Cell Biol 54,626, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puetzer J.L., and Bonassar L.J.High density type I collagen gels for tissue engineering of whole menisci. Acta Biomater 9,7787, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Enobakhare B.O., Bader D.L., and Lee D.A.Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem 243,189, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Neuman R.E., and Logan M.A.The determination of hydroxyproline. J Biol Chem 184,299, 1949 [PubMed] [Google Scholar]
- 47.Okajima T., Nakamura K., Zhang H., Ling N., Tanabe T., Yasuda T., et al. Sensitive colorimetric bioassays for insulin-like growth factor (IGF) stimulation of cell proliferation and glucose consumption: use in studies of IGF analogs. Endocrinology 130,2201, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Sah R.L., Kim Y.J., Doong J.Y., Grodzinsky A.J, Plaas A.H, and Sandy J.D.Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 7,619, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Tran S.C., Cooley A.J., and Elder S.H.Effect of a mechanical stimulation bioreactor on tissue engineered, scaffold-free cartilage. Biotechnol Bioeng 108,1421, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Kelly T.-A.N., Ng K.W., Wang C.C.-B., Ateshian G.A., and Hung C.T.Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech 39,1489, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Handa T., Ishihara H., Ohshima H., Osada R., Tsuji H., and Obata K.Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine (Phila Pa 1976) 22,1085, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Liu G.Z., Ishihara H., Osada R., Kimura T., and Tsuji H.Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine (Phila Pa 1976) 26,134, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Wuertz K., Urban J.P.G., Klasen J., Ignatius A., Wilke H., Claes L., et al. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res 25,1513, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Gokorsch S., Weber C., Wedler T., and Czermak P.A stimulation unit for the application of mechanical strain on tissue engineered anulus fibrosus cells: a new system to induce extracellular matrix synthesis by anulus fibrosus cells dependent on cyclic mechanical strain. Int J Artif Organs 28,1242, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Gillett N.A., Gerlach R., Cassidy J.J., and Brown S.A.Age-related changes in the beagle spine. Acta Orthop Scand 59,503, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Ho M.M., Kelly T.-A.N., Guo X.E., Ateshian G.A., and Hung C.T.Spatially varying material properties of the rat caudal intervertebral disc. Spine (Phila Pa 1976) 31,E486, 2006 [DOI] [PubMed] [Google Scholar]