Abstract
This study aimed to investigate whether pre-chemotherapy anti-mullerian hormone (AMH) is a biomarker for chemotherapy-related amenorrhea (CRA) in breast cancer patients. A multicenter randomized controlled trial, ECOG5103, assigned patients with early stage breast cancer to standard doxorubicin-cyclophosphamide followed by paclitaxel with either placebo or one of two durations of bevacizumab therapy. Five hundred ninety-one patients were part of the decision-making/quality of life substudy, in which there were surveys from baseline through 18-month follow-up. One hundred twenty-four women were included in this analysis of menses data because they were premenopausal at enrollment, responded to the 12-month survey, had not undergone bilateral oophorectomy or ovarian function suppression before that survey, and had serum banked for research before chemotherapy. One hundred of the 124 also responded to the 18-month survey. Median age was 45 years (range 25–55), and median serum AMH level was 0.11 ng/mL (range 0.01–8.63) prior to treatment. Eighty-two percent had CRA at 12 months, and 81 % at 18 months. In multivariate analyses, older age (p = 0.0003) was the only statistically significant predictor of 12-month CRA, but at 18-months, lower pre-chemotherapy AMH (p = 0.04) and older age (p = 0.008) were both statistically significant predictors of CRA. Race, bevacizumab therapy, and tamoxifen use were not statistically significantly associated with CRA after adjustment for AMH and age. Pre-chemotherapy AMH level is a potential novel biomarker for CRA in premenopausal women with early stage breast cancer. Further research to evaluate the clinical utility of AMH testing is warranted.
Keywords: Breast neoplasms, Fertility, Biomarkers, Chemotherapy, Adjuvant
Introduction
Standard adjuvant chemotherapy regimens for breast cancer are gonadotoxic and cause chemotherapy-related amenorrhea (CRA) in many young women. CRA is associated with diminished ovarian reserve, infertility, and premature menopause, which are all of great importance. At present, age and treatment type are the only known predictive markers for CRA, and there is substantial individual heterogeneity that is not accounted for by these two variables [1]. Better prediction of ovarian function after oncologic therapies could help guide fertility preservation decisions, contraceptive choices, and treatment decisions in young women with breast cancer.
Anti-mullerian hormone (AMH), a dimeric glycoprotein produced by ovarian granulosa cells, is a potential biomarker of ovarian reserve [2]. AMH inhibits excessive follicular recruitment by follicle-stimulating hormone (FSH). AMH level appears to reflect ovarian reserve in the general population, and AMH naturally declines over an adult woman's life [3]. Lower AMH level predicts less success with in vitro fertilization [4, 5]. Because AMH remains relatively stable throughout the menstrual cycle, it may be a more clinically useful biomarker of ovarian reserve than other more menstrual-phase dependent measures of ovarian reserve (e.g., estradiol, FSH, and inhibin) in cancer patients making time-sensitive pre-treatment fertility preservation decisions [6].
Lutchman Singh et al. [7] showed that AMH levels were comparable between 24 controls and eight breast cancer patients before chemotherapy, but after chemotherapy, 22 breast cancer patients had lower AMH levels on average (not all of them had AMH measured before treatment). Anderson and colleagues found that 23 breast cancer patients as old as 52 who became amenorrheic during chemotherapy had lower pre-chemotherapy AMH levels than 19 who continued to menstruate (0.58 vs. 1.9 ng/mL, p = 0.0007) [8], and that lower pre-chemotherapy AMH was the only independent predictor of amenorrhea at 4–5 years in long-term follow-up [9]. These investigators confirmed this finding in a later cohort of 39 premenopausal breast cancer patients, in whom only AMH (not age, FSH, or inhibin B) was identified as an independent predictor of CRA at 2 years [10]. Similarly, Anders et al. [11] found that 16 women under 52 who developed CRA had lower AMH levels before chemotherapy (0.16 ng/mL) than five women who resumed menses by one year after chemotherapy (0.16 vs. 1.09 ng/mL, p = 0.02). In fact, Henry et al. identified a 95 % positive predictive value, and an 86 % negative predictive value, of a detectable pre-chemotherapy serum AMH concentration for recovery of ovarian function after chemotherapy among 27 pre- or peri-menopausal breast cancer patients [12]. Further, in our previous work, we demonstrated lower AMH levels and diminished ovarian reserve as measured by transvaginal ultrasound ovarian follicle count in 20 breast cancer survivors who were still menstruating after chemotherapy compared with 20 age- and gravidity-matched pre-menopausal controls [13].
Here, we sought to evaluate the role of pre-chemotherapy AMH level as a biomarker for CRA in a prospective cancer cooperative group clinical trial, Eastern Cooperative Group 5103 (E5103). This trial was well suited for this analysis because it (1) treated patients (some of whom were premenopausal) with at least standard doxorubicin-cyclophosphamide followed by paclitaxel chemotherapy (with some patients also receiving bevacizumab); (2) collected pretreatment serum in which AMH could be measured; and (3) included a mandatory DM/QOL substudy allowing serial menses data collection.
Methods
Study design
E5103 was a large prospective trial that randomized patients with high risk early stage breast cancer to standard adjuvant chemotherapy with doxorubicin-cyclophosphamide (given every 2 or 3 weeks per physician's choice) followed by paclitaxel (AC-T) or to one of two arms that combine AC-T chemotherapy with two different durations of bevacizumab. Institutional review boards approved this study at all participating sites, and all participating patients signed informed consent prior to enrolling on E5103. All E5103 participants were also asked to consent to a baseline blood draw to allow serum banking. For those who consented, four (1 mL) aliquots of serum were banked per patient. Patients who enrolled on E5103 between January 5, 2010 and June 8, 2010 were also enrolled on a longitudinal decision-making and quality of life (DM/QOL) component. These patients were asked to self-report their last menstrual period date as part of telephone-administered surveys administered at 12 and 18 months after enrollment.
Participants
Premenopausal women (those who had menstruated within a year prior to enrollment) in the DM/QOL sub-study were included in this analysis. Patients who had undergone ovarian suppression or bilateral salpingoophorectomy prior to the 12-month survey (as ascertained via follow-up case report forms from E5103) were excluded, as were those who did not consent to future specimen use and/or who had no stored serum available.
Measures
AMH was measured by two-site ELISA (Diagnostic Systems Laboratory, BeckmanCoulter, Webster, TX) from baseline serum samples drawn and banked before chemotherapy. Two 0.25 mL aliquots were extracted from banked serum, thawed, mixed, centrifuged, and transferred to a clearly labeled tube (with de-identified specimen ID and draw date), then rapidly refrozen and shipped on dry ice to the Department of Pathology at Massachusetts General Hospital for the AMH testing. Testing was monitored using quality control sera (two levels); the intra-assay coefficient of variation was <6 % and the inter-assay CV was <12 %.
CRA classifications were based on responses to the items in the 12-month and 18-month DM/QOL surveys regarding last menstrual period. Participants were classified as having had 12-month CRA if they had not experienced a period within the six months prior to the 12-month survey, and as having had 18-month CRA if they had not experienced a period within the six months prior to the 18-month survey.
Statistical analysis
Wilcoxon rank sum and Fisher's exact tests were used to assess for univariate associations between 12- and 18-month CRA and each of the following: AMH (a continuous variable), age (a continuous variable), race (white vs. non-white), receipt of bevacizumab (yes vs. no), and receipt of tamoxifen (yes vs. no). Multivariate logistic regression was used to assess for predictors of CRA at both time points including baseline AMH, age, race, whether or not the patient received bevacizumab, and use of tamoxifen. p values <0.05 were considered statistically significant.
Results
The DM/QOL component of E5103 accrued 591 women, of whom 195 were premenopausal at the time of enrollment. Fifty-one women did not consent to the baseline blood draw, and 1 consented but did not have serum stored. Out of the remaining 143, 17 were excluded due to ovarian suppression or bilateral salpingoophorectomy prior to the 12-month survey, and 2 were excluded due to missing 12-month survey responses. Out of the 124 eligible for analyses at 12 months, 100 (81 % of the 124) also had informative menstrual data available from the 18-month survey. Please see Fig. 1 for a flow diagram of participants. Median age of the 124 eligible women was 45 years old (range 25–55), and only 36 women were 40 years or under. Median pre-chemotherapy AMH in the 124 eligible women was 0.11 ng/mL (range 0.01–8.63). Seventy-six percent was on bevacizumab-containing arms of the trial. Eighty-two percent of the 124 with informative 12-month data had 12-month CRA. Eighty-one percent of the 100 with informative 18-month data had 18-month CRA (Table 1). Figure 2 graphically displays AMH values by age for each woman with and without CRA.
Fig. 1. Flow diagram of participants.
Table 1. Patient characteristics.
| Characteristic | Median (range) |
|---|---|
| Age (in years) | 45 (25–55) |
| Pre-chemotherapy AMH (in ng/mL) | 0.11 (0.01–8.63) |
| N (%) | |
| White race | 106 (85 %) |
| Received bevacizumab | 94 (76 %) |
| Used tamoxifen | 64 (52 %) |
| 12-month CRA | 102/124 (82 %) |
| 18-month CRA | 81/100 (81 %) |
CRA chemotherapy-related amenorrhea, AMH anti-mullerian hormone
Fig. 2. Scatterplot of age (in years) and AMH (in ng/mL) values in patients with CRA (triangles) and without CRA (circles).
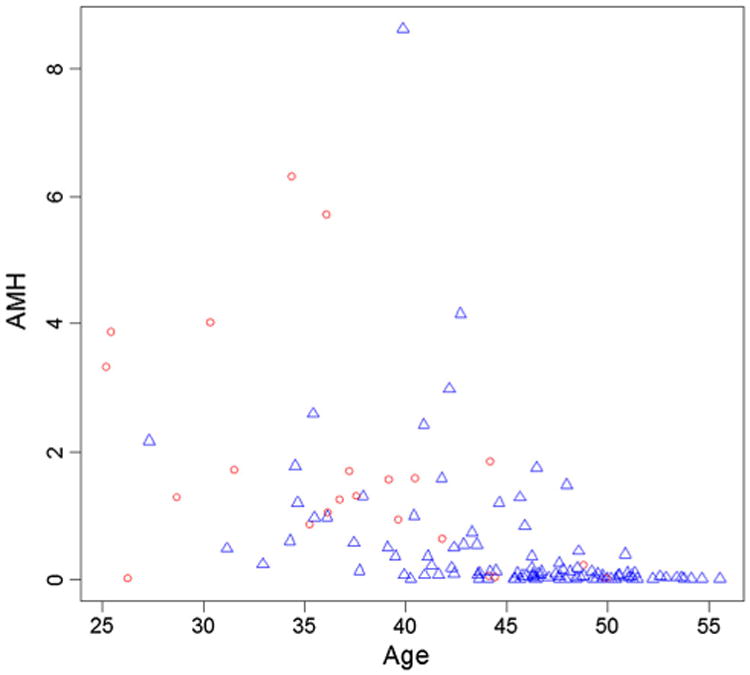
In univariate analyses of data from the 124 women eligible for analysis at 12 months, older age, lower AMH, and receipt of bevacizumab were associated with a greater likelihood of 12-month CRA (Table 2). In multivariate analyses of 12-month CRA, only the association with age remained statistically significant [odds ratio (OR) 1.20, 95 % confidence interval (CI) 1.10–1.33, p = 0.0003]. Please see Table 3. For every one year increase in age, there was a 20 % increase in the odds of developing 12-month CRA. At 18-months, when only 100 women were eligible for analysis, lower AMH and older age were significantly associated with CRA in univariate analyses (please see Table 4). In multivariate analyses of 18-month CRA, both AMH (OR 0.41, 95 % CI 0.18–0.95, p = 0.04) and age (OR 1.18, 95 % CI 1.04–1.34, p = 0.008) were significantly associated with CRA. For every one ng/mL increase in baseline AMH, there was a 59 % decrease in the odds of developing 18-month CRA. For every 1 year increase in age, there was an 18 % increase in the odds of developing 18-month CRA. Race, receipt of bevacizumab, and receipt of tamoxifen were not significantly associated with CRA at either 12 or 18 months in any of the models (Table 5).
Table 2. Univariate predictors of 12-month CRA (n = 124).
| Characteristic | No CRA (n = 22) | CRA (n = 102) | p value |
|---|---|---|---|
| AMH (median) | 1.3 ng/mL | 0.08 ng/mL | <0.0001 |
| Age (median) | 36.5 years | 46 years | <0.0001 |
| Race | 0.31 | ||
| White | 17 | 89 | |
| Other | 5 | 13 | |
| Treatment arm | 0.03 | ||
| No bevacizumab | 10 | 20 | |
| Bevacizumab | 12 | 82 | |
| Tamoxifen use | 1.00 | ||
| No | 11 | 49 | |
| Yes | 11 | 53 |
CRA chemotherapy-related amenorrhea, AMH anti-mullerian hormone
Table 3. Multivariate model for 12-month CRA (n = 124).
| Characteristic | OR | 95 % CI | p value |
|---|---|---|---|
| AMH | 0.83 | 0.58–1.20 | 0.32 |
| Age | 1.20a | 1.10–1.33 | 0.0003 |
| Race (white vs. other) | 1.65 | 0.38–7.17 | 0.50 |
| Received bevacizumab (yes vs. no) | 2.54 | 0.75–8.56 | 0.13 |
| Tamoxifen use (yes vs. no) | 0.66 | 0.20–2.13 | 0.49 |
CRA chemotherapy-related amenorrhea, AMH anti-mullerian hormone
For every 1 year increase in age, there was a 20 % increase in the odds of developing 12-month CRA
Table 4. Univariate predictors of 18-month CRA (n = 120).
| Characteristic | No CRA (n = 19) | CRA (n = 81) | p value |
|---|---|---|---|
| AMH (median) | 1.25 ng/mL | 0.06 ng/mL | <0.0001 |
| Age (median) | 37 years | 46 years | <0.0001 |
| Race | 1.00 | ||
| White | 16 | 69 | |
| Other | 3 | 12 | |
| Treatment arm | 0.36 | ||
| No bevacizumab | 6 | 17 | |
| Bevacizumab | 13 | 64 | |
| Tamoxifen use | 0.13 | ||
| No | 12 | 35 | |
| Yes | 7 | 46 |
CRA chemotherapy-related amenorrhea, AMH anti-mullerian hormone
Table 5. Multivariate model for 18-month CRA (n = 120).
| Characteristic | OR | 95 % CI | p value |
|---|---|---|---|
| AMH | 0.41a | 0.18–0.95 | 0.04 |
| Age | 1.18b | 1.04–1.34 | 0.008 |
| Race (white vs. other) | 0.77 | 0.12–4.84a | 0.79 |
| Received bevacizumab (yes vs. no) | 0.93 | 0.19–4.51 | 0.93 |
| Tamoxifen use (yes vs. no) | 2.25 | 0.59–8.55 | 0.23 |
CRA chemotherapy-related amenorrhea, AMH anti-mullerian hormone
For every 1 ng/mL increase in AMH, there was a 59 % decrease in the odds of developing 18-month CRA
For every 1 year increase in age, there was an 18 % increase in the odds of developing 18-month CRA
Discussion
Our finding of an association between lower baseline AMH level and higher risk of 18-month CRA suggests that pre-chemotherapy AMH may be a valuable biomarker for CRA. Controlling for age, race, bevacizumab, and tamoxifen use, for each 1 ng/mL increase in baseline AMH, the likelihood of 18-month CRA decreased by 59 %. This novel finding has substantial clinical relevance for pre-menopausal women with cancer requiring cytotoxic chemotherapy. Because CRA can impact prognosis [14], fertility [15], and menopausal symptoms [16], in women with breast cancer, pre-chemotherapy AMH measurement may ultimately have utility in clinical decision-making. Furthermore, identifying a pre-treatment predictor of later ovarian dysfunction could be particularly important for reproductive decision-making, as the commonly recommended fertility preservation techniques for breast cancer patients (e.g., oocyte and embryo cryopreservation) are most effective before gonadotoxic therapies are given. Some women who strongly prioritize future biological children might also use a pre-treatment biomarker for CRA to inform decisions about whether to opt against chemotherapy for small reductions in risk of recurrence. In addition, if adjuvant ovarian function suppression (i.e., use of gonadotropin-releasing hormone agonists) is proven beneficial in ongoing clinical trials (e.g., the Suppression of Ovarian Function Trial led by the International Breast Cancer Study Group), it is possible that we will find that those with high pre-treatment AMH are most likely to benefit, and those with low pre-treatment AMH are less likely to benefit and/or are more likely to be able to safely transition from a tamoxifen to an aromatase inhibitor without concurrent ovarian suppression [17].
Although tamoxifen is known to disrupt the menstrual cycle and cause amenorrhea in some women [18–21], we did not find that tamoxifen was associated with CRA in this study. The lack of an association at 12 months likely reflects the fact that many participants would not have been taking tamoxifen long enough to have experienced amenorrhea from this selective estrogen-receptor modulator at that time. The slight trend toward more 18-month CRA (OR 2.25, p = 0.23) in those who were receiving or had received tamoxifen suggests that the amenorrhea related to tamoxifen may be more evident at later time points, and this study's power may not have been adequate to reveal the association.
This study also did not demonstrate that bevacizumab therapy was a predictor of CRA after adjustment for the other covariates though other studies have linked bevacizumab to amenorrhea [22–24]. The non-statistically significant trend toward more 12-month CRA in those who received bevacizumab (OR 2.54, p = 0.13) suggests a potential link between bevacizumab and short-term amenorrhea that might be evident in a larger sample. However, the lack of an association with 18-month CRA (OR 0.93, p = 0.93) suggests that any contribution of bevacizumab to amenorrhea may not persist beyond the duration of the bevacizumab (which ended at approximately the time of the 12-month survey for those on prolonged bevacizumab, and approximately 6–7 months prior to the 12-month survey for those on concurrent bevacizumab).
The fact that multivariate analyses of 12-month CRA data (including a 24 % larger sample than the 18-month CRA data due to interval ovarian suppression and loss to follow-up) revealed only age (not AMH) as a statistically significant predictor is interesting and warrants additional study. It is possible that some of the patients who only completed the 12-month survey, not the 18-month, were less accurate in their reporting of menstrual history than the rest of the group who completed surveys at both time points. Alternatively, it is possible that AMH is truly more informative about the later CRA status.
Though its duration of follow-up for CRA was longer than many others, this study was still limited by its relatively short follow-up period. Long-term data regarding the association of AMH and CRA are clearly warranted. Further, while this is the largest study to date to assess CRA and pre-treatment AMH levels, larger studies are needed to confirm our findings and establish a meaningful threshold for pre-treatment AMH levels below which permanent CRA is highly likely and above which permanent CRA is highly unlikely at a given age. Because the DM/QOL component of E5103 was only designed to survey women for 18 months, we are unable to explore what proportion of women with CRA at 18 months eventually resume menses (estimates of menstrual recovery rates after 12 months in other studies range 3–29 %) [1, 25], nor what proportion of those who desire a future pregnancy are successful in bearing children. Although the median age of women in this study was older than is typical for women pursuing pregnancies (and there were only 36 women at or under age 40 included, too few for a subset analysis focusing on this group), a growing number of women in their forties do aspire to have future biological children thanks to improved reproductive technologies and recent societal trends [26]. Fertility endpoints (pregnancies and live births among those who desire biological children after breast cancer) should be included in future studies because CRA is an imperfect surrogate for infertility. Past gravidity, parity, and infertility data were not collected in this study, but should be in future work, as these may be relevant variables in modeling associations between future fertility endpoints and AMH. A larger patient sample will be needed to assess whether pre-treatment AMH levels are inversely proportional to later menopausal symptoms, prognosis, and infertility, and to measure AMH longitudinally in order to optimize understanding of chemotherapy-related ovarian dysfunction over time. The relationship between AMH and other markers of ovarian reserve (e.g., estradiol, FSH, inhibin), none of which were measured here due to limited sample availability, should be prospectively studied longitudinally in breast cancer patients (ideally utilizing blood draws timed to menstrual cycles). Furthermore, AMH should be investigated as a biomarker in premenopausal patients with other cancers (most importantly in those for whom use of fertility preservation techniques at the time of diagnosis will not be likely to impede prognosis) to potentially inform decision-making in this regard.
In summary, pre-chemotherapy AMH level is a promising biomarker for CRA in women with breast cancer. Future work may prove AMH useful in guiding their reproductive and oncologic therapeutic choices, and possibly also decisions about fertility preservation in other young cancer patients.
Acknowledgments
This study was supported by Public Health Service Grants CA23318, CA66636, CA21115, CA49883, CA14958, CA77651, CA25224, CA32291, as well as a Komen Promise Grant (PI: Schneider) and funds from the Breast Cancer Research Foundation. Chau Dang receives research funding from Roche/Genentech and GlaxoSmithKline and also serves as a consultant/advisor for Pfizer. Kathy Miller, Bryan Schneider, and Joseph Sparano have consultant/advisory roles Genentech, and Dr. Schneider is on the Genentech Speaker's Bureau. George Sledge receives remuneration from Genentech.
Contributor Information
Kathryn J. Ruddy, Email: ruddy.kathryn@mayo.edu, Mayo Clinic, Rochester, MN, USA.
Anne O'Neill, Dana-Farber Cancer Institute, Boston, MA, USA.
Kathy D. Miller, Indiana University Melvin and Bren Simon Cancer Center, Indianapolis, IN, USA
Bryan P. Schneider, Indiana University Melvin and Bren Simon Cancer Center, Indianapolis, IN, USA
Emily Baker, Dana-Farber Cancer Institute, Boston, MA, USA.
Joseph A. Sparano, Montefiore Hospital and Medical Center, Bronx, NY, USA
Chau Dang, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Donald W. Northfelt, Mayo Clinic, Scottsdale, AZ, USA
George W. Sledge, Jr., Stanford University, Palo Alto, CA, USA
Ann H. Partridge, Dana-Farber Cancer Institute, Boston, MA, USA
References
- 1.Sukumvanich P, Case LD, Van Zee K, Singletary SE, Paskett ED, Petrek JA, Naftalis E, Naughton MJ. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer. 2010;116(13):3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- 2.Zec I, Tislaric-Medenjak D, Megla ZB, Kucak I. Anti-Mullerian hormone: a unique biochemical marker of gonadal development and fertility in humans. Biochem Med (Zagreb) 2011;21(3):219–230. doi: 10.11613/bm.2011.031. [DOI] [PubMed] [Google Scholar]
- 3.Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747–750. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Celik E, Bastu E, Dural O, Yasa C, Buyru F. Relevance of anti-Mullerian hormone on in vitro fertilization outcome. Clin Exp Obstet Gynecol. 2013;40(1):66–69. [PubMed] [Google Scholar]
- 5.Wunder DM, Guibourdenche J, Birkhauser MH, Bersinger NA. Anti-Mullerian hormone and inhibin B as predictors of pregnancy after treatment by in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2008;90(6):2203–2210. doi: 10.1016/j.fertnstert.2007.10.078. [DOI] [PubMed] [Google Scholar]
- 6.La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P, Volpe A. Anti-Mullerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004;19(12):2738–2741. doi: 10.1093/humrep/deh508. [DOI] [PubMed] [Google Scholar]
- 7.Lutchman Singh K, Muttukrishna S, Stein RC, McGarrigle HH, Patel A, Parikh B, Groome NP, Davies MC, Chatterjee R. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer. 2007;96(12):1808–1816. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21(10):2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RA, Cameron DA. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96(5):1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RA, Rosendahl M, Kelsey TW, Cameron DA. Pretreatment anti-Mullerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anders C, Marcom PK, Peterson B, Gu L, Unruhe S, Welch R, Lyons P, Behera M, Copland S, Kimmick G, Shaw H, Snyder S, Antenos M, Woodruff T, Blackwell K. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest. 2008;26(3):286–295. doi: 10.1080/07357900701829777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry NL, Xia R, Schott AF, McConnell D, Banerjee M, Hayes DF. Prediction of postchemotherapy ovarian function using markers of ovarian reserve. Oncologist. 2014;19(1):68–74. doi: 10.1634/theoncologist.2013-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partridge AH, Ruddy KJ, Gelber S, Schapira L, Abusief M, Meyer M, Ginsburg E. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94(2):638–644. doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Swain SM, Jeong JH, Geyer CE, Jr, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff J, Vogel VG, Erban JK, Rastogi P, Livingston RB, Perez EA, Mamounas EP, Land SR, Ganz PA, Wolmark N. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore HC. Fertility and the impact of systemic therapy on hormonal status following treatment for breast cancer. Curr Oncol Rep. 2000;2(6):587–593. doi: 10.1007/s11912-000-0114-9. [DOI] [PubMed] [Google Scholar]
- 16.Duffy LS, Greenberg DB, Younger J, Ferraro MG. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304–308. doi: 10.1016/S0033-3182(99)71223-5. [DOI] [PubMed] [Google Scholar]
- 17.Henry NL, Xia R, Banerjee M, Gersch C, McConnell D, Giacherio D, Schott AF, Pearlman M, Stearns V, Partridge AH, Hayes DF. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24(8):2011–2016. doi: 10.1093/annonc/mdt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng K, Tian W, Zhou M, Chen H, Deng Y. Impact of chemotherapy-induced amenorrhea in breast cancer patients: the evaluation of ovarian function by menstrual history and hormonal levels. World J Surg Oncol. 2013;11:101. doi: 10.1186/1477-7819-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou WB, Yin H, Liu XA, Zha XM, Chen L, Dai JC, Tao AD, Ma JJ, Ling LJ, Wang S. Incidence of chemotherapy-induced amenorrhea associated with epirubicin, docetaxel and navelbine in younger breast cancer patients. BMC Cancer. 2010;10:281. doi: 10.1186/1471-2407-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swain SM, Land SR, Ritter MW, Costantino JP, Cecchini RS, Mamounas EP, Wolmark N, Ganz PA. Amenorrhea in pre-menopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113(2):315–320. doi: 10.1007/s10549-008-9937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han HS, Ro J, Lee KS, Nam BH, Seo JA, Lee DH, Lee H, Lee ES, Kang HS, Kim SW. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009;115(2):335–342. doi: 10.1007/s10549-008-0071-9. [DOI] [PubMed] [Google Scholar]
- 22.Newman H, Finger PT, Chin KJ, Pavlick AC. Systemic bevacizumab (Avastin) for exudative retinal detachment secondary to choroidal melanoma. Eur J Ophthalmol. 2011;21(6):796–801. doi: 10.5301/EJO2011.6477. [DOI] [PubMed] [Google Scholar]
- 23.Joshi DD, Banerjee T. Vascular endothelial growth factor (VEGF) receptor antibody bevacizumab (avastin) induces regression of renal cell carcinoma in an adolescent resulting in residual tumorectomy. Pediatr Blood Cancer. 2008;50(4):903–904. doi: 10.1002/pbc.21243. [DOI] [PubMed] [Google Scholar]
- 24.Administration USFaD. [Accessed 30 July 2013];2011 http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm274394.htm.
- 25.Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116(4):791–798. doi: 10.1002/cncr.24835. [DOI] [PubMed] [Google Scholar]
- 26.Armed Forces Health Surveillance Center. Female infertility, active component service women, U.S. Armed Forces, 2000–2012. MSMR. 2013;20(9):8–12. http://www.afhsc.mil/msmrHowTo. [PubMed] [Google Scholar]


