Abstract
Here we review the neurobiology of infant odor learning in rats, and discuss the unique role of the stress hormone corticosterone (CORT) in the learning necessary for the developing rat. During the first 9 postnatal (PN) days, infants readily learn odor preferences, while aversion and fear learning are attenuated. Such restricted learning may ensure that pups only approach their mother. This sensitive period of preference learning overlaps with the stress hyporesponsive period (SHRP, PN4–14) when pups have a reduced CORT response to most stressors. Neural underpinnings responsible for sensitive-period learning include increased activity within the olfactory bulb and piriform “olfactory” cortex due to heightened release of norepinephrine from the locus coeruleus. After PN10 and with the decline of the SHRP, stress-induced CORT release permits amygdala activation and facilitates learned odor aversions and fear. Remarkably, odor preference and attenuated fear learning can be reestablished in PN10–15 pups if the mother is present, an effect due to her ability to suppress pups’ CORT and amygdala activity. Together, these data indicate that functional changes in infant learning are modified by a unique interaction between the developing CORT system, the amygdala, and maternal presence, providing a learning system that becomes more flexible as pups mature.
Keywords: mother–infant interactions, olfactory bulb, norepinephrine, attachment, imprinting, locus coeruleus, amygdala, learning, classical conditioning, corticosterone, stress, fear
INTRODUCTION
“There is continuity in development, such that the organization at one stage provides the basis for organization at the next succeeding stage. This does not mean, however, that all processes persist throughout life, nor does it mean that behaviors must remain stable across stages. On the contrary, development is essentially a dynamic process that promotes reorganization and adaptation across time”
(Levine, 1982).
At birth, altricial infant rats are confined to the nest and exquisitely designed to identify, learn, and remember experiences with their caregivers. Indeed, infants readily learn an attraction to their mother’s odor, which ensures that infants will exhibit approach behaviors toward the mother in order to receive the food, protection, and warmth needed for survival. Perinatal learning of maternal odor is required for pups to approach the mother and attach to her nipples for nursing (Pedersen & Blass, 1982), though somatosensory cues from the nipple are also required for these behaviors (Polan & Hofer, 1999; Stern, 1997). The maternal odor continues to be learned throughout the postnatal period (Cheslock, Varlinskaya, Petrov, & Spear, 2000; Pedersen, Williams, & Blass, 1982), presumably since the mother’s odor can be altered with her diet (Leon, 1992). Overall, infant behavior is centered on maintaining contact with the mother (Galef & Kaner, 1980; Leon, 1992), and, as will be discussed here, this early attachment process is facilitated by infants’ enhanced ability to learn preferences and their decreased ability to learn aversions or fear. Presumably, this constrains infants to form only preferences to caretakers.
Such attachment learning has a wide phylogenetic representation and appears to enable altricial animals to easily form a repertoire of proximity-seeking behaviors toward the primary caregiver, regardless of the quality of care they receive. For example, in avian imprinting, a chick will continue to follow its caregiver even while being shocked (Hess, 1962; Salzen, 1970). A similar experiment in dogs has shown that puppies will display strong attachment to a handler who provides rough treatment or neglect (Rajecki, Lamb, & Obmascher, 1978). Additionally, nonhuman primates and human children will also demonstrate strong attachment to an abusive caregiver (Harlow & Harlow, 1965; Helfer, Kempe, & Krugman, 1997; Maestripieri, Tomaszycki, & Carroll, 1999; Sanchez, Ladd, & Plotsky, 2001).
We have hypothesized that the infant rat learning system is designed to ensure that pups will learn an approach response towards and preference for the mother, regardless of whether she is associated with pain or pleasure (Hofer & Sullivan, 2001). We refer to this period of attachment learning as the “sensitive period.” Furthermore, it is worthwhile to note that postpartum mothers of various mammalian species also display a sensitive period for learning about offspring (Brennan & Keverne, 1997; Insel & Young, 2001; Keverne & de la Riva, 1982; Marlier, Schaal, & Soussignan, 1998; Moffat, Suh, & Fleming, 1993; Okere & Kaba, 2000; Pissonnier, Thiery, Fabre-Nys, Poindron, & Keverne, 1985). Much like that of the infant, mother learning requires unique neural circuitry to facilitate odor preferences, approach responses, and nurturing behavior toward offspring (Brennan & Keverne, 1997; Insel & Young, 2001; Lévy, Gervais, Kindermann, Orgeur, & Piketty, 1990).
In just 3 short weeks, rat pups are transformed into independent organisms with the maturation and experience to survive on their own. These 3 weeks represent a time of transition from maternal dependence to independence that uniquely characterizes the dramatic reorganization and adaptation of learning required of the infant. In this review, we discuss the neural basis that enables pups to transition between readily learning preferences within the context of attachment to learning fear. One prominent characteristic of learning after postnatal day (PN) 10 is the amygdala’s dependence on stress-induced corticosterone (CORT) release. Indeed, the ontogeny of infant stress responsiveness and the hypothalamic–pituitary–adrenal (HPA) system development were two major foci of Seymour Levine’s developmental work. As will be evident below, Levine’s contributions to developmental psychobiology have certainly been instrumental in helping us understand the neurobehavioral basis of infant attachment and the ontogeny of fear learning.
NEUROBIOLOGY OF INFANT RAT ODOR PREFERENCE LEARNING
During the infant sensitive period, PN1–9, pups display an enhanced capacity for preference learning. We have shown that learned odor preferences (conditioned via either positive or aversive stimuli paired with an unfamiliar odor) during this period are in part due to strong noradrenergic input to the olfactory bulb from the locus coeruleus (LC). Infant acquisition (learning) is disrupted if norepinephrine (NE) receptors are blocked in the bulb (Sullivan, Zyzak, Skierkowski, & Wilson, 1992) or if the LC is pharmacologically destroyed (Sullivan, Wilson, Lemon, & Gerhardt, 1994). Presentations of an odor with the activation of olfactory bulb NE β-receptors or stimulation of the LC during this period are sufficient to produce odor preference learning (Sullivan, Stackenwalt, Nasr, Lemon, & Wilson, 2000; Yuan, Harley, Darby-King, Neve, & McLean, 2003). Additionally, we have shown that NE is required for the maintenance of the prolonged mitral cell response characteristic of sensitive-period learning (Wilson, Sullivan, & Leon, 1987).
Unique properties of the LC appear to be responsible for infant preference learning. In effect, the LC of a sensitive-period pup is characterized by prolonged stimulus-evoked excitation, which prompts release of an enormous amount of NE (Nakamura, Kimura, & Sakaguchi, 1987). This is in contrast to the LC of an older pup, in which there is a much shorter evoked physiological response and thus smaller release of NE (Nakamura et al., 1987). The dramatic reduction in NE release at the close of the sensitive period is associated with the functional emergence of LC α2 inhibitory autoreceptors and the downregulation of LC α1 excitatory autoreceptors (Nakamura et al., 1987; Pieribone, Nicholas, Dagerlind, & Hokfelt, 1994; Scheinin et al., 1994). To test whether these developmental changes in LC autoreceptors are important for ending pups’ rapid preference learning, we recreated neonatal levels of these LC autoreceptors’ activity in older pups to reproduce the large NE release of younger pups. Specifically, after stimulating the LC with intra-LC cholinergic infusion, combined with drugs that blocked the autoinhibition (α2 antagonists) and enhanced the autoexcitation (α1 agonists), we successfully reinstated pups’ rapid NE-dependent odor preference learning (Moriceau & Sullivan, 2004b). These data suggest that functional changes in the LC support termination of the rapid and robust preference learning period.
Though early-life learning is characterized by odor preference learning, infants during this developmental period also have a decreased capacity to learn aversions or fear. Specifically, during the sensitive period, neonatal rats readily learn an odor preference even when an unfamiliar odor has been paired with an aversive stimulus, such as .5 mA foot-shock or tail pinch (Camp & Rudy, 1988; Haroutunian & Campbell, 1979; Moriceau & Sullivan, 2006; Moriceau, Wilson, Levine, & Sullivan, 2006; Roth & Sullivan, 2005; Spear, 1978; Sullivan & Hall, 1988; Sullivan, Hofer, & Brake, 1986; Sullivan, Landers, Yeaman, & Wilson, 2000). At the end of the sensitive period, similarly to older animals, pups readily learn to avoid unfamiliar odors paired with the same aversive stimuli (Blozovski & Cudennec, 1980; Camp & Rudy, 1988; Collier, Mast, Meyer, & Jacobs, 1979; Goldman & Tobach, 1967; Haroutunian & Campbell, 1979; Moriceau & Sullivan, 2006; Moriceau et al., 2006; Myslivecek, 1997; Stehouwer & Campbell, 1978; Sullivan, Landers, et al., 2000).
Shock-induced preference learning during the sensitive period is likely not due to the pups’ inability to feel pain since unconditioned responses to shock vary little between sensitive-period pups and older pups (Barr, 1995; Collier & Bolles, 1980; Emerich, Scalzo, Enters, Spear, & Spear, 1985; Fitzgerald, 2005; Shair, Masmela, Brunelli, & Hofer, 1997; Stehouwer & Campbell, 1978; Sullivan, Landers, et al., 2000). Also, pups’ inability to learn aversions or fear is not limited to olfactory-cued fear conditioning, as other learning paradigms that produce learned fear in older animals (such as passive avoidance and inhibitory conditioning) do not readily do so in infant rats (Bialik, Pappas, & Roberts, 1984; Blozovski & Cudennec, 1980; Camp & Rudy, 1988; Collier & Mast, 1979; Myslivecek, 1997).
Due to the known role of the amygdala in supporting learned fear in older animals (Cahill, Weinberger, Roozendaal, & McGaugh, 1999; Debiec & LeDoux, 2006; Fanselow & Gale, 2003; Fanselow & LeDoux, 1999; Goosens & Maren, 2001; Maren, 2003; Sigurdsson, Doyere, Cain, & LeDoux, 2007), we have examined whether the amygdala mediates the developmental transition that permits pups’ emergence of avoidance and fear learning at PN10 (Sullivan, Landers, et al., 2000). Using markers that reflect neural activity (2-deoxyglucose uptake and cfos immunohistochemistry), we have found that the amygdala only appears to be involved in odor-shock conditioning when this conditioning is able to support odor avoidance acquisition—that is, when the sensitive period has ended (Moriceau et al., 2006; Roth & Sullivan, 2005; Sullivan, Landers, et al., 2000; Sullivan & Wilson, 1993, 2003).
As further evidence of its limited role in infant preference learning, amygdala lesions during the sensitive period do not prevent the ability of infants to learn a shock-induced conditioned odor preference (Moriceau et al., 2006; Sullivan & Wilson, 1993). In contrast, amygdala lesions in older pups prevent them from learning a conditioned odor aversion (Maren, 1999; Moriceau et al., 2006; Sullivan, Landers, et al., 2000). Finally, the reduced ability of sensitive-period pups to exhibit amygdala long-term depression (LTD) further suggests that the amygdala is not participating in infant learning (Thompson, Sullivan, & Wilson, 2008). Altogether, these data suggest that the lack of amygdala participation in circuitry mediating sensitive-period learning is key to an infant’s increased capacity to learn a preference. While we first hypothesized that the immaturity of the amygdala (Berdel & Morys, 2000; Berdel, Morys, & Maciejewska, 1997; Bouwmeester, Wolterink, & van Ree, 2002; Cunningham, Bhattacharyya, & Benes, 2002; Morys, Berdel, Jagalska-Majewska, & Luczynska, 1999; Nair & Gonzalez-Lima, 1999) was responsible for its lack of participation, recent studies have since suggested that the amygdala is sufficiently mature to respond to stimuli during the sensitive period (Thompson et al., 2008). Rather, it is increasing CORT levels that play a crucial role in the emergence of fear learning and in the participation of the amygdala after the sensitive period (Barr et al., 2009; Moriceau & Sullivan, 2006; Moriceau et al., 2006; Shionoya, Moriceau, Bradstock, & Sullivan, 2007; Sullivan & Holman, 2010; Sullivan, Landers, et al., 2000).
CORTICOSTERONE, AMYGDALA ACTIVITY, AND THE ONTOGENY OF FEAR
Our interest in CORT was initiated by two areas of research. First, work showing that pups’ ontogenetic emergence of fear to predator odor (unlearned fear) occurs at the same age as learned fear (~PN10) and is controlled by the endogenous increase in CORT during development (Takahashi, 1994). Second, pups’sensitive period for odor preference/attachment learning overlaps with an infant “stress hyporesponsive period” (SHRP, PN4–14), during which pups’ CORT levels are lower than normal and remain either unaffected or are minimally increased by stressors (Grino, Paulmyer-Lacroix, Faudon, Renard, & Anglade, 1994; Levine, 2001; Rosenfeld, Suchecki, & Levine, 1992).
Interestingly, CORT response is functional at birth (Arai & Widmaier, 1991; Martin, Cake, Hartmann, & Cook, 1977; Widmaier, 1990) and the sensory stimulation provided by the mother during nursing and grooming seems to control the pups’ low CORT levels (Levine, 1962; Stanton & Levine, 1990; Van Oers, De Kloet, Whelan, & Levine, 1998). Indeed, sensitive-period pups show increases in CORT in response to intense stressors such as prolonged maternal deprivation or cold, which can be returned to normal low levels with replacement of maternal sensory stimulation or maternal presence (Avishai-Eliner, Yi, Newth, & Baram, 1995; Levine, 2001; Walker, Scribner, Cascio, & Dallman, 1991). Additionally, functional CORT receptors are already present throughout the brain, including within the amygdala (Alexis, Kitraki, Spanou, Stylianopoulou, & Sekeris, 1990; Diorio, Viau, & Meaney, 1993; Kitraki, Alexis, Papalopoulou, & Stylianopoulou, 1996; Rosenfeld, van Eekelen, Levine, & de Kloet, 1993).
Studies utilizing presentations of predator odor have helped provide a causal link between CORT responsivity, amygdala activation, and the ontogeny of natural or unlearned fear. We and others have shown that during the SHRP, predator-odor presentations fail to elicit a CORT response unless it is a very prolonged presentation (Gould, Tanapat, & Cameron, 1997; Moriceau, Roth, Okotoghaide, & Sullivan, 2004; Takahashi, 1994; Wiedenmayer & Barr, 2001; Wiedenmayer, Magarinos, McEwen, & Barr, 2005). Furthermore, these researchers showed that increasing neonatal CORT levels prior to presentation of the predator odor, however, will engage the amygdala and ultimately permit fear expression. Alternatively, depletion of CORT in older pups blocks amygdala responsivity to the male odor presentations, and thus fear expression. Together, these data highlight the importance of CORT in the emergence of natural (unlearned) fear and suggest that changes in the developing CORT system facilitate the transition between sensitive-period preference learning and postsensitive-period fear conditioning.
To investigate this relationship, we gave sensitive-period pups either systemic or intra-amygdala CORT injections prior to odor-shock conditioning. We found that either of these approaches enabled sensitive-period pups to learn an odor aversion. As summarized in Table 1, neural assessment of their brains by 2-deoxyglucose uptake indicated that the learning had evoked significant activity within the amygdala (Sullivan, Landers, et al., 2000). In turn, we can extend the age at which odor-shock conditioning produces an odor preference and prevent amygdala activity by eliminating endogenous CORT in older pups, through either removal of its source, the adrenal glands, or administration of the CORT receptor antagonist, RU 38486 (Moriceau & Sullivan, 2004a, 2006). Preventing the increase of CORT by adrenalectomy has also been shown to delay the emergence of learned fear with aversive conditioning (Bialik et al., 1984; Collier et al., 1979). This developmental effect is in sharp contrast to CORT effects on learning in adults, where it only modifies how well a behavior is learned (Corodimas, LeDoux, Gold, & Schulkin, 1994; Pugh, Tremblay, Fleshner, & Rudy, 1997; Roozendaal, Carmi, & McGaugh, 1996).
Table 1.
Odor-Shock Conditioning in Rat Pups
| Measure | Sensitive period (PN1–9)
|
Conditional sensitive period (PN10–15)
|
||
|---|---|---|---|---|
| Saline | CORT increase (systemic or intra-amygdala CORT) | Saline | CORT reduction (maternal presence, adrenalectomy, amygdala CORT receptor blocker) | |
| Behavior | Preference | Aversion | Aversion | Preference |
| Corticosterone level | Low | High | High | Low |
| 2-DG uptake | ||||
| Olfactory bulb | Increase | No change | No change | Increase |
| Anterior piriform cortex | Increase | No change | No change | Increase |
| Posterior piriform cortex | No change | Increase | Increase | No change |
| Amygdala | No change | Increase | Increase | No change |
Note. The table summarizes our understanding of the brain regions supporting sensitive period (PN1–9) odor preference learning and conditional sensitive period (PN10–15) odor aversion learning. Learned odor preferences during the sensitive period are associated with increased neural activity, as measured by 2-deoxyglucose uptake within the olfactory bulb and anterior piriform “olfactory” cortex. There is no significant activity within the amygdala or posterior piriform cortex. In contrast, when CORTis high endogenously (PN12) or via treatment (PN8), learned odor aversions are associated with significant activity within the amygdala and posterior piriform cortex. Maternal presence during odor-shock conditioning in PN12 pups decreases CORT levels, increases neural activity within the olfactory bulb and anterior piriform “olfactory” cortex, inhibits posterior piriform cortex and amygdala responsivity, and permits odor preference learning. Note, this is the same neural circuitry responsible for PN8 preference learning (Moriceau & Sullivan, 2006; Moriceau et al., 2006; Raineki, Shionoya, Sander, & Sullivan, 2009; Sullivan, Landers, et al., 2000). PN = postnatal day.
Based upon the data discussed above, low CORT levels and the consequent lack of significant amygdala activity during the sensitive period appear to prevent pups from learning aversions or avoidances to odors associated with the mother. Levine and his colleagues have demonstrated that sensory stimulation from the mother is responsible for maintaining low CORT levels during the SHRP (Levine, 2001). For example, removal of maternal sensory stimulation during the SHRP, such as that occurring when pups are separated from the mother for a prolonged period of time (24 hr), produces significant elevations in CORT levels (Levine, 2001). Aberrant maternal care in the rat will produce similar effects (Gilles, Schultz, & Baram, 1996). Furthermore, maternal presence in older pups (>PN12) can blunt CORT release to stressful and painful stimuli (Stanton & Levine, 1990; Stanton, Wallstrom, & Levine, 1987; Suchecki, Rosenfeld, & Levine, 1993). Likewise, our own data illustrated in Figure 1 replicate the remarkable ability of the mother to suppress CORT levels even during stress in PN14 and PN21 pups. The sensory cues capable of blunting the stress-induced CORT release appear to be olfactory and somatosensory (Barr et al., 2009; Moriceau & Sullivan, 2006; Shionoya et al., 2007; Stanton & Levine, 1990; Suchecki et al., 1993; Wiedenmayer, Magarinos, McEwen, & Barr, 2003). The decrease in CORT levels due to social sensory cues has since been referred to as “social buffering” (Hennessy, Kaiser, & Sachser, 2009; Kikusui, Winslow, & Mori, 2006), and has been shown to exist in humans and other animals (DeVries, Glasper, & Detillion, 2003; Kirschbaum, Klauer, Filipp, & Hellhammer, 1995; Thorsteinsson & James, 1999).
FIGURE 1.
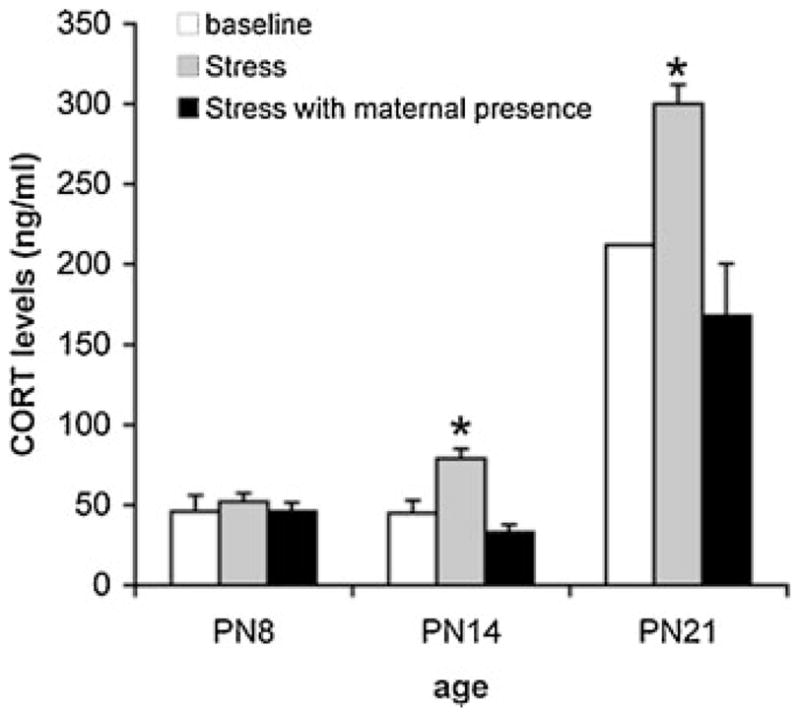
Ontogeny of CORT levels. CORT levels in response to .5 mA foot-shock were measured in PN8, PN14, or PN21 pups with or without maternal presence to assess the ontogeny of social buffering. Immediately following 11 shock presentations with an interval of 4 min (between 12 and 2 pm), pups were anesthetized with pentobarbital and blood was taken from the hearts’ ventricle. Shock elicits a significant increase in CORT in PN14 and PN21 pups, but fails to do so in neonatal pups (PN8). Maternal presence in PN14 and PN21 pups prevents the CORT response to shock (Moriceau & Sullivan, 2006; Moriceau et al., 2006). The mother was anesthetized by urethane to prevent her from interfering with the shock administration, as well as to control for maternal behavior and milk letdown. The pups were, however, free to contact the mother. Stress =foot-shock; PN =postnatal day.
The finding that maternal presence blunts CORT release to stressful and painful stimuli in older pups (Stanton & Levine, 1990; Stanton et al., 1987; Suchecki et al., 1993), prompted us to examine whether maternal presence in older pups (PN10–15) is capable of suppressing amygdala activity and blocking fear learning. We found that indeed maternal presence blocked fear learning (aversion) in response to odor-shock conditioning, as well as prevented significant amygdala activation and permitted significant olfactory bulb activation (Moriceau & Sullivan, 2006). Furthermore, systemic or intra-amygdala CORT infusions allowed the pups to learn odor aversions in the presence of the mother (Moriceau & Sullivan, 2006). As depicted in Table 1, our results indicate that maternal presence in PN10–15 pups reengages the attachment circuitry during learning, effectively preventing them from acquiring an odor aversion or fear. The ability of maternal presence to reengage the attachment circuitry appears to end at PN15, as PN16 pups still learn an odor aversion even in the presence of the mother (Upton & Sullivan, 2010). Based upon these data, we now define PN10–15 as a “conditional sensitive period,” in which odor preference learning and attenuated fear learning can be reestablished if the mother is present.
To summarize, the brain of the developing infant rat is optimized to facilitate attachment during a developmental period when pups are confined to the nest (until PN9), with circuitry providing remarkable constraints on aversion and fear learning. The ecological significance of this may relate to the possible occurrence of rough handling by the mother during normal mother–infant interactions (i.e., stepping on pups while entering/leaving the nest and rough pup retrieval). From an evolutionary perspective, it would be maladaptive for pups to learn to avoid the maternal odor in a situation where the mother is required for milk and warmth, suggesting that this attenuated avoidance learning ensures that pups continue to only approach/follow the caregiver (Hofer & Sullivan, 2001). During the conditional sensitive period (PN10–15), when pups are still dependent on the mother but can begin expanding their environment beyond the nest, they readily demonstrate avoidance and fear learning to aversive stimuli in the absence of the mother. However, the attachment circuitry and restraint on fear learning can be reengaged during this transitional period if the mother is present. This suggests that older pups have a more sophisticated learning system that enables them to respond appropriately to learning situations dependent on whether the mother is present or not. The data summarized here make it clear that the ontogeny of pups’ CORTand amygdala responsivity play a pivotal role in the dramatic reorganization and adaptation of learning necessary for the developing rat.
CONCLUDING REMARKS
Seymour Levine, one of the first to study the role of early experiences in shaping stress responses, has left a lasting legacy regarding the profound influence of the mother on the development of the stress system in numerous species (Levine, 1957, 1967; Lyons, Martel, Levine, Risch, & Schatzberg, 1999; Morgado et al., 2008; Schmidt et al., 2006; Stanton et al., 1987). The data outlined in this review indicate that functional changes in infant learning are modified by a unique interaction between the developing CORT system, the amygdala, and the learning context that depends on whether the mother is present or absent.
While human children show remarkably similar behavior within the realm of attachment (proximity seeking, tolerance of pain), it is unclear if the rat attachment circuitry outlined here exists in human infants. Bowlby’s (1965) use of the animal literature in the construction of his attachment theory would argue the likely evolutionary conservation of attachment circuitry. Following this, it is likely the case that the human infant’s brain is similarly organized to ensure rapid, robust attachment to his or her caregiver.
The importance of a healthy and secure attachment in humans is illustrated by the fact that securely attached children have an increased probability of maturing into mentally healthy adults compared to insecurely attached children (Dozier, Peloso, Lewis, Laurenceau, & Levine, 2008; Gunnar & Quevedo, 2008). Conversely, children in abusive attachment relationships have a greater probability of experiencing adult mental problems (Glaser, 2000; Grossman et al., 2003; Sanchez et al., 2001; Teicher et al., 2003). Presumably, this reflects an altered trajectory of brain development, and it is clear that the brains’ HPA axis and amygdala are particularly vulnerable to early environmental influences (Dent, Smith, & Levine, 2001; Eghbal-Ahmadi, Avishai-Eliner, Hatalski, & Baram, 1999; Francis, Caldji, Champagne, Plotsky, & Meaney, 1999; Swiergiel, Takahashi, & Kalin, 1993). Based upon the data reviewed here, developmental insults to these systems would certainly have the capacity to disrupt early learning processes responsible for securing attachment, thus increasing the risk for poor mental outcomes.
Acknowledgments
This work was funded by grants NIH DC009910, NSF IOB0850527 to RMS and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression to TLR.
Footnotes
This article is a contribution to a Special Issue of Developmental Psychobiology, 52(7), 2010, entitled “Seymour Levine’s Legacy: The Infant’s World and its Consequences.”
References
- Alexis MN, Kitraki E, Spanou K, Stylianopoulou F, Sekeris CE. Ontogeny of the glucocorticoid receptor in the rat brain. Advances in Experimental Medicine Biology. 1990;265:269–276. doi: 10.1007/978-1-4757-5876-4_25. [DOI] [PubMed] [Google Scholar]
- Arai M, Widmaier EP. Activation of the pituitary-adrenocortical axis in day-old rats by insulin-induced hypoglycemia. Endocrinology. 1991;129:1505–1512. doi: 10.1210/endo-129-3-1505. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic–pituitary–adrenal components in the infant rat. Neuroscience Letters. 1995;192:49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA. Ontogeny of nociception and antinociception. NIDA Research Monograph. 1995;158:172–201. [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, et al. Transitions in infant learning are modulated by dopamine in the amygdala. Nature Neuroscience. 2009;12:1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdel B, Morys J. Expression of calbindin-D28k and parvalbumin during development of rat’s basolateral amygdaloid complex. International Journal of Developmental Neuroscience. 2000;18:501–513. doi: 10.1016/s0736-5748(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. International Journal of Developmental Neuroscience. 1997;15:755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Bialik RJ, Pappas BA, Roberts DC. Deficits in conditioned avoidance responding following adrenalectomy and central norepinephrine depletion are dependent on postsurgical recovery period and phase of the diurnal cycle. Behavioral Neuroscience. 1984;98:847–857. doi: 10.1037//0735-7044.98.5.847. [DOI] [PubMed] [Google Scholar]
- Blozovski D, Cudennec A. Passive avoidance learning in the young rat. Developmental Psychobiology. 1980;13:513–518. doi: 10.1002/dev.420130510. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Wolterink G, van Ree JM. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. Journal of Comparative Neurology. 2002;442:239–249. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment. New York: Basic Books; 1965. [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Progress in Neurobiology. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Developmental Psychobiology. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: Promotion of nipple attachment in the newborn rat. Behavioral Neuroscience. 2000;114:484–495. [PubMed] [Google Scholar]
- Collier A, Bolles RC. The ontogenesis of defensive reactions to shock in preweanling rats. Developmental Psychobiology. 1980;13:141–150. doi: 10.1002/dev.420130206. [DOI] [PubMed] [Google Scholar]
- Collier A, Mast J. Alleviation of avoidance deficits by approach alternatives in 10-day old rats. Physiology & Behavior. 1979;23:615–618. doi: 10.1016/0031-9384(79)90068-4. [DOI] [PubMed] [Google Scholar]
- Collier A, Mast J, Meyer DR, Jacobs CE. Approach-avoidance conflict in preweanling rats: A developmental study. Animal Learning and Behavior. 1979;7:514–520. [Google Scholar]
- Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of conditioned fear in rats. Annals of the New York Academy of Sciences. 1994;746:392–393. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. Journal of Comparative Neurology. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: Treatment implications for PTSD. Annals of the New York Academy of Sciences. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- Dent GW, Smith MA, Levine S. Stress-induced alterations in locus coeruleus gene expression during ontogeny. Brain Research Developmental Brain Research. 2001;127:23–30. doi: 10.1016/s0165-3806(01)00108-0. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiology & Behavior. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic–pituitary–adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau JP, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development & Psychopathology. 2008;20:845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. Journal of Neuroscience. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Scalzo FM, Enters EK, Spear NE, Spear LP. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Developmental Psychobiology. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Annals of the New York Academy of Sciences. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nature Reviews Neuroscience. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor—Norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biological Psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Kaner HC. Establishment and maintenance of preference for natural and artificial olfactory stimuli in juvenile rats. Journal of Comparative & Physiological Psychology. 1980;94:588–595. doi: 10.1037/h0077693. [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatric Neurology. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain—A review. Journal of Child Psychology and Psychiatry. 2000;41:97–116. [PubMed] [Google Scholar]
- Goldman PS, Tobach E. Behaviour modification in infant rats. Animal Behaviour. 1967;15:559–562. doi: 10.1016/0003-3472(67)90058-9. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning and Memory. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Cameron HA. Adrenal steroids suppress granule cell death in the developing dentate gyrus through an NMDA receptor-dependent mechanism. Brain Research Developmental Brain Research. 1997;103:91–93. doi: 10.1016/s0165-3806(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha 2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology. 1994;135:2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, McKinney BC, Kodish IM, Otte SL, Greenough WT. Experience effects on brain development: Possible contributions to psychopathology. Journal of Child Psychology and Psychiatry. 2003;44:33–63. doi: 10.1111/1469-7610.t01-1-00102. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: A mechanism for later trauma vulnerability. Progress in Brain Research. 2008;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The affectional systems. In: Schrier A, Harlow HF, Stollnitz F, editors. Behavior of nonhuman primates. Vol. 2. New York: Academic Press; 1965. pp. 287–344. [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Helfer ME, Kempe RS, Krugman RD. The battered child. Chicago: University of Chicago Press; 1997. [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers Neuroendocrinology. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hess E. Ethology: An approach to the complete analysis of behavior. In: Brown R, Galanter E, Hess E, Mendler G, editors. New directions in psychology. New York: Holt, Rinehart and Winston; 1962. pp. 159–199. [Google Scholar]
- Hofer MA, Sullivan RM. Towards a neuro-biology of attachment. In: Nelson CA, Luciana M, editors. Developmental cognitive neuroscience. Cambridge: MIT Press; 2001. pp. 599–616. [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Keverne EB, de la Riva C. Pheromones in mice: Reciprocal interaction between the nose and brain. Nature. 1982;296:148–150. doi: 10.1038/296148a0. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society B Biology Science. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Alexis MN, Papalopoulou M, Stylianopoulou F. Glucocorticoid receptor gene expression in the embryonic rat brain. Neuroendocrinology. 1996;63:305–317. doi: 10.1159/000126971. [DOI] [PubMed] [Google Scholar]
- Leon M. The neurobiology of filial learning. Annual Review of Psychology. 1992;43:377–398. doi: 10.1146/annurev.ps.43.020192.002113. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levine S. Comparative and psychobiological perspectives on development. In: Collins WA, editor. Minnesota Symposia on Child Psychology, Vol. 15. The concept of development. Hillsdale, NJ: Lawrence Erlbaum; 1982. pp. 29–53. [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiology & Behavior. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Lévy F, Gervais R, Kindermann U, Orgeur P, Piketty V. Importance of beta-noradrenergic receptors in the olfactory bulb of sheep for recognition of lambs. Behavioral Neuroscience. 1990;104:464–469. doi: 10.1037//0735-7044.104.3.464. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Martel FL, Levine S, Risch NJ, Schatzberg AF. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Hormones and Behavior. 1999;36:266–275. doi: 10.1006/hbeh.1999.1547. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Developmental Psychobiology. 1999;34:29–35. [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. Journal of Neuroscience. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Annals of the New York Academy of Sciences. 2003;985:106–113. doi: 10.1111/j.1749-6632.2003.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Marlier L, Schaal B, Soussignan R. Bottle-fed neonates prefer an odor experienced in utero to an odor experienced postnatally in the feeding context. Developmental Psychobiology. 1998;33:133–145. [PubMed] [Google Scholar]
- Martin CE, Cake MH, Hartmann PE, Cook IF. Relationship between foetal corticosteroids, maternal progesterone and parturition in the rat. Acta Endocrinology (Copenh) 1977;84:167–176. doi: 10.1530/acta.0.0840167. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Suh EJ, Fleming AS. Noradrenergic involvement in the consolidation of maternal experience in postpartum rats. Physiology & Behavior. 1993;53:805–811. doi: 10.1016/0031-9384(93)90192-i. [DOI] [PubMed] [Google Scholar]
- Morgado E, Gordon MK, del Carmen Minana-Solis M, Meza E, Levine S, Escobar C, et al. Hormonal and metabolic rhythms associated with the daily scheduled nursing in rabbit pups. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2008;295:R690–R695. doi: 10.1152/ajpregu.00162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. International Journal of Developmental Neuroscience. 2004;22:415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on Mammalian neonatal sensitive-period learning. Behavioral Neuroscience. 2004a;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. Journal of Neuroscience. 2004b;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. Journal of Neuroscience. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morys J, Berdel B, Jagalska-Majewska H, Luczynska A. The basolateral amygdaloid complex—Its development, morphology and functions. Folia Morphologica (Warsz) 1999;58:29–46. [PubMed] [Google Scholar]
- Myslivecek J. Inhibitory learning and memory in newborn rats. Progress in Neurobiology. 1997;53:399–430. doi: 10.1016/s0301-0082(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Nair HP, Gonzalez-Lima F. Extinction of behavior in infant rats: Development of functional coupling between septal, hippocampal, and ventral tegmental regions. Journal of Neuroscience. 1999;19:8646–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Kimura F, Sakaguchi T. Postnatal development of electrical activity in the locus ceruleus. Journal of Neurophysiology. 1987;58:510–524. doi: 10.1152/jn.1987.58.3.510. [DOI] [PubMed] [Google Scholar]
- Okere CO, Kaba H. Increased expression of neuronal nitric oxide synthase mRNA in the accessory olfactory bulb during the formation of olfactory recognition memory in mice. European Journal of Neuroscience. 2000;12:4552–4556. doi: 10.1046/j.0953-816x.2000.01325.x. [DOI] [PubMed] [Google Scholar]
- Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Developmental Psychobiology. 1982;15:349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:329–341. [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. Journal of Neuroscience. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissonnier D, Thiery JC, Fabre-Nys C, Poindron P, Keverne EB. The importance of olfactory bulb noradrenalin for maternal recognition in sheep. Physiology & Behavior. 1985;35:361–363. doi: 10.1016/0031-9384(85)90309-9. [DOI] [PubMed] [Google Scholar]
- Polan HJ, Hofer MA. Maternally directed orienting behaviors of newborn rats. Developmental Psychobiology. 1999;34:269–279. doi: 10.1002/(sici)1098-2302(199905)34:2<269::aid-dev3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behavioral Neuroscience. 1997;111:503–511. [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: Similar behaviors but divergent ages of functional amygdala emergence. Learning and Memory. 2009;16:114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki DW, Lamb ME, Obmascher P. Toward a general theory of infantile attachment: A comparative review of aspects of the social bond. Behavioral and Brain Science. 1978;3:417–464. [Google Scholar]
- Roozendaal B, Carmi O, McGaugh JL. Adrenocortical suppression blocks the memory-enhancing effects of amphetamine and epinephrine. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1429–1433. doi: 10.1073/pnas.93.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic–pituitary–adrenal axis during development. Neuroscience Biobehavioral Review. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, van Eekelen JA, Levine S, de Kloet ER. Ontogeny of corticosteroid receptors in the brain. Cellular and Molecular Neurobiology. 1993;13:295–319. doi: 10.1007/BF00711575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Salzen EA. Imprinting and environmental learning. In: Aronson LR, Tobach E, Lehrman DS, Rosensbaltt J, editors. Development and evolution of behavior. San Francisco: W H Freeman; 1970. pp. 158–178. [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development & Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, et al. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Research Molecular Brain Research. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Levine S, Alam S, Harbich D, Sterlemann V, Ganea K, et al. Metabolic signals modulate hypothalamic–pituitary–adrenal axis activation during maternal separation of the neonatal mouse. Journal of Neuroendocrinology. 2006;18:865–874. doi: 10.1111/j.1365-2826.2006.01482.x. [DOI] [PubMed] [Google Scholar]
- Shair HN, Masmela JR, Brunelli SA, Hofer MA. Potentiation and inhibition of ultrasonic vocalization of rat pups: Regulation by social cues. Developmental Psychobiology. 1997;30:195–200. doi: 10.1002/(sici)1098-2302(199704)30:3<195::aid-dev2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Hormones and Behavior. 2007;52:391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: A cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Spear N. Processing memories: Forgetting and retention. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: Specific role of maternal cues. Developmental Psychobiology. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in pre-weanling rats. Developmental Psychobiology. 1987;20:131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ, Campbell BA. Habituation of the forelimb-withdrawal response in neonatal rats. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Stern JM. Offspring-induced nurturance: Animal–human parallels. Developmental Psychobiology. 1997;31:19–37. doi: 10.1002/(sici)1098-2302(199707)31:1<19::aid-dev3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic–pituitary–adrenal axis in the infant rat: The roles of feeding and stroking. Brain Research Developmental Brain Research. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hall WG. Reinforcers in infancy: Classical conditioning using stroking or intra-oral infusions of milk as UCS. Developmental Psychobiology. 1988;21:215–223. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neuroscience & Biobehavioral Reviews. 2010;34:835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behavioral Neuroscience. 2000;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Role of the amygdala complex in early olfactory associative learning. Behavioral Neuroscience. 1993;107:254–263. doi: 10.1037//0735-7044.107.2.254. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Molecular biology of early olfactory memory. Learning and Memory. 2003;10:1–4. doi: 10.1101/lm.58203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Lemon C, Gerhardt GA. Bilateral 6-OHDA lesions of the locus coeruleus impair associative olfactory learning in newborn rats. Brain Research. 1994;643:306–309. doi: 10.1016/0006-8993(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Research Developmental Brain Research. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Research. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Brain Research Developmental Brain Research. 1994;81:121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson DA. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Research. 2008;1200:58–65. doi: 10.1016/j.brainres.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsson E, James J. A meta-analysis of the effects of experimental manipulations of social support during laboratory stress. Psychology Health. 1999;14:869–886. [Google Scholar]
- Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Developmental Psychobiology. 2010;52:453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oers HJ, De Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. Journal of Neuroscience. 1998;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Widmaier EP. Glucose homeostasis and hypothalamic-pituitary-adrenocortical axis during development in rats. The American Journal of Physiology. 1990;259:E601–E613. doi: 10.1152/ajpendo.1990.259.5.E601. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behavioural Brain Research. 2001;126:147–157. doi: 10.1016/s0166-4328(01)00260-1. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Annals of the New York Academy of Sciences. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Age-specific threats induce CRF expression in the paraventricular nucleus of the hypothalamus and hippocampus of young rats. Hormones and Behavior. 2005;47:139–150. doi: 10.1016/j.yhbeh.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM, Leon M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output response patterns to learned attractive odors. Journal of Neuroscience. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Darby-King A, Neve RL, McLean JH. Early odor preference learning in the rat: Bidirectional effects of cAMP response element-binding protein (CREB) and mutant CREB support a causal role for phosphorylated CREB. Journal of Neuroscience. 2003;23:4760–4765. doi: 10.1523/JNEUROSCI.23-11-04760.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


