Summary
Background
The entity pseudoaneurysm arising from the mitral aortic intervalvular fibrosa (P-MAIVF) is a rare cardiac finding caused by multiple factors. This entity is usually diagnosed with echocardiography and confirmed with cardiac computed tomography (CT).
Case Report
We presented a case of congenital P-MAIVF communicating with the left atrium (LA) and an aberrant right subclavian artery, misdiagnosed as primary mitral regurgitation (MR) in transthoracic echocardiogram (TTE) due to relative contraindications to transesophageal echocardiogram (TEE), revealed in a hemophilic patient, and diagnosed with cardiac CT.
Conclusions
In conclusion, cardiac CT plays a definitive role not only in anatomical assessment and confirmation of the lesion but also in primary diagnostics in patients suspected of MAIVF – especially those with relative and absolute contraindications to TEE.
MeSH Keywords: Aneurysm, False; Congenital Abnormalities; Hemophilia A
Background
P-MAIVF is a unique and rare entity especially for a radiologist as it arises from a complex anatomical area in the aorto-mitral continuity region. P-MAIVF is caused by multiple factors grouped into surgical, infective, inflammatory and congenital ones [1,2]. Variable symptoms and dreaded complications are associated with an enlarging aneurysm [3]. The primary screening investigation is TTE and it is complemented by TEE for diagnostic purposes. Confirmation and anatomical assessment are carried out with the use of MDCT and Cardiac Magnetic Resonance Imaging (MRI), which is useful for surgical evaluation [4]. The recommended treatment for P-MAIVF is surgery in both asymptomatic and symptomatic patients [5].
Case Report
A 29-year-old hemophilic male patient was admitted to the cardiology unit of our hospital with complaints of New York Heart Association (NYHA) Grade II dyspnea for three years. There was no past surgical history. On auscultation there was holosystolic murmur with a diastolic component. A provisional diagnosis of mitral regurgitation was made. He had been investigated with TTE in various hospitals for his symptoms, with a consistent diagnosis of primary MR. There was no past history of any ischemic heart disease, rheumatic or infective heart condition. The patient was referred to us for cardiac CT in view of persistent symptoms.
Cardiac CT was performed with a Brilliance™ 64-slice scanner (Philips Medical Systems, Cleveland, Ohio, USA) using an adult cardiac CT protocol and retrospective electrocardiogram gating. A volume of 100 mL of iodinated contrast medium was administered, followed by saline solution. The images were processed on a dedicated Aquarius™ Workstation (TeraRecon, San Mateo, Calif.); reformatted MPR and MIP images in different planes were analyzed.
Cardiac CT revealed an out-pouching coming posteriorly from the mitral aortic intervalvular fibrosa (MAIVF). The apex of the aneurysm was separated from the ascending aorta by the pulmonary trunk. The left coronary artery was abutting the aneurysm anteriorly. The left atrium and the left ventricle were both enlarged and the aneurysm had a fistulous communication with the left atrium (Figure 1A, 1B). Calcification was also noted in the wall of the aneurysm. There was an associated aberrant right subclavian artery (Figure 2). The aortic valve and the membranous portion of the interventricular septum were intact, excluding the diagnosis of a sinus of Valsalva aneurysm or ventricular septal aneurysm, respectively. TTE was carried out retrospectively but due to the posterior location of the pseudoaneurysm and communication with LA, the condition was misdiagnosed as primary MR.
Figure 1.
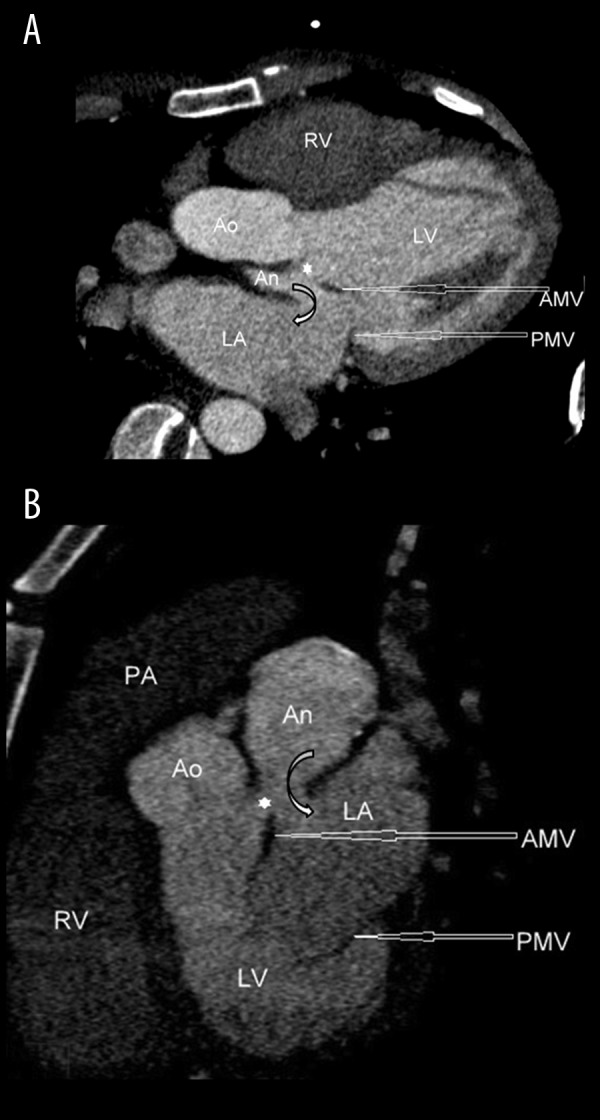
(A, B) ECG-gated contrast-enhanced cardiac CT reformatted images showing a pseudoaneurysm [An] arising from the mitral aortic intervalvular fibrosa [*] and having fistulous communication with the left atrium [LA]. LV indicates the left ventricle. RV indicates the right ventricle. PA indicates the pulmonary artery. Ao indicates the aorta. AMV and PMV indicate the anterior and posterior leaflet of the mitral valve, respectively.
Figure 2.
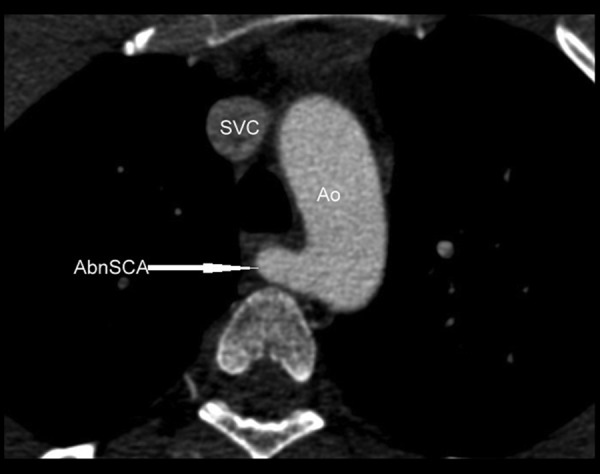
Contrast-enhanced cardiac CT image in the axial plane showing aberrant origin of the right subclavian artery [AbnSCA] from the aorta [Ao], having retrotracheal course. SVC indicates the superior vena cava. TR indicates the trachea.
The absence of spikes of fever, negative blood cultures, normal C-reactive protein level and negative Anti-streptolysin O antibody test result ruled out infective endocarditis as an aetiological factor. Normal erythrocyte sedimentation rate and aortogram also ruled out the inflammatory cause such as Takayasu’s arteritis, which is a possible etiological factor of psuedoaneurysms.
The patient was subsequently operated on and the findings of cardiac CT were confirmed. The histopathological report revealed the fibrocollagenous nature of the wall along with calcifications suggestive of a pseudoaneurysm. No signs of inflammatory activity were noted. Culture of the excised tissue did not yield any growth. Thus, a final diagnosis of congenital pseudoaneurysm of MAIFV communicating with LA along with an aberrant right subclavian artery was made.
Discussion
Congenital subaortic aneurysms are rare. They were initially described in the African population and subsequently reported on in other racial groups. Many of the subaortic aneurysms arise from MAIVF. A comprehensive review study of articles published between 1966–2009 revealed 90 patients with a psuedoaneurysm of MAIVF, out of which 9 cases had communication of the psuedoaneurysm with LA [1].
Left and noncoronary leaflets of the aortic valve share fibrous continuity with the anterior/aortic leaflet of the mitral valve. The thick ends of this fibrous area continue with the ventricular musculature on both sides and are called right and left fibrous trigones. The MAIVF is a central triangular area bounded by these right and left trigones [6].
The causes of P-MAIVF are grouped into surgical, infective, inflammatory and congenital. The most common etiology is the aortic valve surgery and infective endocarditis [1]. Subaortic aneurysms have also been associated with tuberculosis, syphilis, rheumatic carditis and Takayasu’s arteritis [7]. Some of these lesions are thought to be congenital in origin [2]. Being a relatively avascular area, it is prone to weakening and abscess collection after aortic [8,9] and, less commonly, mitral valve surgeries [10,11].
Infective endocarditis of the aortic valve leads to infection of MAIVF. Infection of this area leads to the formation of a subaortic abscess or pseudoaneurysm of the left ventricular outflow tract (LVOT). Therefore, a psuedoaneurysm can rupture in the left atrium due to systolic jet from the left ventricular outflow tract [12].
A patient can be asymptomatic or present with dyspnea, infective symptoms (due to endocarditis), angina (due to compression of coronary arteries) and congestive cardiac failure. Various complications of pseudoaneurysms of MAIVF have been reported [1]. Enlargement of a pseudoaneurysm may lead to compression of adjacent structures including the left atrium, coronary arteries [3], and the pulmonary artery. Proximity to the left atrium and aorta may result in fistulous communications with these structures (Figure 3). Other reported complications include rupture into the pericardial space [13]. Mitral annulus dysfunction may also lead to functional mitral regurgitation [14].
Figure 3.
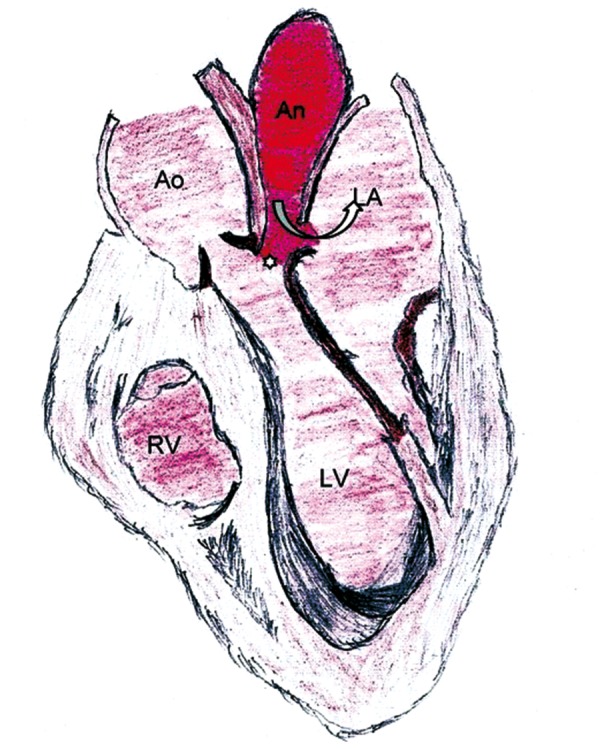
A schematic diagram showing a pseudoaneurysm [An] arising from the mitral aortic intervalvular fibrosa [*], having fistulous communication [curved arrow] with the left atrium [LA]. Ao indicates the aorta. LV indicates the left ventricle. RV indicates the right ventricle.
The first screening investigation to start with is TTE. However, due to posterior location of pseudoaneurysms, TEE is preferred, and communication with LA can mislead the diagnosis to primary MR in TTE, as in our case [1]. A golden standard to confirm the diagnosis is catheter angiography. With an advent of MDCT and cardiac MRI, invasive catheterization is unnecessary. CT angiography has the advantage of simultaneous evaluation of valvular structures, coronary arteries, thrombi, and of a precise location of a pseudoaneurysm with regard to other cardiac chambers, aortic root and pulmonary artery. Transesophageal echo has been used extensively to demonstrate psuedoaneurysms of MAIVF. However, to the best of our knowledge, the use of cardiac CT to delineate the anatomy of P-MAIVF is rare; only a few cases have been reported on with the use of CT [4,9,11,15–19].
The recommended treatment for P-MAIVF is surgery in both asymptomatic and symptomatic patients. The patch repair in P-MAIVF can be carried out with or without aortic root reconstruction. Percutaneous closure of a psuedoaneurysm can also be performed in a candidate surgically not suitable [5].
We presented this unusual and rare case of P-MAIVF communicating with LA (which is never reported on in literature on cardiac CT imaging), with an aberrant right subclavian artery, undiagnosed for several years. Our patient, due to coagulopathy, never underwent TEE, and was diagnosed as primary MR on TTE for years. Psuedoaneurysm of MAIVF is a rare cardiac finding caused by multiple factors and is diagnosed with echocardiography and confirmed with cardiac CT.
Conclusions
We presented a case of a hemophilic patient with a congenital psuedoaneurysm of MAIVF communicating with LA and an aberrant right subclavian artery, diagnosed with the use of cardiac CT, initially misdiagnosed as primary MR on TTE due to relative contraindications to TEE. Cardiac CT plays a definitive role not only in anatomical assessment and confirmation of the lesion type but also in primary diagnostics in patients suspected of MAIVF, especially those with relative and absolute contraindications to TEE.
References
- 1.Sudhakar S, Sewani A, Agrawal M, et al. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF): A comprehensive review. J Am Soc Echocardiogr. 2010;23(10):1009–18. doi: 10.1016/j.echo.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Sivasankaran S, Kannan BR, Kumar A, et al. Coexistence of congenital subaortic and sinus of valsalva aneurysms. Indian Heart J. 2002;54:432–34. [PubMed] [Google Scholar]
- 3.Bier AJ, Lamphere JA, Daily PO. Coronary artery compression caused by a large pseudoaneurysm of the mitral-aortic intervalvular fibrosa. J Am Soc Echocardiogr. 1995;8(5 Pt 1):753–56. doi: 10.1016/s0894-7317(05)80394-3. [DOI] [PubMed] [Google Scholar]
- 4.Ghersin E, Litmanovich D, Agmon Y, et al. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa following aortic valve replacement – diagnosis and dynamic evaluation with multidetector CT and transesophageal echocardiography. Interact Cardiovasc Thorac Surg. 2005;4(6):502–4. doi: 10.1510/icvts.2005.112607. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez Valero S, García E, González Pinto A, et al. [Percutaneous closure of pseudoaneurysm of the mitral-aortic intervalvular fibrosa]. Rev Esp Cardiol. 2005;58(12):1473–75. [in Spanish] [PubMed] [Google Scholar]
- 6.Joshi SS, Jagadeesh AM, Furtado A, et al. Transesophageal echocardiography in surgical management of pseudoaneurysm of mitral-aortic intervalvular fibrosa with aneurysms of right sinus of Valsalva and left main coronary artery. Ann Card Anaesth. 2013;16:40–43. doi: 10.4103/0971-9784.105368. [DOI] [PubMed] [Google Scholar]
- 7.Tufekcioglu O, Ozlu MF, Cay S, et al. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa in a patient with Takayasu’s arteritis. Can J Cardiol. 2008;24:718. doi: 10.1016/s0828-282x(08)70675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyagi S, Fukunaga S, Otsuka H, et al. Left ventricular outflow tract pseudoaneurysm after aortic valve replacement: case report. J Heart Valve Dis. 2004;13(1):145–48. [PubMed] [Google Scholar]
- 9.Entrikin DW, Shroff GS, Kon ND, et al. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa: a delayed complication of aortic root replacement. J Cardiovasc Comput Tomogr. 2011;5(5):333–35. doi: 10.1016/j.jcct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Namboodiri N, Dora SK, Thomas B, et al. Subannular left ventricular pseudoaneurysm following mitral valve replacement. J Cardiothorac Surg. 2008;3:28. doi: 10.1186/1749-8090-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choh NA, Shaheen F, Rather H, et al. Pseudoaneurysm of mitral-aortic intervalvular fibrosa in a child: Demonstration by MDCT and MRI. Ann Pediatr Cardiol. 2013;6(1):80–82. doi: 10.4103/0974-2069.107242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal RC, Graham BM, Jutzy KR, et al. Left ventricular outflow tract to left atrial communication secondary to rupture of mitral-aortic intervalvular fibrosa in infective endocarditis: diagnosis by transesophageal echocardiography and color flow imaging. J Am Coll Cardiol. 1990;15(2):499–504. doi: 10.1016/s0735-1097(10)80082-8. [DOI] [PubMed] [Google Scholar]
- 13.Qizilbash AH, Schwartz CJ. False aneurysm of left ventricle due to perforation of mitral-aortic intervalvular fibrosa with rupture and cardiac tamponade. Rare complication of infective endocarditis. Am J Cardiol. 1973;32(1):110–13. doi: 10.1016/s0002-9149(73)80095-5. [DOI] [PubMed] [Google Scholar]
- 14.Espinosa-Caliani JS, Montijano A, MarõÂa Melero JA, et al. Pseudoaneurysm in the mitral-aortic intervalvular Fibrosa.-A cause of mitral regurgitation. Eur J Cardiothorac Surg. 2000;17(6):757–59. doi: 10.1016/s1010-7940(00)00348-1. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama Y, Tamaki S, Kato N, et al. Pseudoaneurysm from the mitral-aortic intervalvular fibrosa following endocarditis. Jpn J Thorac Cardiovasc Surg. 2003;51(8):374–77. doi: 10.1007/BF02719470. [DOI] [PubMed] [Google Scholar]
- 16.Linhartová K, Veselka J, Adla T. Left ventricular pseudoaneurysm as a late complication of mitral annuloplasty. Eur Heart J. 2007;28(19):2360. doi: 10.1093/eurheartj/ehm120. [DOI] [PubMed] [Google Scholar]
- 17.Berrizbeitia LD, Anderson WA. Ultrafast computed tomography in infectious pseudoaneurysm of the left ventricular outflow tract. J Thorac Cardiovasc Surg. 1997;114(1):138–39. doi: 10.1016/S0022-5223(97)70130-2. [DOI] [PubMed] [Google Scholar]
- 18.Acioli Pereira L, Fontes Gontijo P, Alcântara Farran J, et al. Giant pseudoaneurysm of the left ventricular outflow tract: a rare disease. Rev Port Cardiol. 2013;32(6):541–44. doi: 10.1016/j.repc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Tsai IC, Fu YC, Lin PC, et al. MDCT evaluation of congenital mitral-aortic intervalvular fibrosa aneurysm: implications for the aetiology and differential diagnosis. Pediatr Radiol. 2009;39(1):80–83. doi: 10.1007/s00247-008-1029-0. [DOI] [PubMed] [Google Scholar]


