Abstract
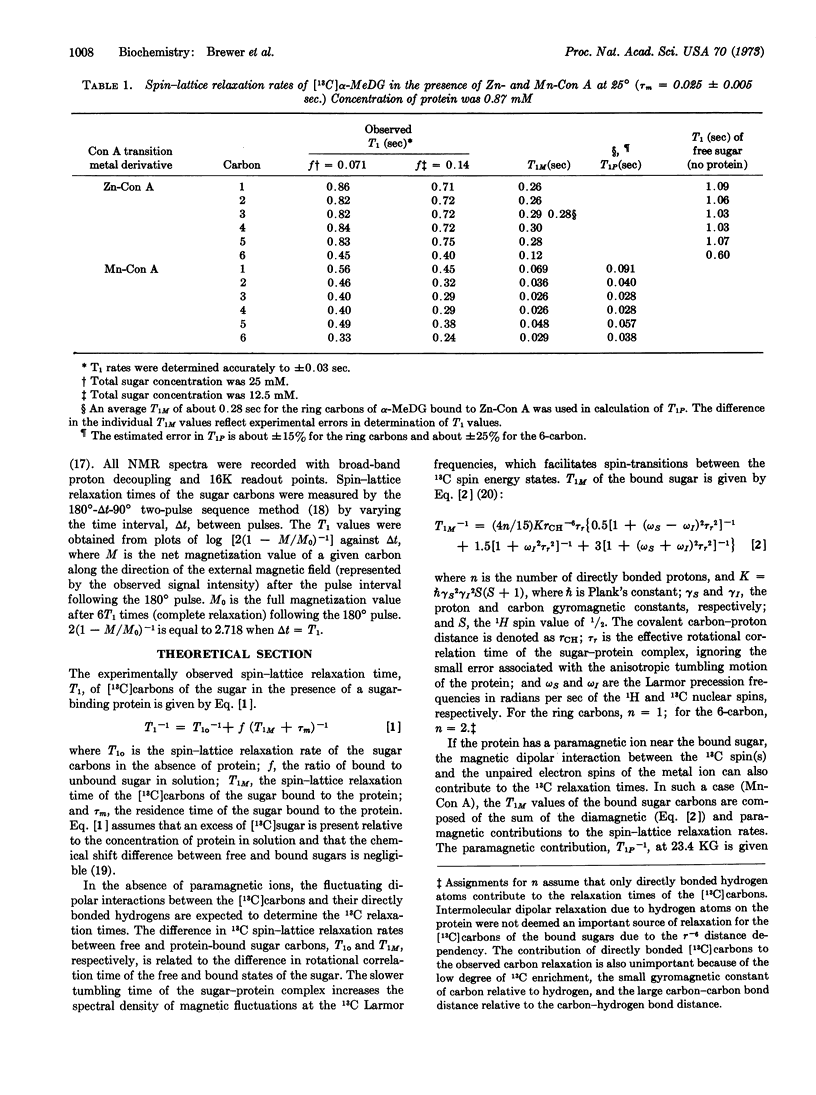
Binding of α-methyl-D-glucopyranoside, uniformly enriched with 14% 13C, to zinc and manganese derivatives of concanavalin A at pH 5.6 was studied by pulsed Fourier transform carbon magnetic resonance techniques. Spin-lattice relaxation (T1) of the [13C]carbons of the sugar was measured in the absence and presence of the two transition metal derivatives of the protein. In the presence of the manganese derivative of concanavalin A, selective relaxation of the sugar carbons was observed. The values for T1 reflect different distances between the carbons of the bound sugar and the manganese ion. Calculation of the distance between the manganese ion and each carbon of the sugar permit the 3-dimensional orientation of the bound sugar to be determined relative to the transition metal site in the protein. The results indicate that α-methyl-D-glucopyranoside binds to the protein in the Cl chair conformation with its 3- and 4- carbons closest to the manganese ion at a mean distance of 10 Å.
Keywords: carbon relaxation, metalloprotein, binding orientation
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Physical and chemical characterization of concanavalin A, the hemagglutinin from jack bean (Canavalia ensiformis). Biochim Biophys Acta. 1967 Feb 21;133(2):376–379. doi: 10.1016/0005-2795(67)90081-5. [DOI] [PubMed] [Google Scholar]
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interactin. XV. The role of bivalent cations in concanavalin A-polysaccharide interaction. Can J Biochem. 1968 Sep;46(9):1147–1150. doi: 10.1139/o68-170. [DOI] [PubMed] [Google Scholar]
- Bennick A., Campbell I. D., Dwek R. A., Price N. C., Radda G. K., Salmon A. G. Relationship between conformationally sensitive probe binding sites on phosphorylase b. Nat New Biol. 1971 Dec 1;234(48):140–143. doi: 10.1038/newbio234140a0. [DOI] [PubMed] [Google Scholar]
- Butchard C. G., Dwek R. A., Kent P. W., Williams J. P., Xavier A. V. A structural study by 19 F-nuclear-magnetic resonance of the binding of sugars to lysozyme. Eur J Biochem. 1972 Jun 9;27(3):548–553. doi: 10.1111/j.1432-1033.1972.tb01871.x. [DOI] [PubMed] [Google Scholar]
- CONCHIE J., LEVVY G. A., MARSH C. A. Methyl and phenyl glycosides of the common sugars. Adv Carbohydr Chem. 1957;12:157–187. doi: 10.1016/s0096-5332(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Levitzki A. Metal-binding sites of concanavalin A and their role in the binding of alpha-methyl d-glucopyranoside. Biochem J. 1968 Oct;109(4):669–672. doi: 10.1042/bj1090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Lustig A. The molecular weight of concanavalin A. Biochim Biophys Acta. 1968 Oct 21;168(2):366–367. doi: 10.1016/0005-2795(68)90161-x. [DOI] [PubMed] [Google Scholar]
- Mildvan A. S., Cohn M. Aspects of enzyme mechanisms studies by nuclear spin relazation induced by paramagnetic probes. Adv Enzymol Relat Areas Mol Biol. 1970;33:1–70. doi: 10.1002/9780470122785.ch1. [DOI] [PubMed] [Google Scholar]
- Reed G. H., Cohn M. Electron paramagnetic resonance spectra of manganese (II)-protein complexes. Manganese (II)-concanavalin A. J Biol Chem. 1970 Feb 10;245(3):662–664. [PubMed] [Google Scholar]
- Shoham J., Inbar M., Sachs L. Differential toxicity on normal and transformed cells in vitro and inhibition of tumour development in vivo by concanavalin A. Nature. 1970 Sep 19;227(5264):1244–1246. doi: 10.1038/2271244a0. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Sternlicht H., Wyluda B. J. Study of metal-ion binding to nucleic acids by 31-P nuclear magnetic resonance. J Chem Phys. 1965 Nov 1;43(9):3116–3122. doi: 10.1063/1.1697285. [DOI] [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]