Abstract
Background
Prior studies on intake of linoleic acid (LA), the predominant n-6 fatty acid, and coronary heart disease (CHD) risk have generated inconsistent results. We performed a systematic review and meta-analysis of prospective cohort studies to summarize the evidence regarding the relation of dietary LA intake and CHD risk.
Methods and Results
We searched MEDLINE and EMBASE databases through June, 2013 for prospective cohort studies that reported the association between dietary LA and CHD events. In addition, we utilized unpublished data from cohort studies in a previous pooling project. We pooled the multivariate-adjusted relative risk (RR) comparing the highest with the lowest categories of LA intake using fixed-effect meta-analysis. We identified 13 published and unpublished cohort studies with a total of 310,602 individuals and 12,479 total CHD events including 5,882 CHD deaths. Comparing the highest to the lowest category, dietary LA was associated with a 15% lower risk of CHD events (pooled RR, 0.85; 95% confidence intervals (95% CI): 0.78–0.92; I2=35.5%) and a 21% lower risk of CHD deaths (pooled RR, 0.79; 95% CI, 0.71–0.89; I2=0.0%). A 5% of energy increment in LA intake replacing energy from saturated fat intake was associated with a 9% lower risk of CHD events (RR, 0.91; 95% CI, 0.86–0.96) and a 13% lower risk of CHD deaths (RR, 0.87; 95% CI, 0.82–0.94).
Conclusion
In prospective observational studies, dietary LA intake is inversely associated with CHD risk in a dose-response manner. These data provide support for current recommendations to replace saturated fat with polyunsaturated fat for primary prevention of CHD.
Keywords: Linoleic acid, coronary heart disease, meta-analysis
INTRODUCTION
The effects of dietary fat intake on primary prevention of coronary heart disease (CHD) have been a long standing interest.1 Greater intakes of trans-fat and saturated fat in comparison to polyunsaturated fatty acids (PUFAs) are associated with increased risk of CHD.2 Therefore, substitution of PUFAs for saturated fatty acids (SFAs) has been recommended to reduce CHD risk.3,4 Previous studies have investigated the effects of n-3 PUFAs (including the long-chain n-3 and alpha-linolenic acid) on CHD risk,5,6 but the relation between intake of n-6 PUFAs and risk of CHD has been less extensively studied.
Some observational studies and many controlled feeding studies have documented that linoleic acid (LA), the predominant n-6 PUFA in the Western diet and primarily from vegetable oils and nuts (e.g. sunflower, safflower, soya, corn and walnuts), reduces major risk factors for CHD. Higher LA intake reduces LDL cholesterol,7–10 promotes insulin sensitivity,7,11 and reduces risk of hypertension.12,13 Therefore, substitution of dietary n-6 PUFAs for SFAs has long been recommended to prevent CHD.1 However, concerns have been raised about higher LA consumption being harmful for heart health because of potential pro-inflammatory and thrombogenic properties.14–17 LA can be elongated to arachidonic acid (AA) and subsequently synthesized to a variety of pro-inflammatory eicosanoids, which may increase CHD risk.18–20 However, this speculation is not supported by randomized controlled feeding studies, in which dietary intake of LA was not found to increase inflammatory markers including C-reactive protein, cytokines, fibrinogen, soluble vascular adhesion molecules, plasminogen activator inhibitor type 1, or tumor necrosis factor-α.21
Recently, a secondary analysis of a small clinical trial conducted in the 1960s suggested that substituting high-LA safflower oil for saturated fat increased cardiovascular mortality.17 In addition, the authors conducted an updated meta-analysis of LA intervention trials (high LA oils without n-3 fatty acids such as corn oil and safflower oil), which showed non-significant trends toward increased risks of CHD death, whereas the trials using high LA oils with n-3 fatty acids (e.g., soybean oil) showed opposite trends.17 However, these trials were small with limited power, and most of them were conducted in patients with existing heart diseases. More recently, Chowdhury et al.22 reported no significant association between n-6 PUFAs and coronary events in a meta-analysis, and concluded that the evidence did not support current recommendations to replace SFAs with PUFAs. However, this analysis was based on a limited number of studies with some erroneous data. Moreover, the meta-analysis could not compare LA with saturated fat or any other specific macronutrient (annals.org.ezp-prod1.hul.harvard.edu/article.aspx?articleid=1846638 comments). To address the role of LA in primary prevention of CHD using published data on LA and CHD events as well as data from unpublished cohort studies, we performed a systematic review and meta-analysis of prospective cohort studies to examine the association between dietary LA intake and CHD endpoints in generally healthy populations.
SUBJECTS AND METHODS
Study Strategy
We followed the checklist of Meta-analysis Of Observational Studies in Epidemiology (MOOSE) for background, design, analysis and interpretation.23 We conducted a systematic literature search of two databases, MEDLINE and EMBASE, related articles, hand-searching of key journals and references and direct author contacts for all cohort studies describing the association of dietary LA intake with incident CHD outcomes, which include myocardial infarction (MI), ischemic heart disease, coronary artery bypass graft, sudden cardiac arrest, acute coronary syndrome and CHD deaths. Our search terms combined the exposure (LA) with various CHD outcomes, and the full details on the search strategy are presented in the Supplemental Material. Searches included the earliest available online indexing year through to June 30, 2013.
Selection of Articles
The titles and abstracts of the identified studies were screened by one investigator (M.S.F.) for potentially relevant articles. Two investigators (M.S.F. and M.D.) independently assessed the full-text of those selected articles to determine relevant articles for inclusion; any discrepancies were resolved by consultation with the third investigator (F.B.H.). Studies were included if they were prospective cohort studies that provided multivariate-adjusted risk estimates [relative risk (RR) or hazard ratio (HR)] for dietary LA consumption as the exposure and CHD endpoints. We excluded retrospective, cross-sectional or ecological studies, studies in non-adults (<19 years old), non-original papers (reviews, editorials, or letters), meeting abstracts and duplicated publications. We also excluded studies conducted in patients with known CHD at baseline. For multiple manuscripts that published from the same cohort, the most up-to-date analyses with highest number of outcomes were included in the meta-analysis.
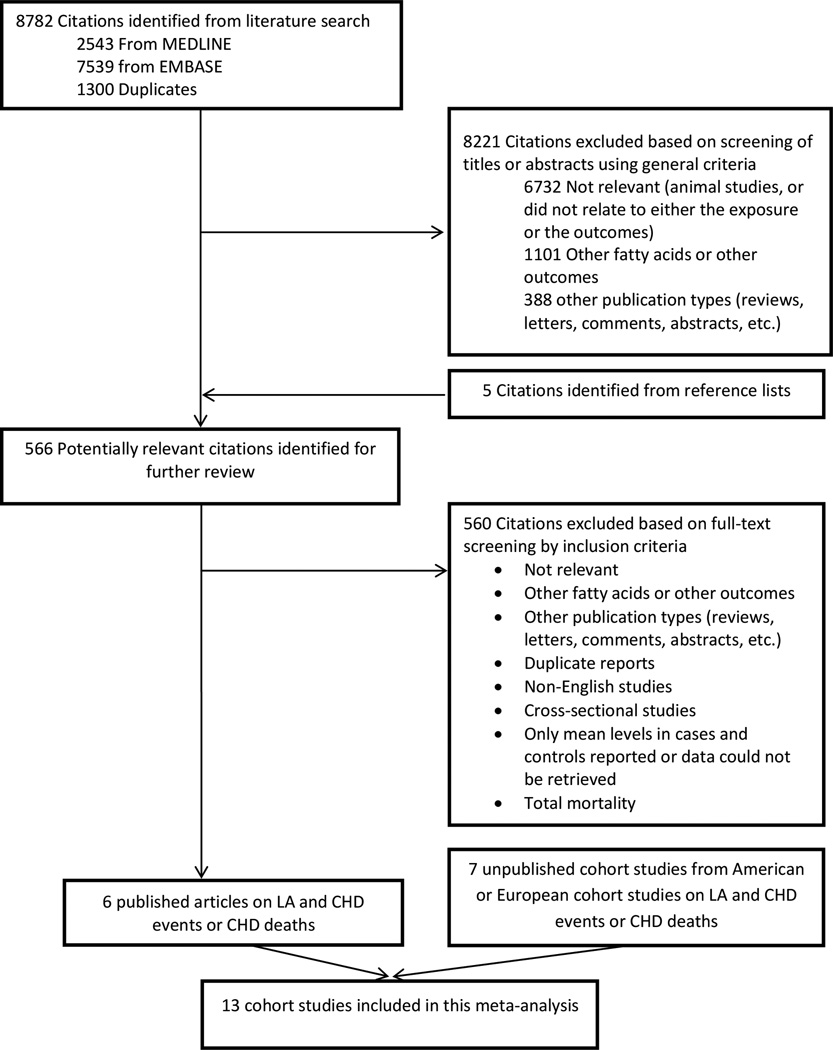
The search strategy identified 8782 unique citations (Figure 1). After screening the titles and abstracts, 566 full-text articles were evaluated which 6 original articles were identified as being appropriate for inclusion in this meta-analysis.24–29 We also obtained permission from principal investigators of the cohort studies presented in the Pooling Project of Cohort Studies on Diet and Coronary Disease,4 to examine the association between LA intake and CHD risk and included these results in the meta-analysis. The characteristics of these cohort studies in the pooling project were described elsewhere in detail.4 Among 11 cohort studies included in Pooling Project of Cohort Studies on Diet and Coronary Disease, we used data of the 6 cohort studies [Atherosclerosis Risk in Communities Study (ARIC); Finnish Mobile Clinic Health Study (FMC); Israeli Ischemic Heart Disease Study (IIHD); Iowa Women’s Health Study (IWHS); Västerbotten Intervention Program (VIP) and Women's Health Study (WHS)], to assess the association between LA and total CHD and CHD deaths.4 The results for associations of LA and CHD events have been previously published in Nurses’ Health Study (NHS), Health Professional Follow-up Study (HPFS) and Alpha-Tocopherol and Beta-Carotene Cancer Prevention Study (ATBC).26–28 Furthermore, the association between dietary n-6 fatty acid and coronary events was evaluated in 8139 men and 12,535 women in the Malmo Diet and Cancer Cohort study.30 The associations between LA and CHD events have been provided by the study investigators through personal correspondence. In addition, we included the results of three other previously published studies.24,25,29 In the NHS and HPFS, we updated the published analyses with longer follow-up: from 20 years in previous publications to 30 years in the NHS, and from 6 years to 24 years in the HPFS. In addition, we reanalyzed data in ATBC study to adjust for the confounding variables similar to other included cohort studies in this meta-analysis (Table 1). In studies where the results on LA were not published before or results were updated in this meta-analysis, the relative risks (RRs) with 95% confidence intervals (95% CIs) for the incidence of CHD events were calculated by Cox proportional hazards regression models with time in study (years) as the time metric. The multivariate model included total energy, age, smoking, body mass index, physical activity, education level, alcohol intake, hypertension, fiber intake, and percent of energy from SFAs, trans fat, monounsaturated fatty acids (MUFAs), alpha-linolenic acid (ALA), PUFAs other than LA and ALA, and protein intake. Due to lack of data on ALA and trans fat intakes in IIHD and MDC studies, we adjusted for PUFAs other than LA. In this model, the relative risk for LA represents its substitution for the same percent of energy from carbohydrate.31 We also estimated HRs of CHD for the substitution of SFAs by LA. To do this, we adjusted for percent of energy from carbohydrates instead of SFAs in the above multivariate model. The analyses were performed by SAS version 9.3 (SAS Institute Inc., Cary, NC).
Figure 1.
Search, screening and selection process of prospective cohort studies of dietary linoleic acid and risk of coronary heart disease.
Table 1.
Characteristics of cohort studies that evaluated dietary LA intake and incidence of coronary heart disease events.
| Author and year |
Study name and country |
Follow- up |
Total No. of participants (No. of cases) |
Disease outcome |
Number of CHD events |
Gender | Baseline age |
LA measures |
Adjustment | |
|---|---|---|---|---|---|---|---|---|---|---|
| CHD events |
CHD deaths |
|||||||||
| Vedtofte et al, 2011 (24) |
MONICA/Denmark | 23.3 | M: 1643 F: 1643 |
Ischemic heart disease (total) |
M: 312 F: 159 |
- | M/F | 50.6 (30.9– 60.8) |
diet/7-day weight food record |
Age, smoking, education, family history of MI, SBP, alcohol use, other PUFA, energy, physical activity, BMI |
| de Goede et al, 2012 (25) |
MORGEN/Netherlands | 10 | 20,000 | Total CHD | 280 | - | M&F | 41.5 (20– 65) |
FFQ | Age, sex, total energy, smoking, BMI, education level, parental history of MI, alcohol, dietary fiber, protein, SFA, MUFA, trans fat and PUFA other than LA |
| Oh et al, 2005 (26) (updated) |
HS/US |
30 | 84,564 | Total CHD/CHD deaths |
3776 | 1111 | F | 42.5 (30– 55) |
FFQ | Age, smoking, BMI, menopausal status, hormone therapy, physical activity, alcohol, total energy, percent of energy from protein, SFA (or carbohydrate), MUFA, ALA, PUFA other than LA and ALA, trans, fiber intake, cholesterol intake, HTN, family history of MI. |
| Ascherio et al, 1996 (27) (updated) |
HPFS/US | 24 | 41,082 | Total CHD/CHD deaths |
3842 | 1971 | M | 42.5 (40– 75) |
FFQ | Age, smoking, BMI, physical activity, alcohol, total energy, percent of energy from protein, SFA (or carbohydrate), MUFA, ALA, PUFA other than LA and ALA and trans, fiber intake, cholesterol intake, HTN, family history f MI. |
| Pietinen et al, 1997 (28) |
ATBC/Finland | 6.1 | 21,930 | Total CHD/CHD deaths |
1339 | 534 | M | 59.5 (50– 69) |
FFQ | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFA (or carbohydrate), MUFA, ALA, PUFA other than LA and ALA and trans, fiber intake, cholesterol intake, HTN, education level. |
| Pooling Project of Cohort Studies on Diet and Coronary Disease (4) |
ARIC/US | 9.2 | M: 5240 F: 6481 |
Total CHD/CHD death |
M:269 F:123 |
M:51 F*:- |
M/F | M: 54 (47–63) F:35 (47– 62) |
FFQ | |
| FMC/Finland | 10 | M:2712 F: 2481 |
Total CHD/ CHD deaths |
M:322 F:162 |
M:147 F:48 |
M/F | M:47 (37–63) F: 49 (38–65) |
DH | ||
| WHS/US | 5.3 | 37,372 | Total CHD/CHD deaths |
152 | 10 | F | 52 (46– 64) |
FFQ | ||
| VIP/Sweden | 10 | M: 9521 F: 10,555 |
Total CHD/CHD deaths |
M:134 F:23 |
M:38 F*:- |
M/F | M: 50 (40–60) F: 50 (40–60) |
FFQ | ||
| IWHS/US | 10 | 30,180 | CHD deaths |
- | 294 | F | 61 (56– 67) |
FFQ | ||
| IIHD/Israel | 10 | 8272 | CHD deaths |
- | 165 | M | 48 (41–59) | FFQ | Age, smoking, BMI, physical activity, alcohol intake, total energy, percent of energy from protein, SFA (or carbohydrate), MUFA, PUFA other than LA, fiber intake, cholesterol intake, HTN, education level. |
|
| Dolecek, 1992 (29) | MRFIT/US | 10.5 | 6250 | CHD deaths |
- | 175 | M | 35–57 | diet/24-hr recall |
Age, race, smoking, baseline SBP, HDL, LDL, alcohol |
ALA, alpha-linolenic acid; ARIC, Atherosclerosis Risk in Communities Study; ATBC, Alpha-Tocopherol and Beta-Carotene Cancer Prevention; BMI, body mass index; CHD, coronary heart disease; DH, dietary history; FFQ: food-frequency questionnaire; FMC, Finnish Mobile Clinic Health Study; HDL, high-density lipoprotein; HPFS, Health Professional s’ Follow-up Study; HTN, hypertension; IIHD, Israeli Ischemic Heart Disease Study; IWHS, Iowa Women’s Health Study; LA, linoleic acid; LDL, low-density lipoprotein; MI, myocardial infarction; MONICA, Multinational Monitoring of Trends and Determinations in Cardiovascular Disease; MORGEN, Monitoring Project on Risk Factors for Chronic Diseases; MRFIT, Multiple Risk Factor Intervention Trial; MUFA, mono-unsaturated fatty acid; MV, multivitamin; NHS, Nurses’ Health Study; PUFA, polyunsaturated fatty acid; SBP, systolic blood pressure; SFA, saturated fatty acid; VIP, Västerbotten Intervention Program; WHS, Women’s Health Study.
Insufficient events for analysis of CHD deaths among women.
Data extraction
Two investigators (M.S.F. and M.D.) independently extracted information on study characteristics (author, study name, country, and publication year), duration of follow-up (mean, median or maximum number of follow-up), sample size, CHD outcomes (specific endpoints), gender, subject age (mean or median), methods for assessing LA consumption, covariates in the statistical models, multivariable-adjusted risk estimates and precision (e.g., 95% CIs, SEs, or P values). When more than one multivariable model was assessed or one article reported multiple RRs for different coronary outcomes, we extracted the RRs for the most specific coronary outcome event (according to the hierarchy: total CHD, CHD deaths, nonfatal CHD) with the largest number of adjustment variables.
Data synthesis
The included studies reported RRs or Hazard Ratios (HRs) of CHD events by categories of dietary LA intake, and RRs are assumed to be unbiased estimates of HRs when events are rare. Studies reported risk estimates based on various categories of LA intake (eg, tertiles, quartiles or quintiles), and we decided to use the RRs comparing the highest versus lowest category. For a study29 that reported RRs without corresponding 95% CIs, standard errors for the RRs were estimated from SE=β/Zp where β is the regression coefficient for 1% increment of energy from LA and Zp is the value of a unit-normal test statistic corresponding to the p value.32
Forest plots were used to evaluate RRs and corresponding 95% CIs for specific studies. Overall RRs were calculated using fixed-effect models (Mantel-Haenszel method), and we also calculated random-effects models (DerSimonian and Laird method) as sensitivity analysis. Potential heterogeneity among studies was assessed using the I2 statistic, and the heterogeneity was further explored using stratified analysis and meta-regression method. Sources of heterogeneity included duration of follow-up (< or ≥ median follow-up years of all cohorts), age (< or ≥ median baseline age of all cohorts), sex (male, female, or both), repeated measures of intake and study quality score. The possibility of publication bias was evaluated by visual inspection of a funnel plot and the Begg's test.
Potential nonlinear relations were examined using restricted cubic spline models with 3 knots at the 25th, 50th and 75th percentiles. We also carried out a dose-response meta-analysis using generalized least-squares regression (2-stage GLST in Stata).33 Using information on risk estimate, standard error, median of LA intake, number of cases, person-year of follow-up or number of subjects from all exposure categories, the log-linear dose-response slope within each study was estimated and then pooled to derive an overall risk estimate. One study29 already reported risk estimates for linear differences in exposure, and data were added to the GLST model at the second stage. All analyses were performed using Stata version 12.0 (STATA Corp, College Station, TX), with a 2-tailed α of 0.05 as statistical significance.
RESULTS
Study characteristics
The characteristics of the 13 identified cohort studies are shown in Table 1. Three cohort studies (IWHS, IIHD and Multiple Risk Factor Intervention Trial (MRFIT)) did not report results for total CHD and four studies (Multinational Monitoring of Trends and Determinations in Cardiovascular Disease (MONICA), ARIC, FMC and VIP) reported results separately for men and women, thus 14 estimates were available for total CHD. Two studies (MONICA and Monitoring Project on Risk Factors for Chronic Diseases (MORGEN)) did not report results for CHD deaths and one study (FMC) reported results separately for men and women, thus 12 estimates were available for CHD deaths. The numbers of participants in each study ranged from 1643 to 84,564, with follow-up durations ranging from 5.3 to 30 years, comprising a total of 310,602 individuals and 12,479 cases of total CHD events and 5,882 CHD deaths. LA consumption varied substantially across studies, with the median intakes across studies ranging from 1.5 to 6.4 percent of energy (10th vs 90th percent range from 1.1 to 9.5 percent of energy) (Supplemental Tables S1–S4). Except for one study conducted in Israel, all other studies were from the North American (n =6) or European countries (n = 6). In all cohort studies, except for two studies (MONICA and MRFIT),24, 29 the RRs for LA can be interpreted as effects of substituting percent of energy intake from LA for the same percent of energy intake from carbohydrates. We were able to estimate the effects of substituting percent of energy intake from LA for the same amount of energy from SFAs for all cohort studies except for MONICA, MORGEN and MRFIT.24, 25, 29
For unpublished and updated cohort studies, the RRs and 95% CIs across the quintiles of LA intake and total CHD and CHD deaths are shown in the Supplemental Tables S1–S4. Due to the small number of CHD events in ARIC study in women, FMC study in women, and VIP study in men and women and small number of CHD death in FMC study in women, WHS study, and VIP study in men, the RRs and 95% CIs across the tertiles of LA intake were provided.
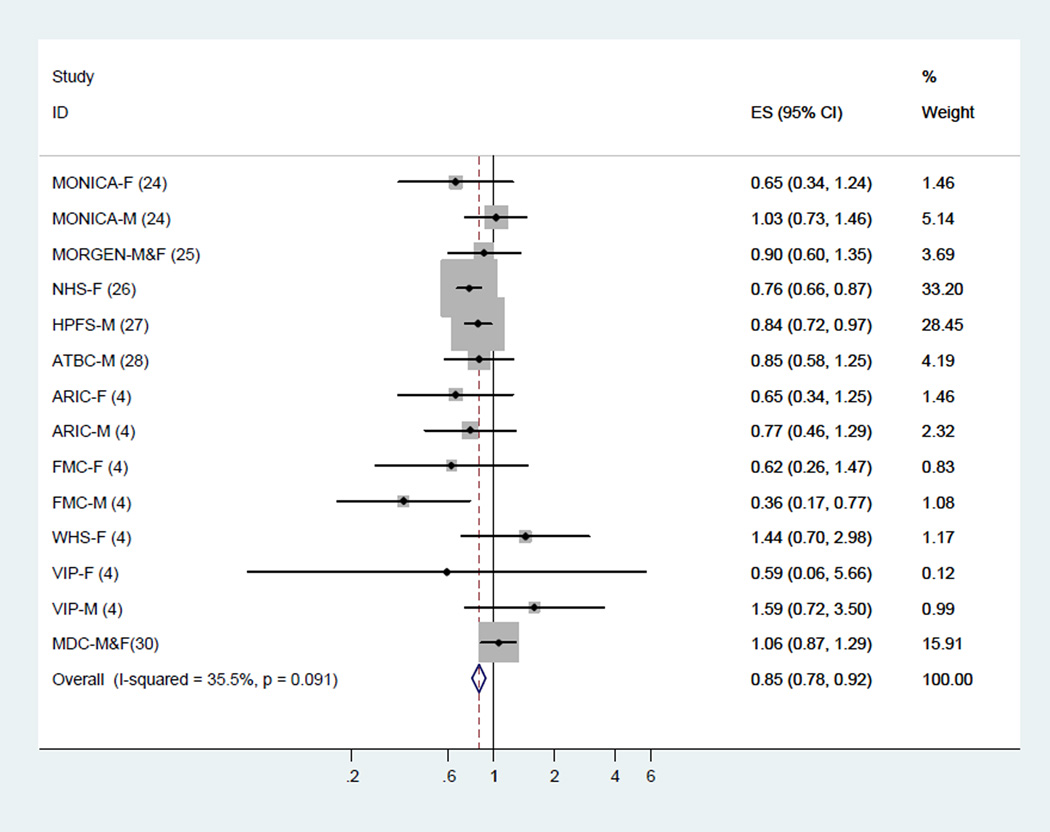
Meta-analysis of LA and total CHD events
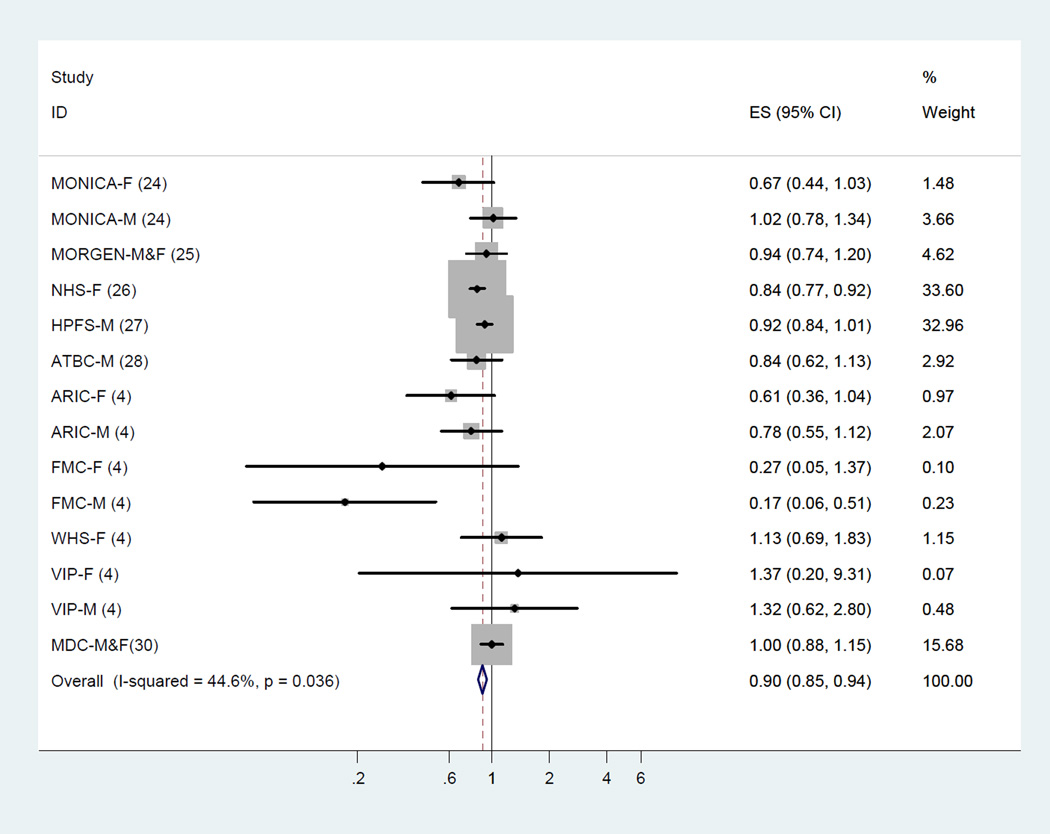
Across 10 cohort studies that examined the association between LA and total CHD events (14 estimates), LA consumption was inversely associated with risk of total CHD events. The fixed-effect summary of RR for comparing the highest with lowest category was 0.85 (95% CI, 0.78–0.92; Figure 2) with medium heterogeneity (I2 = 35.5%). This finding was similar using a random-effects model (RR, 0.86; 95% CI, 0.76–0.97).
Figure 2.
Dietary intake of linoleic acid and relative risk of total coronary heart disease events (highest category versus lowest category). The relative risk was pooled by using fixed effects meta-analysis.
The meta-regression analysis did not identify statistically significant sources of heterogeneity, including the mean age of the participants, gender, study duration, repeated measures of intake or study quality score (all P >0.10).
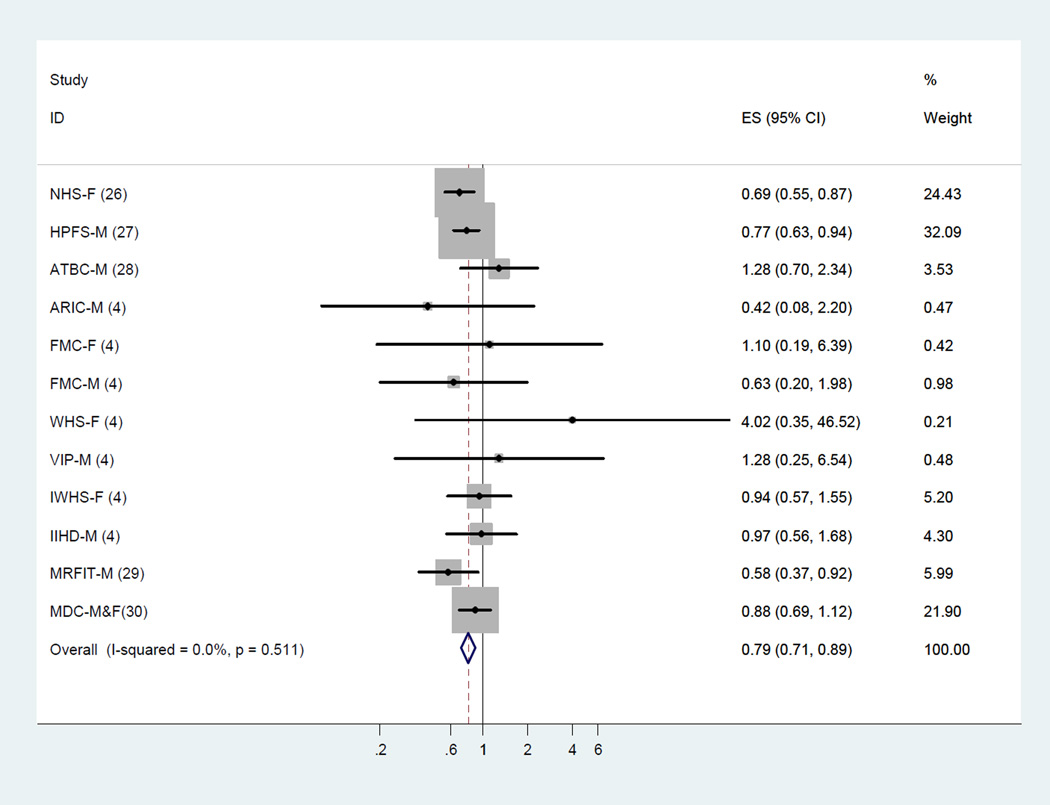
Meta-analysis of LA and CHD deaths
Among 11 cohort studies (12 estimates) that examined the association between LA and CHD deaths, higher LA intake was associated with a lower risk of CHD deaths. The fixed-effect summary of RR for comparing the highest with lowest category was 0.79 (95% CI, 0.71–0.89; Figure 3).
Figure 3.
Dietary intake of linoleic acid and relative risk of coronary heart disease deaths (highest category versus lowest category). The relative risk was pooled by using fixed effects meta-analysis.
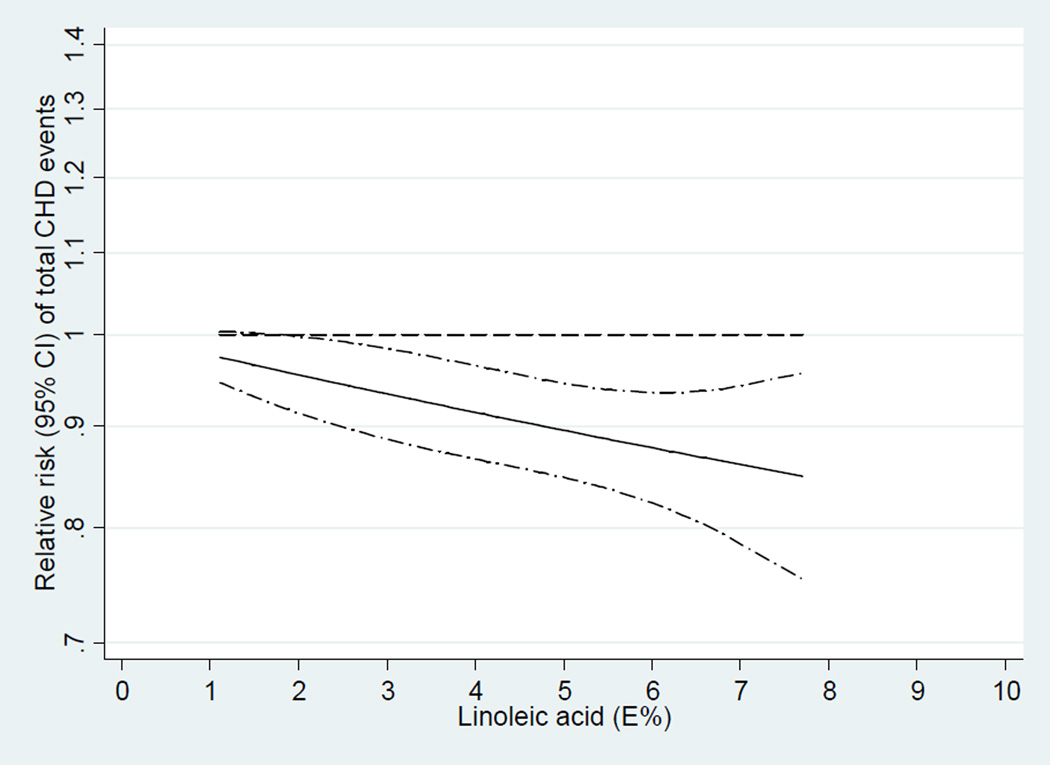
Dose-response meta-analyses
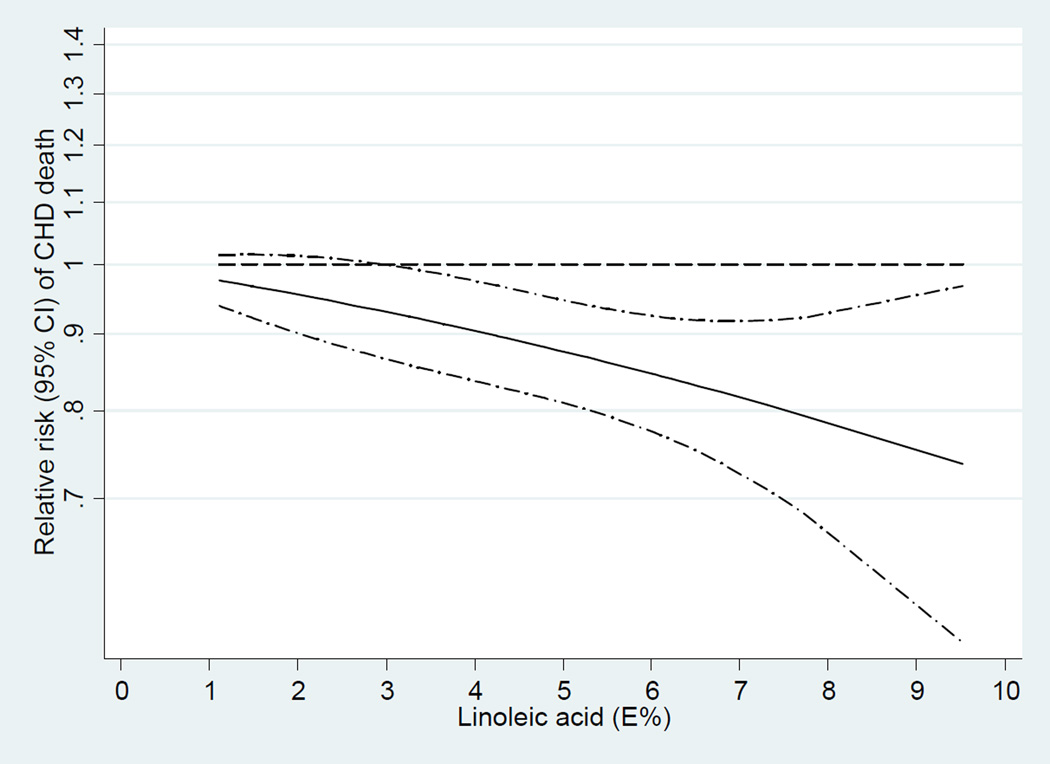
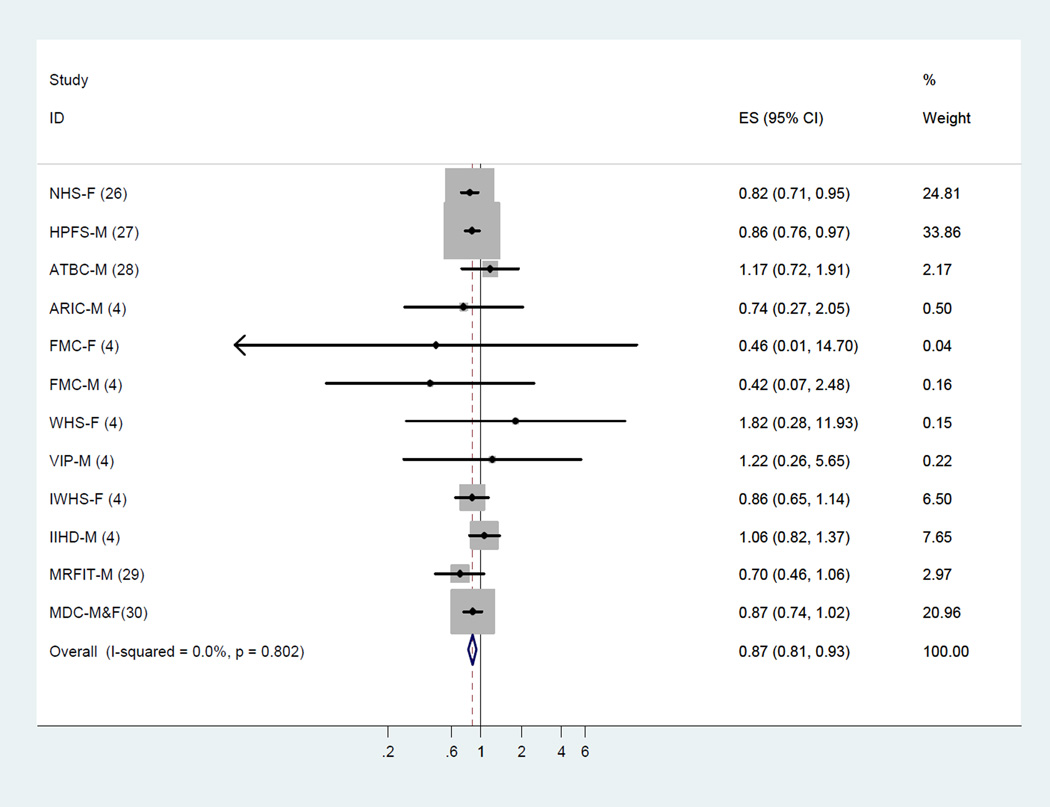
In the dose-response analysis, we found a linear association between LA intake and CHD events (P=0.91 for non-linearity; Figure 4) and CHD deaths (P=0.72 for non-linearity; Figure 5). An increment of 5% of energy intake from LA was associated with a 10% lower risk of CHD events (RR, 0.90; 95% CI, 0.85–0.94;I2 = 44.6%; Figure 6) and a 13% lower risk of CHD deaths (RR, 0.87; 95% CI, 0.81–0.93; Figure 7). Among 9 cohort studies (12 estimates) that evaluated substitution of LA for carbohydrate, the risk estimate for substituting 5% energy intake from LA for carbohydrates was 0.90 (95% CI, 0.85–0.94;I2 = 47.3%; Supplemental Figure S1) with a fixed-effect model and 0.89 (95% CI, 0.80–0.98) with a random-effects model. Among 8 cohort studies (11 estimates) that evaluated substitution of LA for SFA, the risk estimate for substituting 5% energy intake from LA for SFAs was 0.91 (95% CI, 0.87–0.96;I2 = 55.9%; Supplemental Figure S2) with a fixed-effect model and 0.90 (95% CI, 0.80–1.01) with a random-effects model. Substituting 5% energy intake from LA for the same amount of energy from carbohydrates was associated with an 13% lower risk of CHD deaths (RR, 0.87; 95% CI, 0.81–0.94; Supplemental Figure S3) and an 13% lower risk of CHD deaths when substituting for the same amount of energy from SFAs (RR, 0.87; 95% CI, 0.82–0.94; Supplemental Figure S4).
Figure 4.
Dose-response analysis for curvilinear association between dietary intake of linoleic acid and total coronary heart disease events. P=0.91 for non-linearity relationship, indicating a linear relationship.
Figure 5.
Dose-response analysis for curvilinear association between dietary intake of linoleic acid and coronary heart disease deaths. P=0.72 for non-linearity relationship, indicating a linear relationship.
Figure 6.
Two-stage dose-response meta-analysis of each 5% energy increment of dietary intake of linoleic acid and relative risk of total coronary heart disease events. The relative risk was pooled by using fixed effects meta-analysis.
Figure 7.
Two-stage dose-response meta-analysis of each 5% energy increment of dietary intake of linoleic acid and relative risk of coronary heart disease deaths. The relative risk was pooled by using fixed effects meta-analysis.
Publication bias
For dietary LA intake, visual inspection of a funnel plot (see Supplemental Figures S5 and S6) and Begg's test suggested no evidence of publication bias for either CHD events (P = 0.25) or CHD deaths (P = 1.00).
DISCUSSION
This systematic review and meta-analysis support a significant inverse association between dietary LA intake, when replacing either carbohydrates or saturated fat, and risk of CHD. Our dose-response analyses identified a lower risk of both total CHD events and CHD deaths with increasing LA intake in a linear fashion. These associations were independent of traditional CHD risk factors and other dietary factors such as fiber and ALA.
An inverse association between intake of PUFAs and CHD was reported in a pooled analysis of 11 prospective cohort studies in the US, Europe and Israel.4 In that pooled analysis with a wide range of PUFA intake (from 1.7% to 10.6%), each 5% lower energy intake from SFAs and a concomitant higher energy from PUFAs was associated with 13% lower risk of coronary events and 26% lower risk of coronary death.4 Consistent with that analyses, our meta-analysis suggests that intake of LA, the predominant n-6 PUFA, has cardio-protective effects: a 5% increase in energy from LA, replacing SFAs, was associated with 9% lower risk of total CHD and 13% lower risk of CHD deaths. A comparison of our findings to those of clinical trials of PUFA consumption is also informative. In a meta-analysis of eight RCTs, Mozaffarian et al.3 found that each 5% increase in energy from PUFAs, replacing SFAs, would reduce occurrence of coronary events by 10%.
An inverse association between LA and CHD has also been seen in most previous studies of LA biomarkers and CHD events. In a meta-analysis of 22 studies including case-control and prospective cohort data, blood/tissue LA concentrations were inversely associated with non-fatal CHD endpoints.34 Even in a population with high PUFAs intake (mean intake 10.1% of energy) and very high LA adipose tissue content (25.6% of adipose tissue composition), an inverse association was found between adipose LA and acute MI after controlling for other n-6 PUFAs.35 Concerns about recommendations for diets high in LA have been based on the assumption that AA produced from metabolism of LA is the main precursor of eicosanoids with inflammatory and thrombogenic properties such as prostaglandin E2, thromboxane A2 and Leukotriene B4.15,36 However, conversion of LA to AA is tightly controlled, variations in dietary LA intake do not appreciably modify tissue AA content;37,38 and adipose tissue AA concentrations are not associated with LA dietary intake or adipose tissue levels of LA.19,20 Also, some molecules that reduce inflammation and thrombosis such as prostacyclin, epoxyeicosatrienoic acids and lipoxin A4 are produced from AA.39,40 In an Italian adult population, higher plasma levels of AA were associated with lower plasma levels of pro-inflammatory and higher levels of anti-inflammatory markers.41 Although in several studies adipose tissue content of AA was positively correlated with risk of MI,19,20 this was not supported in a meta-analysis of 20 prospective and case-control studies where tissue AA was unrelated to either non-fatal or fatal CHD outcomes.34 Furthermore, consumption of an AA-enriched oil did not increase urinary and plasma AA metabolites or blood biomarkers of cardiovascular, inflammatory or allergic diseases.42 Independent of effects on AA, diets high in n-6 PUFAs, might decrease beneficial effects of n-3 PUFAs on CHD risk by competition in elongation and desaturation pathways.43,44 Because of multiple, potentially competing effects on many metabolic pathways, the effects of LA on heart disease risks are difficult to predict by these mechanistic considerations. A diet high in LA is considered to increase lipid susceptibility to free radical oxidation and lipid peroxidation that may play a role in etiology of cancer.45 However, in the extensive literature on this topic, including many prospective studies and meta-analyses, there is little evidence that higher intake of LA is associated with an increased risk of cancer in prospective studies.46–48
Recommendations to reduce LA in the diet are based on minimal direct evidence.14–17 Ramsden et al.17 investigated the effects of increasing LA alone and LA in combination with ALA on CHD mortality in men with recent coronary events in a single-blinded, randomized controlled trial that was conducted in the 1960’s in Australia. They suggested that n-6 LA alone increased the risk of CHD mortality. However, the study sample size was very small (n = 221) and the 95% CIs for the estimates were wide (RR, 1.74; 95% CI, 1.04–2.91). The duration of the study was also short (39 months). In addition, partial hydrogenation of vegetable oils was a common practice in the 1960’s and the results may have been confounded by trans fat in the special margarines high in LA. Also, by replacing dietary fats as much as possible with oils high in LA but with little n-3 PUFAs, any adverse effects may have been due to low n-3 PUFAs rather than high LA.
Potential limitations to this study should be considered. As in any meta-analysis, publication bias is possible. However, significant publication bias was not indicated through visual inspection of funnel plots and Begg’s test. We cannot exclude the possibility of residual confounding due to the observational nature of the included studies. However, most of the studies adjusted for major CHD risk factors and other dietary sources of energy. In most of the studies, diet was assessed using a FFQ, thus measurement errors may be introduced by the under- or over-reporting of the amounts of food groups usually eaten per day. In addition, since FFQs used in some studies did not query brand names of margarine, cooking oils, salad dressings, and other foods containing linoleic acid, the intake levels of LA may be underestimated in these studies.
Our analysis has several strengths. Our comprehensive search methods, personal contacts with authors and experts, and inclusion of unpublished data in several cohorts (ARIC, FMC, IIHD, IWHS, VIP, WHS and MDC) as well as updated analyses in some cohorts (NHS, HPFS, and ATBC) minimized misclassification and potential for publication bias. We limited our analysis to prospective cohort studies to minimize the influence of recall and selection biases that are common in case-control studies. Also, most studies adjusted for potential CHD risk factors and other types of dietary fat. Despite variations in LA measurement methods, population characteristics, study design, and specific outcomes evaluated in the included cohort studies, there was low to moderate heterogeneity across studies, which supports the validity of the pooled results. In our meta-analysis, except for the MONICA and MRFIT studies,24,29 all other cohort studies adjusted for energy from other fat components (SFAs, trans fat, MUFAs, and PUFAs other than LA). It is worth noting that the inverse association between LA and CHD was independent of ALA intake. In these analyses, the association between LA and CHD was compared to that for CHD with both carbohydrate and saturated fat intakes. Other advantages included the ability to evaluate the association of LA intake and CHD events in different populations with different diets including large variations in intakes of LA.
CONCLUSIONS
This current systematic review and meta-analysis of prospective cohort studies of LA consumption provides robust evidence that higher LA intake is associated with lower risk of CHD in a dose-response fashion. These data support current recommendations to replace saturated fat with LA for primary prevention of CHD in the general population.
Supplementary Material
Acknowledgments
Funding Sources: This study is supported by NIH grants HL60712, UM1 CA167552 and P01 CA055075.
F.B.H has received research support from California Walnut Commission.
Footnotes
Conflict of Interest Disclosures: None of the other authors had a conflict of interest to declare.
References
- 1.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Bälter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 6.Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB. α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96:1262–1273. doi: 10.3945/ajcn.112.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, Rudling M, Arner P, Cederholm T, Ahlström H, Risérus U. Effects of n-6 PUFAs compared with SFAs on liver fat lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–1012. doi: 10.3945/ajcn.111.030114. [DOI] [PubMed] [Google Scholar]
- 8.Hodson L, Skeaff CM, Chisholm WA. The effect of replacing dietary saturated fat with polyunsaturated or monounsaturated fat on plasma lipids in free-living young adults. Eur J Clin Nutr. 2001;55:908–915. doi: 10.1038/sj.ejcn.1601234. [DOI] [PubMed] [Google Scholar]
- 9.Rassias G, Kestin M, Nestel PJ. Linoleic acid lowers LDL cholesterol without a proportionate displacement of saturated fatty acid. Eur J Clin Nutr. 1991;45:315–320. [PubMed] [Google Scholar]
- 10.Singer P, Jaeger W, Berger I, Barleben H, Wirth M, Richter-Heinrich E, Voigt S, Gödicke W. Effects of dietary oleic, linoleic and alpha-linolenic acids on blood pressure, serum lipids, lipoproteins and the formation of eicosanoid precursors in patients with mild essential hypertension. J Hum Hypertens. 1990;4:227–233. [PubMed] [Google Scholar]
- 11.Kurotani K, Sato M, Ejima Y, Nanri A, Yi S, Pham NM, Akter S, Poudel-Tandukar K, Kimura Y, Imaizumi K, Mizoue T. High levels of stearic acid, palmitoleic acid, and dihomo-γ-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr Res. 2012;32:669–675. doi: 10.1016/j.nutres.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Miura K, Stamler J, Nakagawa H, Elliott P, Ueshima H, Chan Q, Brown IJ, Tzoulaki I, Saitoh S, Dyer AR, Daviglus ML, Kesteloot H, Okayama A, Curb JD, Rodriguez BL, Elmer PJ, Steffen LM, Robertson C, Zhao L International Study of Macro-Micronutrients and Blood Pressure Research Group. Relationship of dietary linoleic acid to blood pressure. The International Study of Macro-Micronutrients and Blood Pressure Study [corrected] Hypertension. 2008;52:408–414. doi: 10.1161/HYPERTENSIONAHA.108.112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolin G, Huster G, Glueck CJ, Speirs J, Vandegrift J, Illig E, Wu J, Streicher P, Tracy T. Blood pressure lowering in elderly subjects: a double-blind crossover study of omega-3 and omega-6 fatty acids. Am J Clin Nutr. 1991;53:562–572. doi: 10.1093/ajcn/53.2.562. [DOI] [PubMed] [Google Scholar]
- 14.Hamazaki T, Okuyama H. The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid: a review and critique of the scientific evidence. World Rev Nutr Diet. 2003;92:109–132. doi: 10.1159/000073796. [DOI] [PubMed] [Google Scholar]
- 15.Simopoulos AP, Leaf A, Salem N., Jr Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab. 1999;43:127–130. doi: 10.1159/000012777. [DOI] [PubMed] [Google Scholar]
- 16.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 17.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013;346:e8707. doi: 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol. 2011;668(Suppl 1):S50–S58. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 19.Baylin A, Campos H. Arachidonic acid in adipose tissue is associated with nonfatal acute myocardial infarction in the central valley of Costa Rica. J Nutr. 2004;134:3095–3099. doi: 10.1093/jn/134.11.3095. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen MS, Schmidt EB, Stegger J, Gorst-Rasmussen A, Tjonneland A, Overvad K. Adipose tissue arachidonic acid content is associated with the risk of myocardial infarction: a Danish case-cohort study. Atherosclerosis. 2013;227:386–390. doi: 10.1016/j.atherosclerosis.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029–1041. 1041.e1–1041.e15. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth A, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J, Angelantonio AD. Association of Dietary, Circulating, and Supplement Fatty Acids With Coronary Risk. A Systematic Review and Meta-analysis. Ann Intern Med. 2014;160 doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Vedtofte MS, Jakobsen MU, Lauritzen L, Heitmann BL. Dietary α-linolenic acid, linoleic acid, and n-3 long-chain PUFA and risk of ischemic heart disease. Am J Clin Nutr. 2011;94:1097–1103. doi: 10.3945/ajcn.111.018762. [DOI] [PubMed] [Google Scholar]
- 25.de Goede J, Geleijnse JM, Boer JM, Kromhout D, Verschuren WM. Linoleic acid intake, plasma cholesterol and 10-year incidence of CHD in 20,000 middle-aged men and women in the Netherlands. Br J Nutr. 2012;107:1070–1076. doi: 10.1017/S0007114511003837. [DOI] [PubMed] [Google Scholar]
- 26.Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses’ health study. Am J Epidemiol. 2005;161:672–679. doi: 10.1093/aje/kwi085. [DOI] [PubMed] [Google Scholar]
- 27.Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ. 1996;313:84–90. doi: 10.1136/bmj.313.7049.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, Virtamo J. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol. 1997;145:876–887. doi: 10.1093/oxfordjournals.aje.a009047. [DOI] [PubMed] [Google Scholar]
- 29.Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200:177–182. doi: 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]
- 30.Wallström P, Sonestedt E, Hlebowicz J, Ericson U, Drake I, Persson M, Gullberg B, Hedblad B, Wirfält E. Dietary fiber and saturated fat intake associations with cardiovascular disease differ by sex in the Malmö Diet and Cancer Cohort: a prospective study. PLoS One. 2012;7:e31637. doi: 10.1371/journal.pone.0031637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willett W, Lenart E. Nutritional Epidemiology. Oxford University Press; 2013. [Google Scholar]
- 32.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 33.Orsini N, Bellocco R. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 34.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10. doi: 10.1016/j.atherosclerosis.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Kark JD, Kaufmann NA, Binka F, Goldberger N, Berry EM. Adipose tissue n-6 fatty acids and acute myocardial infarction in a population consuming a diet high in polyunsaturated fatty acids. Am J Clin Nutr. 2003;77:796–802. doi: 10.1093/ajcn/77.4.796. [DOI] [PubMed] [Google Scholar]
- 36.Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 37.Sarkkinen ES, Agren JJ, Ahola I, Ovaskainen ML, Uusitupa MI. Fatty acid composition of serum cholesterol esters, and erythrocyte and platelet membranes as indicators of long-term adherence to fat-modified diets. Am J Clin Nutr. 1994;59:364–370. doi: 10.1093/ajcn/59.2.364. [DOI] [PubMed] [Google Scholar]
- 38.Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 2005;46:269–280. doi: 10.1194/jlr.M400225-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Samuelsson B. Arachidonic acid metabolism: role in inflammation. Z Rheumatol. 1991;50(Suppl 1):3–6. [PubMed] [Google Scholar]
- 40.Brezinski ME, Gimbrone MA, Jr, Nicolaou KC, Serhan CN. Lipoxins stimulate prostacyclin generation by human endothelial cells. FEBS Lett. 1989;245:167–172. doi: 10.1016/0014-5793(89)80214-5. [DOI] [PubMed] [Google Scholar]
- 41.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 42.Kakutani S, Ishikura Y, Tateishi N, Horikawa C, Tokuda H, Kontani M, Kawashima H, Sakakibara Y, Kiso Y, Shibata H, Morita I. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: a randomized controlled study. Lipids Health Dis. 2011;10:241. doi: 10.1186/1476-511X-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grønn M, Gørbitz C, Christensen E, Levorsen A, Ose L, Hagve TA, Christophersen BO. Dietary n-6 fatty acids inhibit the incorporation of dietary n-3 fatty acids in thrombocyte and serum phospholipids in humans: a controlled dietetic study. Scand J Clin Lab Invest. 51:255–263. doi: 10.3109/00365519109091612. 199. [DOI] [PubMed] [Google Scholar]
- 44.Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–946. doi: 10.1016/j.bcp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 45.Nam TG. Lipid peroxidation and its toxicological implications. Toxicol Res. 2011;27:1–6. doi: 10.5487/TR.2011.27.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Institute for Cancer Research and the World Cancer Research Fund. Food, Nutrition, Physical Activity and the Prevention of Cancer. A global Prospective. 2007 [Google Scholar]
- 47.Wang J, John EM, Horn-Ross PL, Ingles SA. Dietary fat, cooking fat, and breast cancer risk in a multiethnic population. Nutr Cancer. 2008;60:492–504. doi: 10.1080/01635580801956485. [DOI] [PubMed] [Google Scholar]
- 48.Murff HJ, Shu XO, Li H, Yang G, Wu X, Cai H, Wen W, Gao YT, Zheng W. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: a prospective cohort study. Int J Cancer. 2011;128:1434–1441. doi: 10.1002/ijc.25703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.