Abstract
Chronic obstructive pulmonary disease (COPD) is a pathologic pulmonary condition characterized by expiratory airflow obstruction due to emphysematous destruction of the lung parenchyma and remodeling of the small airways. While spirometry is a very useful diagnostic tool for screening large groups of smokers, it cannot readily differentiate the etiologies of COPD and thus has limited utility in characterizing subjects for clinical and investigational purposes. There has been a longstanding interest in thoracic imaging and its role in the in-vivo characterization of smoking related lung disease. Research in this area has spanned readily available modalities such as chest x-ray and computed tomography to more advanced imaging techniques such as optical coherence tomography and magnetic resonance imaging. While chest x-ray is almost universally available, it lacks sensitivity in detecting both airway disease and mild emphysema, and is not generally amenable to objective analysis. Computed tomography has become the standard modality used for objective visualization of disease. It can provide useful measures of emphysema, airway disease, and more recently pulmonary vascular disease for clinical correlation. It does, however, face limitations in standardization across brands and generations of scanners, and the ionizing radiation associated with image acquisition is of concern to both patient and health care provider. Newer techniques such as OCT and MRI offer exciting in-vivo insight into lung structure and function that was previously available only in necropsy specimens and physiology labs. Given the more limited availability of these techniques, they are at present viewed as adjuncts to CT imaging.
Keywords: Emphysema, COPD, Imaging, computed tomography, airway disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a pathologic condition of the lung characterized by emphysematous destruction of the lung parenchyma and remodeling of the small airways. The admixture of these two processes leads to what is clinically observed as expiratory airflow obstruction that is not completely reversible with the use of inhaled bronchodilating medications.(1) Despite ongoing refinements in the spirometric classification of disease, marked heterogeneity still exists in both subject symptoms and response to therapeutic intervention.(2, 3) This inconsistent association between lung function and disease manifestations has led to increasing interest in image based methods for the diagnosis and classification of COPD.
The following is a review of the role of chest imaging in the detection and quantification of the structural and functional abnormalities of a lung affected by COPD. The history of this effort extends from the use of the Roentgenologic exam, through the evolution of Computed Tomography (CT) and Optical Coherence Tomography (OCT), into more functional imaging modalities such as contrast enhanced (both intravenous and inhaled) Magnetic Resonance Imaging (MRI). The primary focus of this review will be applications of CT scanning in clinical investigation with lesser mention of additional techniques because of their more limited ability to be performed in very large cohorts of subjects with COPD.
Chest X-Ray
Posteroanterior and lateral chest x-ray (CXR) is a standard part of the clinical evaluation of subjects with COPD. Such images are inexpensive, easily obtained, and involve minimal radiation exposure. Prior work by several groups has led to several proposed criteria for the detection of emphysema on CXR (Table 1, Figure 1). These include
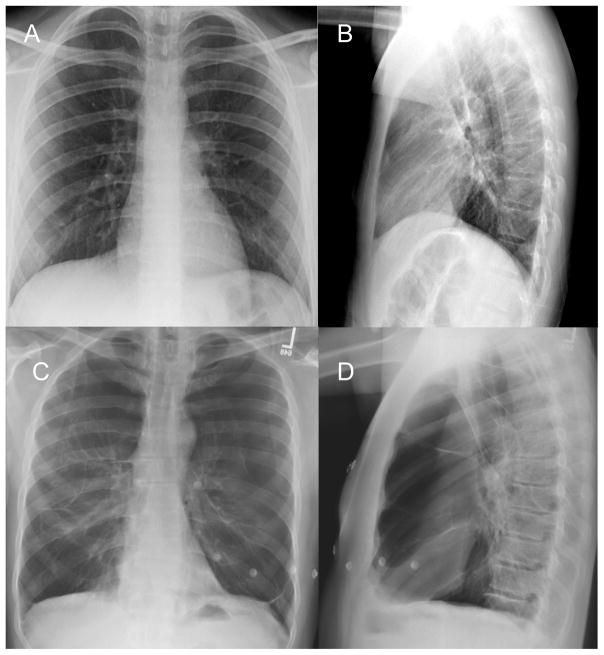
Figure 1.
Posteroanterior and Lateral CXR of a normal healthy subject (panels A and B) and one with several emphysematous destruction of the lung parenchyma (Panels C and D).
Increased radiolucency of the lung fields
Flattening of the diaphragms
Pruning of the peripheral vasculature
Increased retrosternal airspace
Widening of the intercostal spaces
Narrowed and more vertical cardiac silhouette.
While the application of such criteria to CXR for the detection of emphysema has historically had mixed success in correlations to histopathologic examination (4, 5), recent investigation suggests that the semi-objective visual interpretation of chest radiographic images may have clinical utility. In 2008, Miniati and colleagues demonstrated that both experienced and inexperienced viewers could identify the presence of moderate and severe emphysema with over 90% sensitivity and specificity with minimal training.(6) While such an approach is not amenable to the detection of subtle changes on longitudinal examination or a regional assessment of disease, it does suggest that in the clinical setting, CXR may provide useful subjective phenotypic information in subjects with COPD.
Computed Tomography: Emphysema
The introduction of computed tomographic imaging has facilitated the in-vivo examination of the most fundamental aspect of lung structure, the secondary pulmonary lobule. In it can be found the juxtaposition of the pulmonary vessels, airways, lymphatics, and lobular septa that maintain normal lung function. It is also the site most recognizable on CT scan for its appearance in both health and disease.(7)
Emphysema is defined as the abnormal enlargement of the airspaces distal to the respiratory bronchioles resulting from the destruction of the septal walls.(8) On CT scan, this process results in visually apparent regions of low density tissue surrounded by more normal lung. The distribution of these regions of low attenuation and degree to which they involve the secondary pulmonary lobule can be characterized as centrilobular, panlobular, and paraseptal emphysema. As its name implies, centrilobular emphysema typically manifests as central destruction of the secondary pulmonary lobular parenchyma surrounding the centrilobular artery. In contrast, panlobular emphysema can be identified on CT scan as uniform destruction of the lobule. Finally, paraseptal emphysema is a form of panlobular emphysema localized primarily to the parenchyma adjacent to the pleural surface.(7) Examples of these conditions can be found in Figure 2.
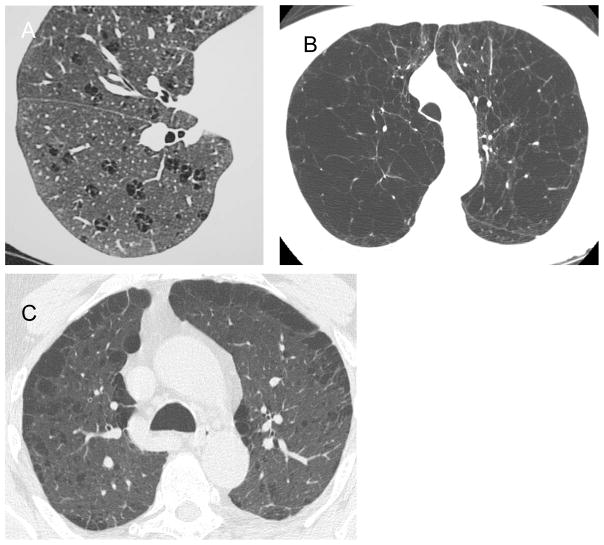
Figure 2.
Examples of centrilobular (A), panlobular (B), and paraseptal emphysema (C). Images provided courtesy of the COPDGene® Study.
There are several methods for both the detection and quantification of emphysema on CT scan. These can be preliminarily divided into two categories, visual detection schemes and more objective techniques based upon lung density. Typically, subjective approaches to analysis such as visual interpretation involve either a global or regional assessment of the lung using an ordinal scoring system (i.e. 0–4) to reflect disease severity. Using visual interpretation schemes, multiple investigators have demonstrated a correlation to histopathology (9–12), lung function (13, 14), and even response to therapeutic intervention.(3) Limitations to these approaches are, however, their susceptibility to intra and inter-observer variability (15), sensitivity to the viewing conditions such as window width and level (16, 17), and potential insensitivity for the detection of disease progression in longitudinal studies. Paradoxically, a potential strength to such analysis is that same ability of the user to be either consciously or unconsciously influenced by their visual perception of disease. Depending on the experience of the user, subtle patterns of disease may be observed that are not readily amenable to objective quantification.
In principle, a CT scanner is a densitometer where the brightness or attenuation of each pixel is a product of the density of the tissue it encompasses. These densities are expressed numerically in Hounsfield Units (HU) and generally range from −1000 HU (air) through 0 HU (water) to +1000 HU (bone) although the extremes can vary based upon CT scanner brand. Using this information, one can generate a histogram of the distribution of tissue densities in the lung, where each point is defined by the HU value of that pixel or voxel (3 dimensional pixel). Examples of these are shown in Figure 3. Multiple techniques for objectively identifying meaningful points on the CT lung histogram exist and include defining the mean lung density (18, 19), percentile points such as the Perc15 (HU threshold that delineates the lowest 15% of the histogram from the denser 85%) (20), and a fixed HU threshold such as −910 or −950 to identify the low attenuation regions of emphysema.(21, 22)
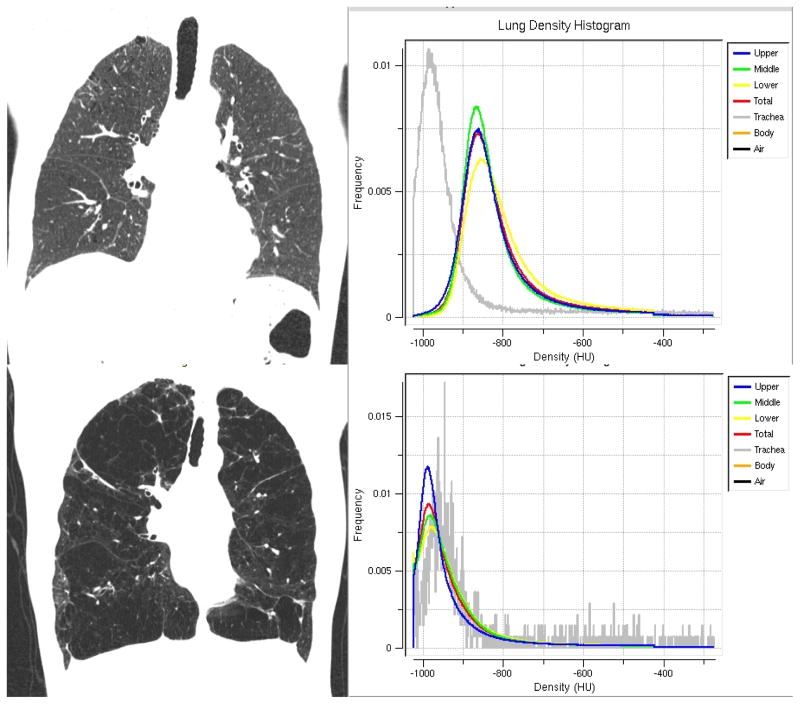
Figure 3.
Coronal images of a subject with minimal emphysema (top panels) and severe emphysema (bottom panels). For each subject, density histograms are presented for the upper (blue), middle (green), and lower (yellow) regions of lung divided by lung volume. Note the leftward shift in the density histograms of the subject with severe emphysema compared to the more normal smoker.
Since their inception over 30 years ago the afore mentioned methods of densitometric analysis have become standard for the detection and quantification of emphysema on CT scan and in the case of HU thresholding has been demonstrated on several occasions to offer correlates to the results of tissue necropsy.(21, 22) Through its application, investigators have found an objective tool for the prediction of surgical outcomes (23, 24) began to test drugs for their efficacy in the attenuation of disease progression (25), and have become increasingly aware of gender differences in the manifestations of smoking related lung disease.(26, 27) Despite these advances, lung densitometry is vulnerable to several influences including the lung volume at which the CT scan was obtained (28, 29) and the protocol used to acquire and reconstruct the images.(30) While the former may be addressed by a calculated volume correction (28), the latter still offers an unanswered challenge to discover one or several “correction factors” by which one set of images can be adjusted so that they are comparable to those obtained by a different brand of scanner which has generated similar but not exactly the same set of images.
Computed Tomography: Airway Disease
The site of expiratory airflow obstruction in smokers is believed to be the peripheral small airways.(31) While the size of these airways precludes their direct assessment on clinical CT scans, recent investigation has demonstrated that the morphology of the central cartilaginous airways reflect the distal remodeling process. In 2000, Nakano and colleagues demonstrated that in smokers, subjects with the greatest degree of mural thickening and lumenal occlusion of the apical segment of the right upper lobe tended to have the lowest FEV1 expressed as a percent of predicted (32) and a greater burden of distal small airway disease on histopathologic examination.(33) Further, the combination of CT measures of emphysema and densitometric measures of emphysema provided additive information when predicting lung function. Interestingly, there was no relationship between absolute airway wall thickness and lung function which alludes to the overall variability native airway morphology in this group. To address this issue investigators employed a measure called the Wall Area Percent (WA%) which is calculated as 100 times the airway wall area divided by the total bronchial cross sectional area.(32) This has become the standard CT based measure of airway disease in smokers.
There have since been several similar investigations of the correlation between CT measures of airway disease and lung function.(34–36) Among the most notable of these was work done by Hasegawa and colleagues who demonstrated that the quantitative assessment of the WA% of more distal airway generations (5th) provided stronger correlations to lung function than the proximal segmental airways (3rd generation) suggesting that a more robust biological signal could be detected in the most peripheral aspects of the bronchial tree.(34) This finding has been further extended by demonstrating that distal measures of WA% provided the strongest correlate to inhaled bronchodilator response. (37)
To this point, CT measures of airway disease have been defined as mural thickening with encroachment on the lumen. While useful for functional correlation, their relationship to another clinically significant occurrence, acute exacerbations of COPD, is currently undefined. In contrast, another radiographic form of airway disease, bronchiectasis, is increasingly being recognized for its association with elevated biomarkers of inflammation and the severity of respiratory events.(38)
Bronchiectasis is characterized radiographically as the abnormal dilation of the airway lumen with concomitant wall thickening.(39, 40) Its prevalence in the general population of smokers with COPD is unknown. In one of the largest reported CT based studies of smokers to date, Patel and colleagues found that approximately 2.5% of subjects had moderate to severe disease.(41) In contrast, Parr and colleagues found that almost 95% of their study cohort of subjects with AATD exhibited some bronchiectatic changes in their airways.(42) There are 2 notable differences to these studies. The first is that the prevalence estimate published by Parr and colleagues included subjects with even mild, regionally limited disease while those estimates reported by Patel and colleagues were based on severe disease that precluded quantitative airway analysis. The second is related to the unique pulmonary manifestations of smoking related lung disease in smokers with or without AATD. Further investigation is needed to determine the prevalence and clinical associations of bronchiectatic airway disease detected on CT scan in smokers.
Computed Tomography: Expiratory/Dynamic Imaging
While HRCT images provide detailed structural information, they are generally acquired in a single breath hold at full inflation. To fully exploit the potential of CT, investigators have begun to utilize static expiratory scans and in the case of more central airway disease such as tracheobronchomalacia, dynamic expiratory imaging.(43) One of the most striking findings on expiratory CT imaging in subjects with expiratory airflow obstruction is mosaic perfusion of the secondary pulmonary lobule (Figure 4). Regions of low attenuation represent relative oligemia due to local obstruction of either the vasculature or small airway which in the latter case results in focal gas trapping.(44) While recognized visually, little has been done to objectively quantify this distinct radiographic pattern.
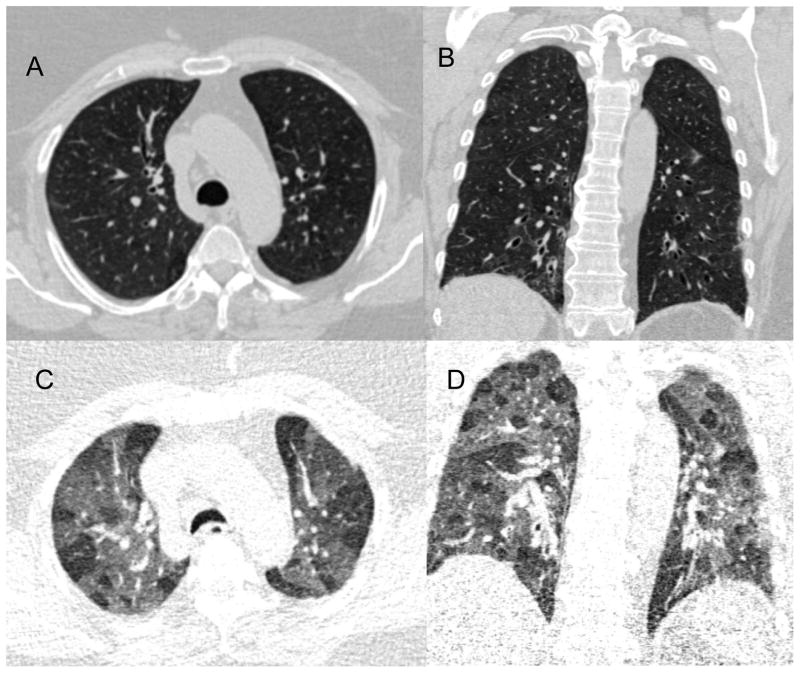
Figure 4.
Examples of axial and coronal CT images obtained at full inflation (panels A and B) and at relaxed exhalation (panels C and D). Note the mosaic attenuation of the bottom 2 expiratory panels suggesting oligemia or gas trapping within the secondary pulmonary lobule.
Computed Tomography: Pulmonary Vascular Disease (PVD)
Clinicians and investigators have long recognized the significance of pulmonary vascular disease in subjects with COPD. In 1972, Burrows and colleagues performed right heart catheterization in 50 subjects with severe COPD revealing that 26% of them had resting mean pulmonary artery (Ppa) pressures ≥ 26 mmHg.(45) Subsequent investigations have estimated the prevalence of elevated pulmonary vascular pressures to be between 35% and 90%, the higher estimates reported in subjects with severe emphysema.(46, 47) In addition to the disabling dyspnea on exertion ascribed to pulmonary hypertension in subjects with COPD, the presence of pulmonary vascular disease in this cohort has been demonstrated to be a poor prognostic determinant of survival (45, 46, 48, 49) and is associated with increased utilization of healthcare resources.(50)
While CT scanning is a standard of care for the investigation of pulmonary thromboembolic disease, there has been little application of CT to conditions such as PVD associated with COPD. Recently, Matsuoka and colleagues conducted an investigation of the relationship between CT burdens of emphysema and PVD in smokers using a metric termed the CSA or cross sectional area.(51) In an axial image, the authors hypothesized that some of the apparent high density structures are small vessels captured orthogonal to their long axis. By examining the cross sectional area of those round structures, one may have an objective measure of the aggregate small pulmonary vasculature available for lung perfusion. Further, when constraining this analysis to those structures that are each less than 5 mm2 in cross sectional area, one can assess the CSA of the sub-sub segmental pulmonary vessels.
Using this metric of disease, Matsuoka and colleagues found that the CSA of those pulmonary vessels less than 5mm2 was inversely related to the CT burden of emphysema.(51) Not surprisingly, subjects with greater burdens of parenchymal destruction tended to have lower aggregate cross sectional area of the small pulmonary vasculature. In a subsequent investigation, the same CSA measure was applied to a subset of subjects enrolled in the National Emphysema Treatment Trial (NETT) who underwent high resolution CT scanning and right heart catheterization for the invasive assessment of pulmonary artery pressures. In this cohort of subjects with severe emphysema, there was a marked inverse relationship between the vascular CSA and PA pressures while there was no evidence of a similar relationship between CT burdens of emphysema and PA pressures.(52) Further work needs to be done to replicate and validate these findings but CSA may prove to be a minimally invasive CT based assessment of pulmonary vascular disease in smokers.
Computed Tomography: Summary and Limitations
There are several technical as well as biologic limitations to the use of CT as a measure of lung disease in subjects with COPD. The first pertains to such considerations as the lack of manufacturer standardization of CT acquisition and reconstruction protocols across multiple brands and generations of scanners. Such systematic issues can bias data so that subjects at a given center may appear to have more or less emphysema for a given degree of tobacco smoke exposure or expiratory airflow obstruction. A second well documented limitation of quantitative CT scan analysis is the resolution imposed by clinical imaging protocols. Standard theory in image analysis states that structures smaller than 2 pixel widths in size cannot be resolved with accuracy. (53) Since the wall thicknesses of the 4th and 5th generation airways tend to fall in this range, consideration must be given to the accuracy of such measures prior to clinical application despite their providing a more robust biologic signal.
Additional seemingly simple biologic considerations that must be acknowledged include the level of inspiratory effort a subject exerts to achieve a full inflation scan. The lung (and in turn the airways) expand isotropically with inflation (54) so that for a given subject, the greater the degree of inflation, the greater the apparent burden of emphysema (by a corresponding reduction in lung density) and potentially diminished CT burden of airway disease (by dilating the airway lumen used to calculate cross-sectional measures of WA%). While the impact of such variability is recognized, and efforts are being made to adjust CT measures of emphysema for inspiratory effort (55), thus far there are no similar adjustments for CT measures of airway disease.
Finally, while CT imaging is a relatively safe, non-invasive tool that can be used on a large scale for genetic and epidemiologic studies, as a modality, it is limited in its ability to asses such things as the detailed mural remodeling process found in airway disease or the regional function of seemingly emphysematous tissue. In addition, general tendencies for subjects with thicker walls and smaller airway lumens to have greater expiratory airflow obstruction have been reproduced, but a single reported value of WA% at a single site in a single subject has little clinical meaning. Because of this, there is great interest in additional imaging modalities such as optical coherence tomography (OCT) and magnetic resonance imaging (MR) that may provide more detailed structural information and possibly insight into tissue function without the potential risks of ionizing radiation exposure inherent in CT.(56)
Optical Coherence Tomography (OCT)
Optical coherence tomography is a newer imaging technique that can provide detailed information of mural structures to a depth of several mm with a resolution of less than 5 μm.(57–59) By advancing a fiber optic probe bronchoscopically, investigators can selectively examine detailed regions of the tracheobronchial tree. OCT functions very similarly to ultrasound in that a tissue or region of interest is exposed a signal (in this case infrared light) and the reflected or back-scattered signals are reconstructed to generate an image. Again, similar to ultrasound, the density and depth of these tissues will influence the back-scattered signal such that they can be differentiated on the final reconstructed image.
Clinical application of this technique in subjects with COPD has provided new insight into the mural remodeling process characteristic of airway disease. Given the orders of magnitude increase in image resolution offered by OCT over CT, Coxson and colleagues were able to demonstrate the degree of overestimation of CT measures of airway disease and were further able to demonstrate that OCT may be a more sensitive imaging modality to assess disease progression.(60) Additional investigation is ongoing in such areas as the use of Doppler and OCT in objectively examining microvascular blood flow (61, 62) and the spectroscopic examination of in-vivo molecular structure.(63)
Magnetic Resonance Imaging
Unlike CT, magnetic resonance imaging does not require exposure to ionizing radiation; rather it is dependent upon the application of an external magnetic field to align the nuclear magnetization of the hydrogen atoms in a structure of interest. The resulting behavior of these atoms can then be detected by the scanner and reconstructed to generate an image. A major limitation of MRI imaging of the lung is the relatively high ratio of air to tissue. The resulting low density of pulmonary hydrogen atoms limits image resolution for the quantitative assessment of structure without the use of intravenous or inhaled contrast enhancing agents.(64)
The discovery and implementation of new contrast agents for MRI is an area of ongoing research. Some of the newest and most exciting agents under investigation are the hyperpolarized noble gases such as helium (3He) and xenon (129Xe). While both gases provide detailed images of the tracheobronchial tree and allow global and regional measurement of surface to volume ratio(65, 66), 129Xe has the added property of being diffusible across the alveolar capillary membrane and can therefore be used to examine the septal thickness and diffusion capacity.(66–68) Further work is needed using these and other contrast agents however such techniques may truly allow in-vivo assessment of lung function in both health and disease.
Conclusions
Imaging and image analysis offers new insight into pulmonary disease processes that were previously available only on tissue necropsy. Current techniques can offer detailed measures of lung structure and with newer modalities previously immeasurable things like regional lung function. Imaging in the context of clinical investigation may offer the ability to define more homogeneous subsets of subjects with COPD and to potentially provide an intermediate biomarker of disease progression in lieu of a declining FEV1. In the case of CT, there are several limitations that must be overcome prior to its universal adoption in clinical investigation. These include the noise introduced by such non-disease related variables as subject inspiratory level and inter scanner reproducibility. Newer modalities such as OCT and MRI have distinct advantages to CT in such areas as image resolution and functional assessment of lung tissue. They, however, suffer for requiring more training to perform and are more burdensome for clinical study. Despite acknowledged limitations in all techniques, there is tremendous opportunity for further application of multimodality imaging in ongoing large clinical investigations.
Acknowledgments
Funding: This work is supported by NIH Grant Numbers HL089353-01A1, U01-HL089897, U01-HL08956, and a grant from the Parker B. Francis Foundation.
References
- 1.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46(8):798–825. [PubMed] [Google Scholar]
- 2.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56(11):880–7. doi: 10.1136/thorax.56.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 4.Sutinen S, Christoforidis AJ, Klugh GA, Pratt PC. Roentgenologic Criteria for the Recognition of Nonsymptomatic Pulmonary Emphysema. Correlation between Roentgenologic Findings and Pulmonary Pathology. Am Rev Respir Dis. 1965;91:69–76. doi: 10.1164/arrd.1965.91.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Nicklaus TM, Stowell DW, Christiansen WR, Renzetti AD., Jr The accuracy of the roentgenologic diagnosis of chronic pulmonary emphysema. Am Rev Respir Dis. 1966;93(6):889–99. doi: 10.1164/arrd.1966.93.6.889. [DOI] [PubMed] [Google Scholar]
- 6.Miniati M, Monti S, Stolk J, Mirarchi G, Falaschi F, Rabinovich R, Canapini C, Roca J, Rabe KF. Value of chest radiography in phenotyping chronic obstructive pulmonary disease. Eur Respir J. 2008;31(3):509–15. doi: 10.1183/09031936.00095607. [DOI] [PubMed] [Google Scholar]
- 7.Webb WR. Thin-section CT of the secondary pulmonary lobule: anatomy and the image--the 2004 Fleischner lecture. Radiology. 2006;239(2):322–38. doi: 10.1148/radiol.2392041968. [DOI] [PubMed] [Google Scholar]
- 8.Snider GL. Emphysema: the first two centuries--and beyond. A historical overview, with suggestions for future research: Part 1. Am Rev Respir Dis. 1992;146(5 Pt 1):1334–44. doi: 10.1164/ajrccm/146.5_Pt_1.1334. [DOI] [PubMed] [Google Scholar]
- 9.Foster WL, Jr, Pratt PC, Roggli VL, Godwin JD, Halvorsen RA, Jr, Putman CE. Centrilobular emphysema: CT-pathologic correlation. Radiology. 1986;159(1):27–32. doi: 10.1148/radiology.159.1.3952318. [DOI] [PubMed] [Google Scholar]
- 10.Bergin C, Muller N, Nichols DM, Lillington G, Hogg JC, Mullen B, Grymaloski MR, Osborne S, Pare PD. The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis. 1986;133(4):541–6. doi: 10.1164/arrd.1986.133.4.541. [DOI] [PubMed] [Google Scholar]
- 11.Hruban RH, Meziane MA, Zerhouni EA, Khouri NF, Fishman EK, Wheeler PS, Dumler JS, Hutchins GM. High resolution computed tomography of inflation-fixed lungs. Pathologic-radiologic correlation of centrilobular emphysema. Am Rev Respir Dis. 1987;136(4):935–40. doi: 10.1164/ajrccm/136.4.935. [DOI] [PubMed] [Google Scholar]
- 12.Hayhurst MD, MacNee W, Flenley DC, Wright D, McLean A, Lamb D, Wightman AJ, Best J. Diagnosis of pulmonary emphysema by computerised tomography. Lancet. 1984;2(8398):320–2. doi: 10.1016/s0140-6736(84)92689-8. [DOI] [PubMed] [Google Scholar]
- 13.Washko GR, Criner GJ, Mohsenifar Z, Sciurba FC, Sharafkhaneh A, Make BJ, Hoffman EA, Reilly JJ. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. Copd. 2008;5(3):177–86. doi: 10.1080/15412550802093025. [DOI] [PubMed] [Google Scholar]
- 14.Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology. 1999;211(2):541–7. doi: 10.1148/radiology.211.2.r99ma52541. [DOI] [PubMed] [Google Scholar]
- 15.Hersh CP, Washko GR, Jacobson FL, Gill R, Estepar RS, Reilly JJ, Silverman EK. Interobserver variability in the determination of upper lobe-predominant emphysema. Chest. 2007;131(2):424–31. doi: 10.1378/chest.06-1040. [DOI] [PubMed] [Google Scholar]
- 16.Muller NL, Coxson H. Chronic obstructive pulmonary disease. 4: imaging the lungs in patients with chronic obstructive pulmonary disease. Thorax. 2002;57(11):982–5. doi: 10.1136/thorax.57.11.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern EJ, Frank MS. CT of the lung in patients with pulmonary emphysema: diagnosis, quantification, and correlation with pathologic and physiologic findings. AJR Am J Roentgenol. 1994;162(4):791–8. doi: 10.2214/ajr.162.4.8140992. [DOI] [PubMed] [Google Scholar]
- 18.Guenard H, Diallo MH, Laurent F, Vergeret J. Lung density and lung mass in emphysema. Chest. 1992;102(1):198–203. doi: 10.1378/chest.102.1.198. [DOI] [PubMed] [Google Scholar]
- 19.Heremans A, Verschakelen JA, Vanfraeyenhoven L, Demedts M. Measurement of lung density by means of quantitative CT scanning. A study of correlations with pulmonary function tests. Chest. 1992;102(3):805–11. doi: 10.1378/chest.102.3.805. [DOI] [PubMed] [Google Scholar]
- 20.Gould GA, Redpath AT, Ryan M, Warren PM, Best JJ, Flenley DC, MacNee W. Lung CT density correlates with measurements of airflow limitation and the diffusing capacity. Eur Respir J. 1991;4(2):141–6. [PubMed] [Google Scholar]
- 21.Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988;94(4):782–7. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 22.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187–92. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 23.Nakano Y, Coxson HO, Bosan S, Rogers RM, Sciurba FC, Keenan RJ, Walley KR, Pare PD, Hogg JC. Core to rind distribution of severe emphysema predicts outcome of lung volume reduction surgery. Am J Respir Crit Care Med. 2001;164(12):2195–9. doi: 10.1164/ajrccm.164.12.2012140. [DOI] [PubMed] [Google Scholar]
- 24.Rogers RM, Coxson HO, Sciurba FC, Keenan RJ, Whittall KP, Hogg JC. Preoperative severity of emphysema predictive of improvement after lung volume reduction surgery: use of CT morphometry. Chest. 2000;118(5):1240–7. doi: 10.1378/chest.118.5.1240. [DOI] [PubMed] [Google Scholar]
- 25.Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DC, Ulrik CS, Skovgaard LT, Kok-Jensen A, Rudolphus A, Seersholm N, Vrooman HA, Reiber JH, Hansen NC, Heckscher T, Viskum K, Stolk J. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1468–72. doi: 10.1164/ajrccm.160.5.9901055. [DOI] [PubMed] [Google Scholar]
- 26.Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, Kazerooni E, Murray S, Criner GJ, Sin DD, Hogg J, Ries AL, Han M, Fishman AP, Make B, Hoffman EA, Mohsenifar Z, Wise R. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176(3):243–52. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest. 2007;132(2):464–70. doi: 10.1378/chest.07-0863. [DOI] [PubMed] [Google Scholar]
- 28.Stoel BC, Putter H, Bakker ME, Dirksen A, Stockley RA, Piitulainen E, Russi EW, Parr D, Shaker SB, Reiber JH, Stolk J. Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc. 2008;5(9):919–24. doi: 10.1513/pats.200804-040QC. [DOI] [PubMed] [Google Scholar]
- 29.Gierada DS, Yusen RD, Pilgram TK, Crouch L, Slone RM, Bae KT, Lefrak SS, Cooper JD. Repeatability of quantitative CT indexes of emphysema in patients evaluated for lung volume reduction surgery. Radiology. 2001;220(2):448–54. doi: 10.1148/radiology.220.2.r01au46448. [DOI] [PubMed] [Google Scholar]
- 30.Boedeker KL, McNitt-Gray MF, Rogers SR, Truong DA, Brown MS, Gjertson DW, Goldin JG. Emphysema: effect of reconstruction algorithm on CT imaging measures. Radiology. 2004;232(1):295–301. doi: 10.1148/radiol.2321030383. [DOI] [PubMed] [Google Scholar]
- 31.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355–60. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 32.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Pare PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–8. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Pare PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142–6. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, Ito Y, Betsuyaku T, Nishimura M. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(12):1309–15. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 35.Kim WJ, Silverman EK, Hoffman E, Criner GJ, Mosenifar Z, Sciurba FC, Make BJ, Carey V, Estepar RS, Diaz A, Reilly JJ, Martinez FJ, Washko GR. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136(2):396–404. doi: 10.1378/chest.08-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuoka S, Kurihara Y, Yagihashi K, Hoshino M, Nakajima Y. Airway dimensions at inspiratory and expiratory multisection CT in chronic obstructive pulmonary disease: correlation with airflow limitation. Radiology. 2008;248(3):1042–9. doi: 10.1148/radiol.2491071650. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa M, Makita H, Nasuhara Y, Odajima N, Nagai K, Ito Y, Betsuyaku T, Nishimura M. Relationship between improved airflow limitation and changes in airway calibre induced by inhaled anticholinergic agents in COPD. Thorax. 2009;64(4):332–8. doi: 10.1136/thx.2008.103671. [DOI] [PubMed] [Google Scholar]
- 38.Patel IS, Vlahos I, Wilkinson TM, Lloyd-Owen SJ, Donaldson GC, Wilks M, Reznek RH, Wedzicha JA. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):400–7. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 39.Grenier PA, Beigelman-Aubry C, Fetita C, Preteux F, Brauner MW, Lenoir S. New frontiers in CT imaging of airway disease. Eur Radiol. 2002;12(5):1022–44. doi: 10.1007/s00330-002-1342-1. [DOI] [PubMed] [Google Scholar]
- 40.McGuinness G, Naidich DP. CT of airways disease and bronchiectasis. Radiol Clin North Am. 2002;40(1):1–19. doi: 10.1016/s0033-8389(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 41.Patel BD, Coxson HO, Pillai SG, Agusti AG, Calverley PM, Donner CF, Make BJ, Muller NL, Rennard SI, Vestbo J, Wouters EF, Hiorns MP, Nakano Y, Camp PG, Nasute Fauerbach PV, Screaton NJ, Campbell EJ, Anderson WH, Silverman EK, Lomas DA. Airway Wall Thickening and Emphysema Show Independent Familial Aggregation in COPD. Am J Respir Crit Care Med. 2008 doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 42.Parr DG, Guest PG, Reynolds JH, Dowson LJ, Stockley RA. The Prevalence and Impact of Bronchiectasis in Alpha-1 Antitrypsin Deficiency. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200703-489OC. [DOI] [PubMed] [Google Scholar]
- 43.Boiselle PM, O’Donnell CR, Bankier AA, Ernst A, Millet ME, Potemkin A, Loring SH. Tracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CT. Radiology. 2009;252(1):255–62. doi: 10.1148/radiol.2521081958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webb WR. High-resolution computed tomography of obstructive lung disease. Radiol Clin North Am. 1994;32(4):745–57. [PubMed] [Google Scholar]
- 45.Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, Kessler R. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107(5):1193–8. doi: 10.1378/chest.107.5.1193. [DOI] [PubMed] [Google Scholar]
- 46.Weitzenblum E, Sautegeau A, Ehrhart M, Mammosser M, Hirth C, Roegel E. Long-term course of pulmonary arterial pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;130(6):993–8. doi: 10.1164/arrd.1984.130.6.993. [DOI] [PubMed] [Google Scholar]
- 47.Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166(3):314–22. doi: 10.1164/rccm.2107027. [DOI] [PubMed] [Google Scholar]
- 48.Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener CF. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med. 1972;286(17):912–8. doi: 10.1056/NEJM197204272861703. [DOI] [PubMed] [Google Scholar]
- 49.Traver GA, Cline MG, Burrows B. Predictors of mortality in chronic obstructive pulmonary disease. A 15-year follow-up study. Am Rev Respir Dis. 1979;119(6):895–902. doi: 10.1164/arrd.1979.119.6.895. [DOI] [PubMed] [Google Scholar]
- 50.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(1):158–64. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 51.Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San Jose Estepar R, Diaz A, Silverman EK, Patz S, Hatabu H. Quantitative CT Measurement of Cross-sectional Area of Small Pulmonary Vessel in COPD: Correlations with Emphysema and Airflow Limitation(1) Acad Radiol. 2009 doi: 10.1016/j.acra.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuoka SWG, Yamashiro T, San Jose Estepar R, Diaz A, Silverman EK, Hoffman EA, Fessler HE, Criner GJ, Marchetti N, Scharf SM, Martinez FJ, Reilly JJ, Hatabu H. Pulmonary hypertension and CT measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200908-1189OC. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyquist H. Certain topics in telegraph transmission theory. Trans AIEE. 1928;47:617–644. [Google Scholar]
- 54.Hughes JM, Hoppin FG, Jr, Mead J. Effect of lung inflation on bronchial length and diameter in excised lungs. J Appl Physiol. 1972;32(1):25–35. doi: 10.1152/jappl.1972.32.1.25. [DOI] [PubMed] [Google Scholar]
- 55.Staring M, BME, Shamonin DP, Stolk J, Reiber JHC, Stoel BC. Towards local estimation of emphysema progression using image registration. Proc of SPIE. 2009;7259:72590O-1–9. [Google Scholar]
- 56.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 57.Fujimoto JG, Brezinski ME, Tearney GJ, Boppart SA, Bouma B, Hee MR, Southern JF, Swanson EA. Optical biopsy and imaging using optical coherence tomography. Nat Med. 1995;1(9):970–2. doi: 10.1038/nm0995-970. [DOI] [PubMed] [Google Scholar]
- 58.Tearney GJ, Brezinski ME, Bouma BE, Boppart SA, Pitris C, Southern JF, Fujimoto JG. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997;276(5321):2037–9. doi: 10.1126/science.276.5321.2037. [DOI] [PubMed] [Google Scholar]
- 59.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coxson HO, Quiney B, Sin DD, Xing L, McWilliams AM, Mayo JR, Lam S. Airway wall thickness assessed using computed tomography and optical coherence tomography. Am J Respir Crit Care Med. 2008;177(11):1201–6. doi: 10.1164/rccm.200712-1776OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang VX, Tang SJ, Gordon ML, Qi B, Gardiner G, Cirocco M, Kortan P, Haber GB, Kandel G, Vitkin IA, Wilson BC, Marcon NE. Endoscopic Doppler optical coherence tomography in the human GI tract: initial experience. Gastrointest Endosc. 2005;61(7):879–90. doi: 10.1016/s0016-5107(05)00323-8. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z, Milner TE, Srinivas S, Wang X, Malekafzali A, van Gemert MJ, Nelson JS. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography. Opt Lett. 1997;22(14):1119–21. doi: 10.1364/ol.22.001119. [DOI] [PubMed] [Google Scholar]
- 63.Short MA, Lam S, McWilliams A, Zhao J, Lui H, Zeng H. Development and preliminary results of an endoscopic Raman probe for potential in vivo diagnosis of lung cancers. Opt Lett. 2008;33(7):711–3. doi: 10.1364/ol.33.000711. [DOI] [PubMed] [Google Scholar]
- 64.Kauczor HU, Ley-Zaporozhan J, Ley S. Imaging of pulmonary pathologies: focus on magnetic resonance imaging. Proc Am Thorac Soc. 2009;6(5):458–63. doi: 10.1513/pats.200901-002AW. [DOI] [PubMed] [Google Scholar]
- 65.Yablonskiy DA, Sukstanskii AL, Leawoods JC, Gierada DS, Bretthorst GL, Lefrak SS, Cooper JD, Conradi MS. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI. Proc Natl Acad Sci U S A. 2002;99(5):3111–6. doi: 10.1073/pnas.052594699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patz S, Muradian I, Hrovat MI, Ruset IC, Topulos G, Covrig SD, Frederick E, Hatabu H, Hersman FW, Butler JP. Human pulmonary imaging and spectroscopy with hyperpolarized 129Xe at 0.2T. Acad Radiol. 2008;15(6):713–27. doi: 10.1016/j.acra.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc Natl Acad Sci U S A. 2006;103(48):18278–83. doi: 10.1073/pnas.0608458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mugler JP, 3rd, Driehuys B, Brookeman JR, Cates GD, Berr SS, Bryant RG, Daniel TM, de Lange EE, Downs JH, 3rd, Erickson CJ, Happer W, Hinton DP, Kassel NF, Maier T, Phillips CD, Saam BT, Sauer KL, Wagshul ME. MR imaging and spectroscopy using hyperpolarized 129Xe gas: preliminary human results. Magn Reson Med. 1997;37(6):809–15. doi: 10.1002/mrm.1910370602. [DOI] [PubMed] [Google Scholar]