Abstract
Electrogenic communication appears to have evolved independently in a variety of animal and plant lineages. Considered here are metazoan cells as disparate as the loose three-dimensional parenchyma of glass sponges, the two-dimensional epithelial sheets of hydrozoan jellyfish and the egg cell membranes of the ctenophore Beroe ovata, all of which are capable of generating electrical impulses. Neuronal electrogenesis may have evolved independently in ctenophores and cnidarians but the dearth of electrophysiological data relating to ctenophore nerves means that our attention is focused on the Cnidaria, whose nervous systems have been the subject of extensive study. The aim here is to show how their active and passive neuronal properties interact to give integrated behaviour. Neuronal electrogenesis, goes beyond simply relaying ‘states of excitement’ and utilizes the equivalent of a set of basic electrical ‘apps’ to integrate incoming sensory information with internally generated pacemaker activity. A small number of membrane-based processes make up these analogue applications. Passive components include the decremental spread of current determined by cellular anatomy; active components include ion channels specified by their selectivity and voltage dependence. A recurring theme is the role of inactivating potassium channels in regulating performance. Although different aspects of cnidarian behaviour are controlled by separate neuronal systems, integrated responses and coordinated movements depend on interactions between them. Integrative interactions discussed here include those between feeding and swimming, between tentacle contraction and swimming and between slow and fast swimming in the hydrozoan jellyfish Aglantha digitale.
KEY WORDS: Cnidaria, Ctenophores, Electrogenesis, Ion channel, Ionic currents, Sponges
Introduction: electrogenesis in early Metazoa
It is evident that many of the ion channel variants involved in signal propagation in multicellular life forms have their origins in bacteria and other single-celled species. In fact, propagating impulses arise in a number of different evolutionary lineages. In each case, natural selection co-opted much the same set of ion channels but inserted them into a different cell matrix. Besides networks of neuronal-like cells, these matrices include the loose three-dimensional parenchyma of glass sponges such as Rhabdocalyptus (Leys and Mackie, 1997; Leys et al., 1999) and the two-dimensional epithelial sheets found in the Hydrozoa (Mackie, 1965; Mackie, 1976; Mackie and Passano, 1968). The following is a brief description of four classes of non-neuronal electrogenesis followed by a more detailed account of integrative aspects of neuronal electrogenesis as exhibited by the Cnidaria. For an account that includes aspects of electrogenesis in the Protozoa, see Meech and Mackie (Meech and Mackie, 2007), for a more general account, see Meech (Meech, 2015).
Electrogenesis in the glass sponge Rhabdocalyptus dawsoni
Electrical events propagate within Rhabdocalyptus because its trabecular reticulum is a syncytium with cytoplasmic bridges that allow the free flow of ionic current. Like other sponges Rhabdocalyptus is a filter feeder that uses delicate flagellated chambers to pump sea water. Stimuli such as a current pulse or, more naturally, a local mechanical disturbance such as sediment in the inflowing water, are associated with a protective response – an immediate cessation of pumping (Lawn et al., 1981; Mackie et al., 1983). These are not the easiest animals to record from and thus far all attempts to penetrate glass sponge cells with microelectrodes have failed. The cells also resist forming high-resistance seals for patch clamp experiments, but they do recognize, and accept, grafted clumps of cells prepared from their own tissue. It is from these grafted attachments that ‘all-or-nothing’ action potentials have been recorded in response to electrical stimulation (Leys and Mackie, 1997). In Rhabdocalyptus, action potentials last for at least 5s and propagate at a speed of 0.17–0.3 cm s−1. Substituting 50% of the sodium chloride in the bathing medium with choline chloride had little effect but the action potentials were reversibly blocked by 10 mmol l−1 Co2+ as if the inward current is carried by Ca2+ (Leys et al., 1999; Leys and Meech, 2006). By analogy with Paramecium (Naitoh and Eckert, 1969), it seems likely that it is the influx of Ca2+ into the cells of the flagellated chamber that stops their pumping.
Electrogenesis in an excitable two-dimensional sheet
Impulse propagation in the exumbrella of hydrozoan jellyfish such as Sarsia and Nanomia (Mackie, 1964; Mackie and Passano, 1968; Passano et al., 1967) is made possible by the presence of epithelial gap junctions [which are absent in other forms of jellyfish such as the scypho- and cubo-medusae (Mackie et al., 1984]. Action potentials are elicited by electrical or mechanical stimulation and spread throughout the epithelium at a speed of 24–31 cm s−1 (at 12°C). If a Nanomia colony meets an obstruction, an impulse in the exumbrella causes contraction in its bell-like nectophores; a jet of water is directed forwards and the colony is propelled backwards out of harm's way (Mackie, 1965). The whole of the exumbrella behaves like a sensory surface.
The delicate nature of the epithelial tissue means that the ionic currents generated by a depolarizing command are best studied with the ‘loose patch clamp’ technique (Stühmer et al., 1983). Using this technique on Nanomia, we applied a relatively large micropipette (about 10 μm tip diameter) to the surface of the epithelium where it formed a seal that was about four times the pipette resistance (R.W.M. and G. O. Mackie, unpublished results). Pipettes were filled with seawater and so pipette resistances were in the range 180–280 KΩ. Seals were encouraged to form using a maintained negative pipette pressure of about 5 mmHg. Leakage current flowing between the pipette tip and the surrounding medium through the loose seal were subtracted by a combination of an analogue circuit (see Stühmer et al., 1983) and digital processing (P4 technique) (Armstrong and Bezanilla, 1974). In nerve-free regions, Nanomia was found to exhibit two inward currents: one carried by Na+, activated around 0 mV, the other appeared at potentials closer to rest and was carried by Ca2+. Thus, one might expect to see action potentials in either Na+-free or Ca2+-free saline but not in Na+/Ca2+-free saline, just as in the exumbrellar epithelium of Euphysa japonica (Schwab and Josephson, 1982).
Electrogenesis in oocytes
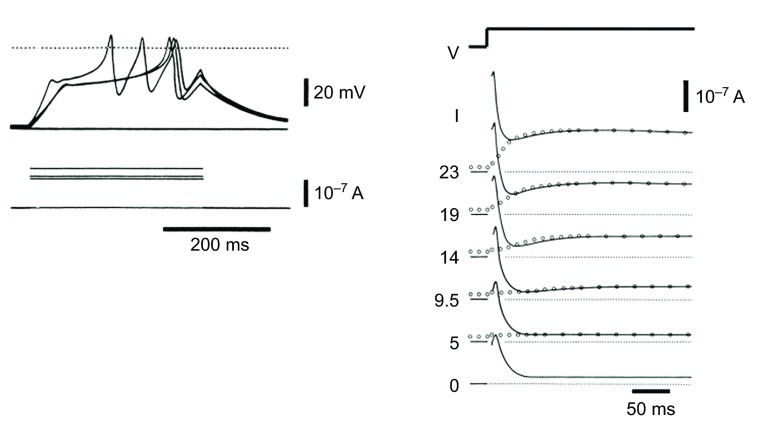
Fig. 1 shows records from an oocyte of the cnidarian Renilla reniformis, the ‘Sea Pansy’ (Hagiwara et al., 1981). Injection of a maintained depolarizing current eventually generates an action potential or even a series of action potentials. In many fertilized oocytes the action potential is part of the mechanism that prevents polyspermy. Hagiwara et al. (Hagiwara et al., 1981) studied the currents using a two-electrode voltage clamp and found that they were dominated by a rapidly activating and inactivating outward potassium current. The function of this current is to prevent the oocyte generating an action potential unless the stimulus is persistent. With a maintained stimulus the outward current gradually inactivates so that any remaining inward current will depolarize the cell.
Fig. 1.
Electrogenesis in a Renilla oocyte. Action potentials (shown at left) elicited during a prolonged depolarization are delayed by a rapidly activating transient K+ current recorded during depolarizing commands under voltage clamp (shown at right). Depolarizing command voltage in mV shown at the start of each current trace. Holding potential for continuous traces, −76 mV; holding potential for data shown by open circles, −30 mV. From Hagiwara et al. (Hagiwara et al., 1981).
Using techniques similar to those of Hagiwara et al., it was possible to record action potentials in oocytes from different species of Beroe (see Fig. 2) (A. Bilbaut, M.-L. Hernandez-Nicaise and R.W.M., unpublished results). When depolarized under a two-electrode voltage clamp Beroe oocytes exhibited a rapidly activating transient outward potassium current like that in Renilla. Interestingly, we found that the kinetics of the current in each of the two species we studied (B. ovata and B. mitrata) was markedly different. The current in B. mitrata inactivated so quickly that one would expect a maintained stimulation to generate a series of action potentials as in Renilla oocytes (Fig. 1A).
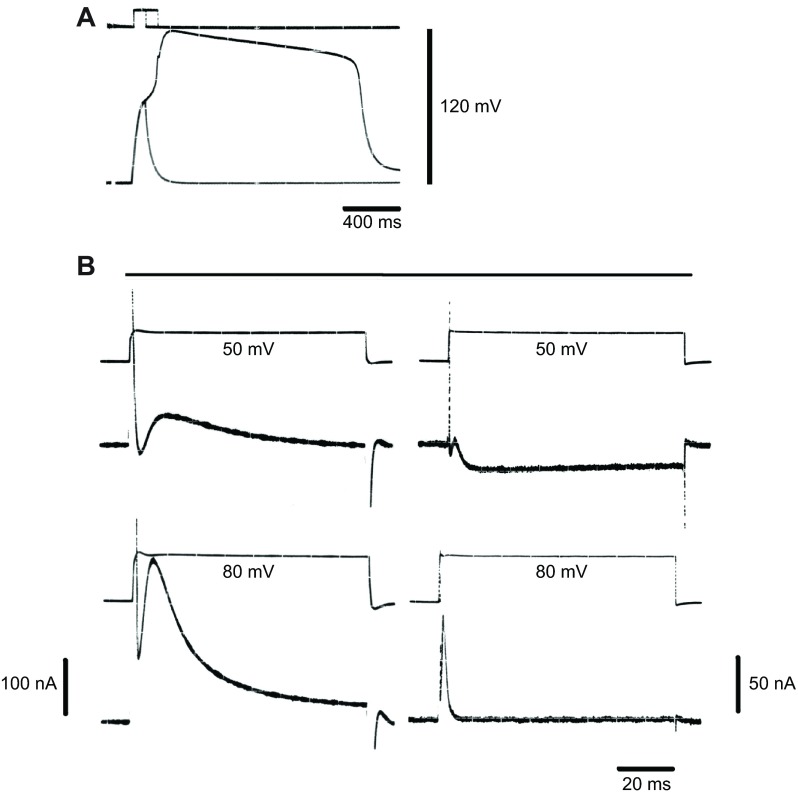
Fig. 2.
Electrogenesis in a Beroe egg. (A) Action potential recorded (bottom trace) in response to a depolarizing current pulse (top trace). Two superimposed traces; the shorter current pulse is sub-threshold. (B) Ionic currents (bottom traces) elicited by voltage commands under voltage clamp (top traces). Traces on the left are from Beroe ovata; those on the right hand are from Beroe mitrata. Holding potential, −60 mV (B. ovata); −70 mV (B. mitrata). Measurements at room temperature in artificial sea water. Data from A. Bilbaut, M.-L. Hernandez-Nicaise and R.W.M., unpublished results.
Electrogenesis in ctenophore muscle cells
Analysis of the genomes of Mnemiopsis leidyi (Ryan et al., 2013) and Pleurobrachia bachei (Moroz et al., 2014) supports the possibility that ctenophore neurons evolved independently of other nervous systems (see Moroz, 2015). Electron microscopy shows synapse-like and neuron-like structures (Hernandez-Nicaise, 1973; Hernandez-Nicaise, 1991) while immunofluorescence studies demonstrate the existence of two distinctly separate nerve nets (Jager et al., 2011). Unfortunately, we can say little about how ctenophore nerves function because of the marked absence of electrophysiological recordings (Tamm, 2014). This is largely because of the size of the supposed neuronal elements, but also because they are buried within a stiff mesoglea. Ctenophore muscles, however, although highly stretch sensitive, are large enough to be accessible to intracellular micropipettes either in situ (Hernandez-Nicaise et al., 1980) or after isolation (Bilbaut et al., 1988a; Bilbaut et al., 1988b; Anderson, 1984; Stein and Anderson, 1984). In Beroe ovate, three main classes of fibre may be distinguished: radial, longitudinal and intestinal. Longitudinal fibres generate single, long-lasting action potentials in response to a depolarizing command, whereas radial fibres produce multiple impulses. Two-electrode voltage-clamp records show that although their electrophysiology differs markedly, the inward calcium current that provides the signal for contraction, forms an important component in each (Bilbaut et al., 1988a). These properties correlate with their separate functions, which in the case of the radial fibres is to maintain the tension across the mesoglea to stiffen it into a hydroskeleton.
Although a study of the Beroe nervous system was not the primary focus of our work, an incidental observation is of interest in view of the absence of other published work. Fig. 3 shows what appear to be excitatory synaptic events recorded in an enzymically isolated fragment of muscle (A. Bilbaut, M.-L. Hernandez-Nicaise and R.W.M., unpublished results). Some fragments have been observed, in EM sections, to have retained their synapses despite the enzyme treatment (M.-L. Hernandez-Nicaise, personal communication). Flurries of excitatory depolarizations were observed to summate and give rise to overshooting action potentials.
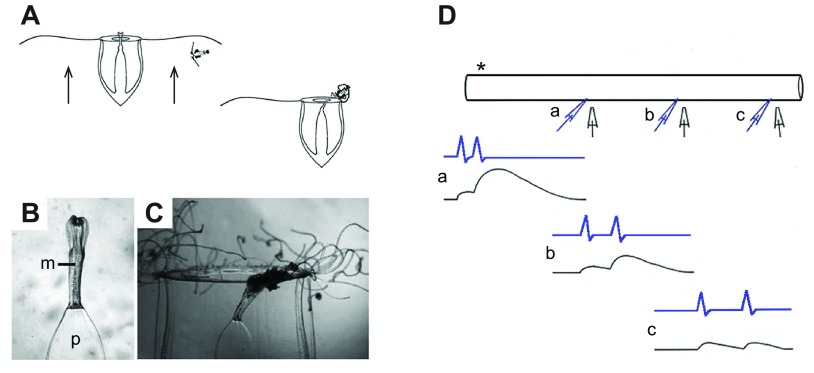
Fig. 3.
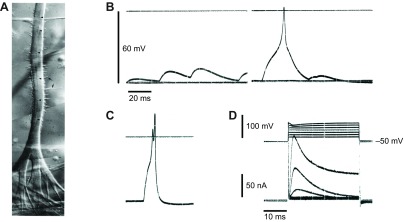
Electrogenesis in enzymically isolated smooth muscle from Beroe ovata. (A) Pharyngeal end of a living radial muscle cell. The fibre branches extensively; the endings anchor upon the epithelium, which is hidden by the pharyngeal circular muscles. Nomarski interference contrast. Scale bar, 20 μm. Note the multiple nuclei (arrowheads) and the thin sarcolemmal evaginations (arrows). From Hernandez-Nicaise et al. (Hernandez-Nicaise et al., 1980). (B,C) Series of spontaneous depolarizing events, possibly synaptic in origin, recorded from a fragment of longitudinal muscle produced by enzymic digestion (see Bilbaut et al., 1988a). In C, falling phase of the EPSP (and its short-circuiting effect) appears to end just before the peak of the action potential giving the appearance of a double spike. (D) Voltage-clamp records from same muscle fragment showing the typical response of a longitudinal muscle to depolarizing commands. Note that the clamp was imperfect at positive potentials because the micropipette could not supply the large currents necessary to control the membrane potential. Holding potential −50 mV; experiments conducted in artificial sea water at room temperature. Data from A. Bilbaut, M.-L. Hernandez-Nicaise and R.W.M., unpublished results.
The elements of electrogenesis
The frequency with which natural selection assembled the building blocks for impulse generation might seem surprising were it not for the modular nature of ion-selective channels. The definition and significance of modularity in evolution has been reviewed by Schlosser and Wagner (Schlosser and Wagner, 2004). For them, a module is ‘a component of a system that operates largely independently of other components’. Such is the case with the many classes of ion-selective channel that contribute to the complex process of electrogenesis. Not only is each individual channel molecule to a large extent a stand-alone unit, but channel functions (such as selectivity, gating etc.) are also separated into specific structural domains.
The advantage of a modular system of construction was highlighted by Simon (Simon, 1962) in the form of a parable about two highly regarded watchmakers. The problem with their being so highly sought after was that they were constantly being interrupted. When they were interrupted, their partly assembled watches fell to pieces. The successful watchmaker was the one who designed his watch to be made of subassemblies. That way, were he to be interrupted, he lost only a small proportion of his work and the completed subassemblies could be quickly put together to make a fully operational watch.
The apparent ease with which natural selection generates electrogenesis from ion-channel modules is consistent with its independent evolution in ctenophores and cnidarians. That being so, comparisons between different neural systems should increase our understanding of the factors that determine their form and function. Moroz (Moroz, 2015) has compared the transmitter make-up predicted by the ctenophore genome with that of other nervous systems. Here, I discuss the performance of the hydrozoan nervous system, in particular the contribution of its electrical properties to neural integration.
Implications for neuronal integration
Electrogenesis, in the different forms we have examined thus far, is purely reactive; a glass sponge takes in sediment, the message spreads and the whole structure stops pumping water; Nanomia bumps into a barrier and the colony immediately propels itself backwards. Neuronal electrogenesis, however, goes beyond simply relaying ‘states of excitement’ as Sherrington (Sherrington, 1906) expressed it. To be a fully mobile exploratory unit, an animal must take stock of its environment; it must sample multiple modalities and integrate the information obtained so that responses can be coordinated and executed in an efficient manner. Neural integration requires that all-or-nothing signals be translated into graded responses; coordination requires the operation of one function at the expense of another.
To begin this section of the review, I focus on the role of ion channels in mechanisms of integration and coordination in hydrozoan jellyfish; links with the scypho- and cubo-medusae are necessarily restricted to a short section at the end. I follow others in identifying four main areas of interest: swimming, control of tentacle length, defensive responses [called ‘crumpling’ (Hyman, 1940)] and escape, mouth manipulation during feeding [described as ‘pointing’ (Romanes, 1877)].
Hydromedusae are such a diverse group of animals that it has been a challenge to define a ‘ground plan’ for their neural circuitry. However, one common feature is the production of a regular swim rhythm by a distinct set of electrically coupled neurons – the pacemaker system (Satterlie and Spencer, 1983). Many hydromedusae stop swimming while they are feeding (Horridge, 1955b), suggesting that an inhibitory input to the pacemaker system is another common feature. In many species, swimming is preceded by tentacle retraction as if a common mechanism ensures a delay in the initiation of swimming until after the tentacles have contracted. Variations in the ground plan include the circuitry for ‘escape swimming’, which in Aglantha and Nanomia replace crumpling (Donaldson et al., 1980; Mackie, 1964) and the different ways of reducing tentacle drag exhibited by Chelophyes and Muggiaea (see later).
The nervous control of these different aspects of jellyfish behaviour is somewhat modular in nature in that each is under the influence of a separate neuronal system. Clune et al. (Clune et al., 2013) consider a network to be modular if it contains ‘highly connected clusters of nodes that are sparsely connected to nodes in other clusters’. Such nerve circuit modules may have arisen as a way of minimizing the cost of making nerve connections (see Laughlin and Sejnowski, 2003) but thereafter they are likely to be favoured during the trial and error process of natural selection (Simon, 1962). Despite this, the requirement for integration and coordination depends on interaction between modules. Here, I focus on three such interactions: between feeding and swimming, between tentacle contraction and swimming and between slow and fast swimming in Aglantha.
Interaction between feeding and swimming
The way in which the tentacles capture small planktonic organisms and bring them to the margin has been described previously (Romanes, 1877; Horridge, 1955a). In Aglantha digitale, the graded movements of the tentacles are coordinated by the ‘tentacle conduction system’ (Mackie and Meech, 1995a; Mackie and Meech, 1995b). Once the prey is at the margin, the manubrium (mouth) bends toward the food (points) and engulfs it with its lips prior to ingestion as shown in Fig. 4. In less prolate species such as Aequorea, a local contraction of the margin serves to bring the food towards the manubrium during the transfer process (Horridge, 1955b). The radial, smooth muscles responsible for the local contraction are located in the ectoderm where they overlie the circular, striated swim muscles. There are no local contractions in Aglantha for the good reason that radial muscles are absent from the underside of the bell. They do, however, persist (as longitudinal muscles) in the manubrium where they are associated with bundles of small axons with FMRFamide-like immunoreactivity. These axons are shown as part of the ‘F’ system in Fig. 5; they run down the bell towards the margin alongside each radial canal (Mackie et al., 2003).
Fig. 4.
Food capture in Aglantha digitale. (A) Ciliary beating drives water past the extended tentacles (arrows) bringing them into contact with prey (a copepod). Tentacular contraction and flexion brings the prey to the margin while the manubrium points across to it with flared lips. (B) Peduncle (p) and manubrium (m) of an unfed animal at rest. (C) Lateral flexion (pointing) to a site where food is held by the tentacles. (D) Mechanism for gradation of manubrial muscle response with distance from the prey site (*). Each set of traces (a–c) represents a pair of F impulses (top) and the graded muscle responses (bottom) at each of the recording sites shown. The second F impulse travels more slowly than the first and summation becomes less effective as the action potentials become increasingly separated. See text for more details. A–C from Mackie et al. (Mackie et al., 2003); D adapted and redrawn from Mackie (Mackie, 1984).
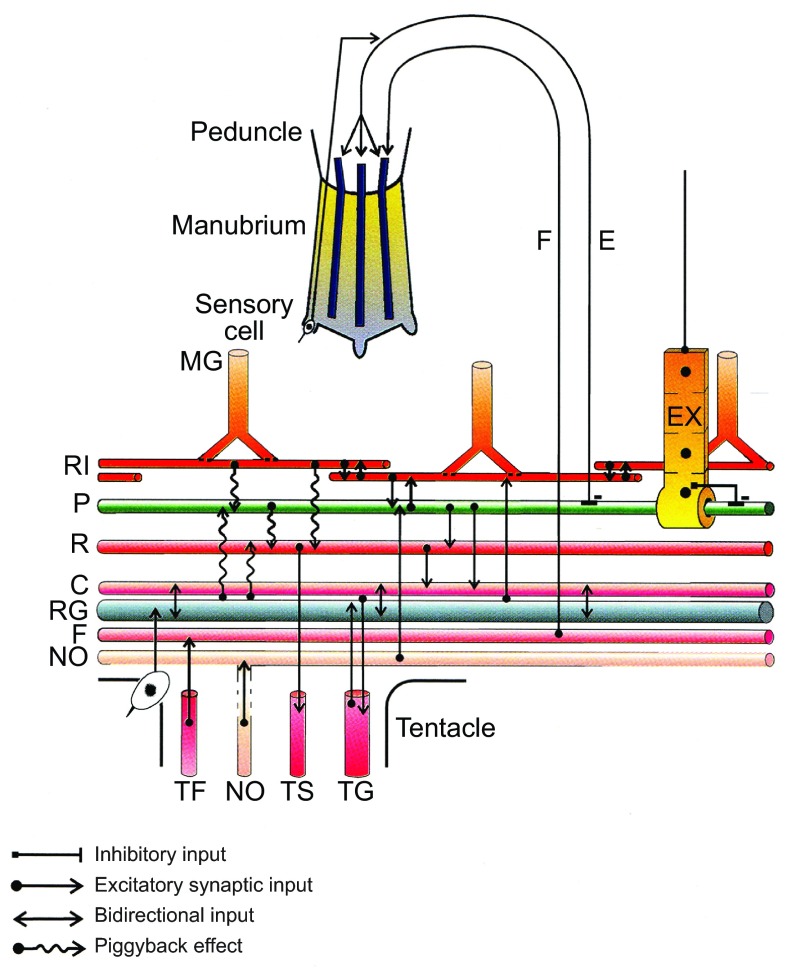
Fig. 5.
Nerve circuits in Aglantha digitale. An outline of the principal pathways involved in swimming, the control of tentacle length and mouth manipulation. Three of the eight longitudinal muscle bands (blue) lying in the wall of the peduncle and manubrium are shown. Gap junctions are shown as incomplete membrane partitions at the junctions of the rootlet interneurone system (RI) with the motor giant axons (MG) and between the epithelial cells. A minus sign indicates inhibitory input; all others are excitatory. The piggyback effect is described in Mackie and Meech (Mackie and Meech, 1995a). The pathways include: (1) the flexion system (F) that mediates the pointing movements seen during feeding (Mackie et al., 2003); (2) the endodermal system (E) that mediates lip flaring and swimming inhibition (Mackie et al., 2012); (3) the swim pacemaker system (P) (Mackie and Meech, 2000); (4) the exumbrellar conduction system (EX) which also inhibits swimming (Mackie and Singla, 1997); the cells in this epithelial pathway are coupled by gap junctions; conduction is unpolarized so that impulses spread in all directions; (5) the ring giant axon (RG) excited by vibration receptors at the bell margin mediates escape swimming via the carrier (C) system and the motor giant axon (MG) (Mackie and Meech, 1995a; Mackie and Meech, 1995b); (6) the nitric oxide pathway (NO) (Moroz et al., 2004); (7) the relay system (R) (Mackie and Meech, 1995a); (8) tentacle nerves include those nerves that feed into the F system (TF), the tentacle giant axon (TG) and the slow tentacle system (TS) (see Mackie and Meech, 1995a; Mackie and Meech, 1995b, Mackie and Meech, 2000).
When Aglantha preparations were stimulated on the outer nerve ring at the base of one of the radial canals, i.e. at a point where a small axon bundle joins the margin, the manubrium ‘pointed’ to the stimulation site. Such pointing was found to be accurate to within 15 deg, 70% of the time (Mackie et al., 2003). It was just as accurate when the stimulating electrode was moved to an intermediate position between radial canals as if the radial pathways were linked by circular pathways within the nerve ring (shown as a horizontal cylinder, F, in Fig. 5). We suppose that when food is captured at a point on the margin near the origin of a small axon bundle, pointing to that site results from impulses travelling directly to the associated muscle band in the manubrium.
The muscle bands closest to the food site will be the first to be excited, but impulses will spread to other small axon bundles by way of the F system in the nerve ring. It appears, however, that this excitation may fail to be conveyed to the associated muscle bands. A possible explanation stems from the observation that pointing seems to require multiple firing of the small axon bundle and that the second of two impulses evoked 250 ms apart travels 73% more slowly than the first (Mackie et al., 2003). This means that the further the impulses travel the more separated they will be. As Fig. 4B illustrates, if successive impulses produce summating responses in the manubrium, the contraction of the muscle band closest to the food site will be enhanced relative to contraction at other muscle bands further away. An integrative mechanism similar to this (but involving two nerves with different conduction velocities) has been demonstrated in the stem of Nanomia (see Mackie, 1984).
During feeding, nerve impulses not only travel towards the manubrium, they also go from the manubrium to the margin via the ‘E’ system (see Fig. 5). At the margin the E system is believed to make synaptic contact with the pacemaker system. The pacemaker neurons that generate rhythmic swimming in hydromedusae have been identified and studied in most detail in Polyorchis penicillatus (see Fig. 6A) (Anderson and Mackie, 1977; Satterlie and Spencer, 1983). These ‘swim motor neurons’, which are located in the inner nerve ring at the base of the bell, are directly linked to the subumbrellar musculature via chemical synapses (Spencer, 1975). Electrical coupling (via gap junctions) means that intracellularly recorded action potentials are synchronous throughout the swim motor neuron system. In many hydrozoan species, swim motor neurons show what appear to be slow endogenously generated baseline oscillations, but the precise pattern of activity depends on input from other sources such as the ‘B’ and ‘O’ systems. Fig. 6B is a schematic diagram to show the relationships between these different systems (Spencer and Arkett, 1984).
Fig. 6.
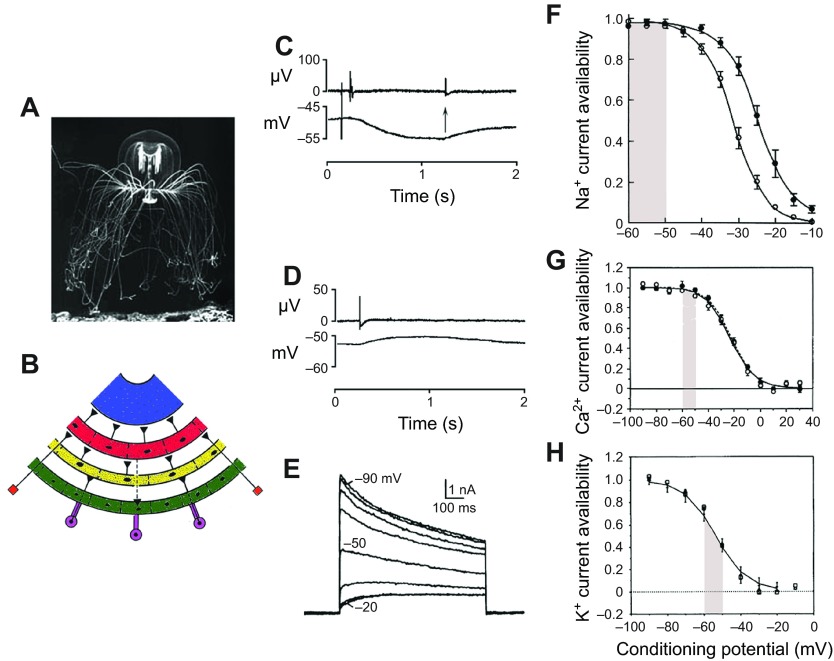
Excitatory synaptic input to Polyorchis penicillatus swim motor neurons. (A) Polyorchis (photograph provided by Claudia Mills). (B) Partial representation of the ring-shaped neuronal networks showing known synaptic contacts. The muscle epithelium (blue) is directly excited by pacemakers in the inner nerve ring, the swim motor neurons (red). They, in turn, receive an excitatory synaptic input from neurons of the ‘B’ system (yellow) in the outer nerve ring and from unicellular receptors (orange). Excitatory inputs to the ‘B’ system are shown arising in the ocelli (purple). Also shown is an excitatory pathway from the swim motor neurons to the ‘O’ system (green) in the outer nerve ring; other connections may be present. Excitatory synapses are indicated by filled triangles. All three networks consist of a ring of electrically coupled nerve cells. Adapted from Spencer and Arkett (Spencer and Arkett, 1984). (C) Intracellularly recorded inhibitory potential (bottom) in response to an externally recorded E event (top) after an electrical stimulus to the nearby radial canal. The IPSP was terminated by a spontaneous depolarizing excitatory postsynaptic potential (EPSP; arrow) that is coincident with an externally recorded B system pulse. (D) Excitatory synaptic potential (lower trace) coincident with an externally recorded ‘B’ system impulse; four excitatory events were aligned and averaged. D and E are from Mackie et al. (Mackie et al., 2012). (E) Inactivating K+ currents recorded under voltage clamp in response to test commands to +50 mV. Each test command preceded by a conditioning command lasting 1 s. The superimposed current traces show the effect of the conditioning level (range −90 to −20 mV; 10 mV steps) on the availability of the inactivating current. At −20 mV the inactivating component (IKfast) is absent, leaving IKslow. From Przysiezniak and Spencer (Przysiezniak and Spencer, 1994). (F) Availability of INa at different conditioning potentials. Each test command (to +10 mV) preceded by a 2 s conditioning command. Fast (open circles) and slow (filled circles) components obtained by fitting exponential curves to raw data. Data normalized to maximal current and averaged ± s.e.; N=14). The curves are fitted Boltzmann functions. Holding potential; −80 mV. For more details, see Grigoriev et al. (Grigoriev et al., 1996). (G) Availability of ICa at different conditioning potentials. Each test command (to +10 mV) preceded by a 1 s conditioning command. Peak currents measured 2.2–9.2 ms from the beginning of the test pulse. Average values for ICa are shown by filled circles (n=6); for IBa open circles (n=7). Data normalized to maximal current after subtracting the non-inactivating component. The curves are Boltzmann functions fitted to the average peak current. Holding potential, −80 mV. For more details see Przysiezniak and Spencer (Przysiezniak and Spencer, 1992). (H) Availability of IKfast at different conditioning potentials. Each test command (to +50 mV) preceded by a 1 s conditioning command. Peak currents measured at 12 ms after the command onset. Average values are shown by filled circles (N=8). Data normalized to the maximal current after subtracting the non-inactivating component. The solid curve is a Boltzmann function fitted to the average peak current. For more details, see Przysiezniak and Spencer (Przysiezniak and Spencer, 1994).
Many hydromedusae stop swimming while they are feeding (Horridge, 1955b) and Aglantha is no exception (Mackie et al., 2003). Swimming was inhibited when food was presented directly to the lips or following electrical stimulation of the manubrium; the period of inhibition depended on the stimulus duration. Perhaps the strong water currents generated during swimming tend to dislodge trapped prey and inhibition serves to facilitate the transfer of food to the mouth.
Analysis of the inhibitory pathway concerned depended on work with three different hydrozoan species; an example of the way in which similarities of ground plan can aid the experimentalist. In Neoturris breviconis the trains of action potentials (E impulses) that arise in the manubrium during ingestion are conducted in a nerve plexus located in the endodermal walls of the stomach and radial and ring canals (Mackie and Meech, 2008). Neurites belonging to this E system run around the margin adjacent to the inner nerve ring, where the pacemaker neurons are located but direct synaptic contact has yet to be demonstrated anatomically (Mackie et al., 2003).
There appears to be the equivalent of an E system in other medusae, such as Sarsia (Hernandez-Nicaise and Passano, 1967), Aglantha (shown in Fig. 5) and Polyorchis (Mackie et al., 2012). In Polyorchis the swim motor neurons are larger than in other species and it has proved possible to record from them intracellularly while stimulating the E system axons (Mackie et al., 2012). Following stimulation, the appearance of the E impulse at the periphery was associated with an inhibitory postsynaptic potential (IPSP) in a nearby swim motor neuron (Fig. 6C). In a typical example, the hyperpolarization reached a maximum after about 700 ms and stayed there for about 500 ms. Its reversal potential was about −69 mV.
The significance of this unusually prolonged IPSP becomes apparent when we consider the pacemaker mechanism. In rhythmically firing cells, the inter-pulse interval is determined by a number of ‘background’ currents (Connor and Stevens, 1971), one of which is a rapidly activating transient K+ current first identified in molluscan neurons (Hagiwara et al., 1961) and commonly called IKA. The properties of a similar current (here called IKfast) in Polyorchis swim motor neurons (see Fig. 6E) have been examined by Przysiezniak and Spencer (Przysiezniak and Spencer, 1994). The availability of this current depends on preconditioning potentials in the range −20 to −90 mV (see Fig. 6H); the more depolarized the membrane, the more inactivated the channels become. As recovery from inactivation is complete within 1 s (Przysiezniak, 1993), a typical IPSP will increase the availability of IKfast and as a consequence, tend to delay the next pacemaker impulse. This effect is in addition to the increase in membrane conductance, which will also oppose the depolarizing effect of any background inward current.
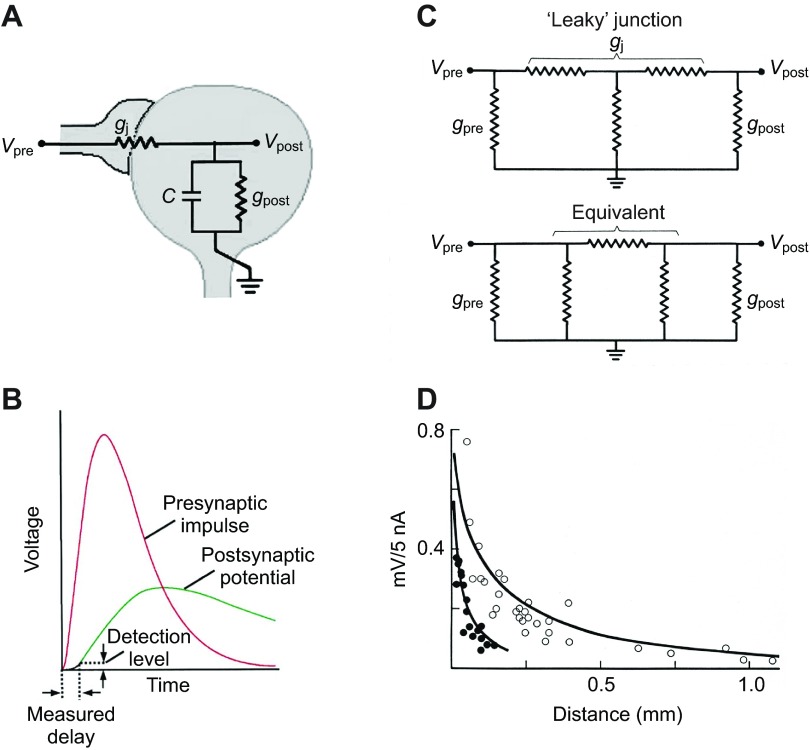
The slow time course of the swim motor neuron IPSPs (and excitatory post-synaptic potentials, EPSPs – see below) is a result of electrical coupling, which makes the entire system behave like a low-pass filter (see Fig. 7A,B) (Bennett, 1966; Bennett and Zukin, 2004). Spencer (Spencer, 1981) has measured the frequency cut-off for the swim system in Polyorchis and finds that signals are progressively attenuated and slowed at frequencies above about 1 Hz.
Fig. 7.
Low-pass filtering in electrically coupled neurons. (A) Equivalent circuit. A process from the presynaptic neuron connects with the post-synaptic cell through a junctional conductance gj. Current through the junctional conductance flows to ground through the post-synaptic membrane conductance and capacitance acting in parallel. From Bennett and Zukin (Bennett and Zukin, 2004). (B) A rapidly rising pre-synaptic impulse is attenuated and slowed because of the time needed to charge and discharge the capacitance; assuming a just-measurable detection limit a significant synaptic delay is introduced. From Bennett and Zukin (Bennett and Zukin, 2004). (C) Equivalent circuits for a ‘leaky’ junction. The current path from the junction is represented either by a resistance originating mid-way through the junction (top) or to resistances in parallel with pre- and post-junctional resistances (bottom; Wye-delta transformation). Leakage of current is therefore equivalent to a membrane shunt in the vicinity of the junction. It is not known if such a shunt is a physical reality. From Bennett (Bennett, 1966). (D) The anisometric nature of the spread of current in Aglantha myoepithelium. Mean membrane potential displacement produced by current injected at different distances from the recording site. In a thin sheet of coupled cells current flow is restricted to two dimensions and the steady state change in membrane potential declines with distance from a point source as a modified Bessel function (solid line) (see Jack et al., 1975). Measurements in a circular direction (open circles) and radial direction (closed circles). The difference in current spread suggests that the current path in the circular direction has fewer leaky gap junctions as might be expected from the elongated form of the individual cells. From Kerfoot et al. (Kerfoot et al., 1985).
Interaction between tentacle contraction and swimming
Polyorchis and other hydromedusae [Eperetmus typus (Mackie and Mackie, 1963); Leuckartiara octona (Russell, 1953)] that engage in ‘sink-fishing’ and spend much of their time drifting with their tentacles extended, shorten them just before swimming. Presumably, this serves to remove the drag that they would otherwise generate. In Proboscidactyla flavicirrata, synchronous tentacle shortening occurs less than 1 s before a swim burst (Spencer, 1975) but in the siphonophore Muggiaea, with its more extensive array of tentacles, the delay can last for as long as 3 s (R.W.M., unpublished results).
In Polyorchis, tentacle length is under the control of the ‘B’ system, which provides an input to the swim system from all around the nerve ring (see Fig. 6B). These B system impulses have been variously called ‘marginal’ or ‘pre-tentacle’ pulses (Passano, 1965; Passano et al., 1967; Ohtsu and Yoshida, 1973; Mackie, 1975; Spencer, 1975). They originate from a system of electrically coupled neurons that lie along the mid-line of the outer nerve ring just below the epithelial cell layer (Satterlie and Spencer, 1983). Action potential spiking in B neurons, like that in the swim system, appears to have an endogenous component and it too is synchronous throughout. B system pulse frequencies of 1 s−1, or more, cause maintained tentacle shortening as well as generating synchronized and slowly depolarizing EPSPs (Fig. 6D; mean duration, 1.3 s; time to half-decay, 0.5 s) in the swim system (Anderson and Mackie, 1977; Spencer and Arkett, 1984; Mackie et al., 2012).
In Sarsia, activity in the B system is often at too low a frequency to cause the tentacles to respond directly (Passano, 1973). Nevertheless, a single B system spike appears to advance the swim system pacemaker by some hidden mechanism. It is as if a single EPSP resets the pacemaker without raising the membrane to threshold. A pair of B system impulses, however, will initiate a swim system impulse after a characteristic delay that depends on the inter-pulse interval. If the B system pulses are 1 s apart, the delay is about 750 ms. Presumably, in this case, summating EPSPs in the swim system eventually exceed the action potential threshold.
EPSPs in Polyorchis swim motor neurons (Spencer and Arkett, 1984; Mackie et al., 2012) reach a broad flat maximum after about 750 ms (see Fig. 6D), long enough to partially inactivate IKfast, the fast inactivating K+ current (Przysiezniak and Spencer, 1994). IKfast is half-inactivated with the membrane at −52 mV and is almost fully inactivated after 1 s at −30 mV (Fig. 6H; Przysiezniak and Spencer, 1994) whereas the inward calcium and sodium currents show little inactivation in the range −50 to −60 mV (Fig. 6F,G; Przysiezniak and Spencer, 1992; Grigoriev et al., 1996). As the shaded region in Fig. 6F–H shows, swim system EPSPs act within the range of membrane voltage that reduces the availability of IKfast while having little effect on the channels carrying inward current. It appears therefore that the EPSP input has two effects: EPSPs not only summate and take the membrane towards threshold, but they also inactivate IKfast and reset the pacemaker by reducing the level of available potassium current. This explanation would account for Passano's (Passano, 1973) conclusion that the B system and swim system are linked by two processes, each following a different time course.
Tentacle drag in siphonophores
In Chelophyes appendiculata, another siphonophore that deploys an elaborate tentacle ‘net’ to trap its prey, swims are graduated in strength during a swim sequence (Bone, 1981; Chain et al., 1981). Weaker swims during the early stages of the sequence reconfigure the tentacle net as it is drawn into a more streamlined shape. The energy expended is the product of drag and velocity (Vogel, 1994) and so when the drag is high, energy can be conserved by keeping the swim velocity low; later in the swim sequence, when the stem and tentacles of the animal have had time to contract and the overall configuration is more streamlined, a greater velocity of movement can be achieved with the same expenditure of energy.
Action potentials recorded from the myoepithelium of Chelophyes during a series of swims, show a gradual increase in duration (Bone, 1981). A voltage-clamp analysis of enzymically isolated myoepithelial cells by Inoue et al. (Inoue et al., 2005) shows that the potassium channels responsible for repolarizing the membrane during an action potential, recover only slowly from inactivation (τ=13.2 s at −70 mV). This means that with each succeeding impulse, less and less K+ current becomes available for repolarization. The inward Na+ and Ca2+ currents recover in time for the next impulse (i.e. within 200 ms) and so Ca2+ entry is enhanced as the plateau phase of the action potential becomes progressively longer. It is the enhanced Ca2+ entry that leads to the increased strength of contraction.
The two siphonophores, Chelophyes and Muggiaea appear to have evolved different ways to manage their tentacle nets. In recent studies, Muggiaea was found to delay swimming until its tentacles were fully contracted, then, when it did finally swim, each contraction was of uniform strength. When we examined its myoepithelium using the loose patch clamp technique, we found in addition to the currents reported by Inoue et al. (Inoue et al., 2005), a second outwardly directed K+ current. By contributing to repolarization, this current would ensure a greater uniformity in action potential duration (R.W.M. and G. O. Mackie, unpublished results). Unlike Chelophyes, therefore, tentacle contraction in Muggiaea precedes swim activation, which occurs only after a considerable inbuilt delay.
Tentacle drag in Aglantha digitale
Although the tentacle net in Aglantha digitale is not as extensive as it is in the siphonophores its management is surprisingly complex. Aglantha is unusual in that it exhibits both fast swimming, when escaping from predators, and slow swimming, when gathering food (see Fig. 8 and Fig. 9A). The trailing tentacles would compromise both forms of swimming. During an escape swim, the tentacles rapidly shorten into tight coils as a result of activity in a large axon (the tentacle giant in Fig. 5) that runs within the tentacle itself (Donaldson et al., 1980). Roberts and Mackie (Roberts and Mackie, 1980) suggest that tentacle and ring giants are coupled electrically to minimize any synaptic delay. However, if the ring giant is destroyed, the tentacle giants can be excited by stimulating a system of small neurons in the outer nerve ring (the ‘carrier system’ in Fig. 5). This carrier system acts as an intermediary between the ring and tentacle giant axons and also between the ring giant and the motor giant axons in the subumbrella. One effect of the carrier system is that it delays the message getting to the bell musculature, thereby ensuring that the tentacles contract first. It also acts as a one-way ‘valve’, so that activity initiated in the giant motor axons is not transmitted to the ring giant and so back to the tentacles.
Fig. 8.
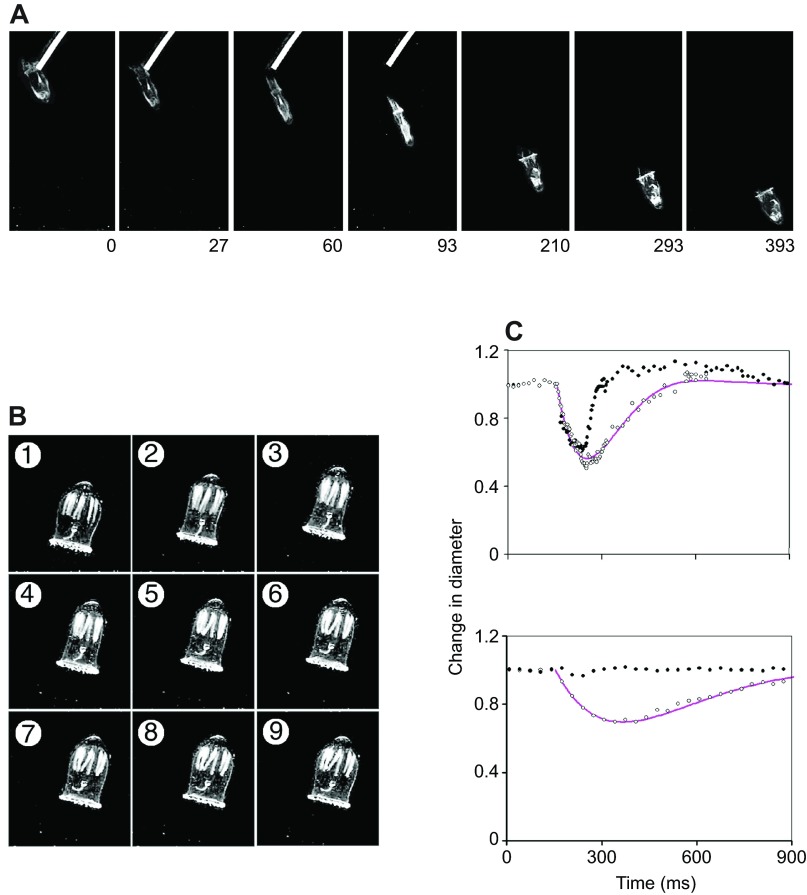
Two modes of swimming in Aglantha digitale. (A) Video frames of an escape swim elicited by a glass probe used to disturb vibration receptors at the base of the tentacles. Time (ms) after stimulus is shown at the bottom of each frame. (B) Video frames at 0.1 s intervals of a single slow swim. Frames were captured at 1/300 s. (C) Changes in bell diameter over time. During an escape swim (top) the jellyfish bell contracts uniformly along its length; during recovery the base diameter (closed circles) recovers more rapidly than the mid-bell diameter (open circles). Data measured from individual video frames including those in A. During a slow swim (bottom) the change in diameter at the base of the bell (closed circles) is significantly less than that at the mid point (open circles). Data measured from individual video frames including those in B. Lines through the data drawn according to an equation for the performance of a damped oscillator: x=A0exp−bt(sin 2πt/τ), where x is the reduction in bell diameter, A0 is the maximum diameter change in an un-damped system, b is the damping coefficient, t is time and τ is the period of the swim. Mid-way up the bell the values for A0 are 0.675 (fast swim), 1.0 (slow swim); b is 0.0038 in each case; τ is 480 ms (fast swim), 1750 ms (slow swim). At the base of the bell, the values during the slow swim are 0.18 (A0), 0.0038 (b), 1250 (τ). Temperature, 10°C. Data from R.W.M., unpublished results.
Fig. 9.
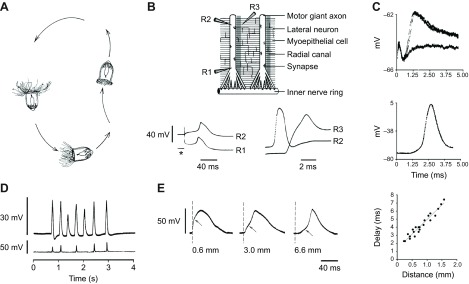
Electrophysiology of swimming in Aglantha digitale. (A) Aglantha is shown sinking passively with its tentacles extended ready to trap prey (left); asymmetrical swims bring it into an upright position (bottom); the tentacles become progressively contracted as an extended series of slow swims (right) takes the animal up the water column until the sequence restarts. From Mackie (Mackie, 1980). (B) (Top): Diagram of a section of the body wall at the base of the bell; the inner nerve ring, containing a variety of different systems including pacemaker neurones and the ring giant axon (see Fig. 5), is represented by a single cylinder. Synapses are shown between inner nerve ring and motor giant axons, and between motor giant axons and myoepithelium. Eight motor giant axons are distributed round the bell running beside a radial canal; only two are shown here. Lateral neurons are electrically coupled to the motor axon. Intracellular recording sites R1, R2 in the motor giant axon are 2 mm apart; R3 is in the myoepithelium, 80 μm from R2. (Bottom left): A propagating Ca2+ spike is recorded at R1 and R2 following a brief depolarizing current pulse (*). (Bottom right): An overshooting Na+-dependent action potential is recorded at R2 during a fictive escape swim; the response of the myoepithelium at R3 appears after a short synaptic delay (0.7±0.1 ms s.d.; n=6). Axon resting potential −72 mV (left); −62 mV (right); myoepithelium resting potential −77 mV. From Mackie and Meech (Mackie and Meech, 1985) and Kerfoot et al. (Kerfoot et al., 1985). (C) Properties of lateral neurons. (Top): Intracellular signal recorded from motor giant axon following 1 ms stimulus to nearby lateral nerve; three superimposed traces; in one, the stimulus was subthreshold and the stimulus artefact is seen alone. The suprathreshold signal (amplitude, 3.8±0.12 mV; mean ± s.d.; N=4) appears to be an attenuated version of the action potential in the lateral neuron. If the junctional resistance is low and axon (30 μm diameter) and neuron (3 μm diameter) have similar membranes, the attenuation factor should be about 30 (equivalent to the ratio of input resistances i.e. diameter3/2). (Centre): Action potential recorded intracellularly in a particularly large lateral nerve (10 μm diameter). (Bottom): Graph of conduction velocity of action potential along lateral neuron monitored intracellularly at sites in the adjacent myoepithelium. Abscissa: distance (mm) between the intracellular recording site in the myoepithelium and the site of stimulation of the lateral neuron; ordinate: delay (ms) between the stimulus and the appearance of the depolarizing post-synaptic potential. Stimulation site fixed; stimulus just supra-threshold. The line through the points gives a conduction velocity of 34 cm s−1 (mean, 39 cm s−1; N=3). Extrapolation suggests a constant delay of 1.2 ms; taken to be the time to initiate the action potential plus the synaptic delay. Preparations immobilized in 82 mmol l−1 Mg2+ seawater; temperature 10°C. (Data from G.O. Mackie and R.W.M., unpublished.) (D) Sequence of spontaneous low-threshold Ca2+ spikes (top trace) at recording site in the motor giant axon about 5.5 mm from the nerve ring; bottom trace shows corresponding postsynaptic electrical events recorded from adjacent myoepithelium. Preparation partially immobilized in 65 mmol l−1 Mg2+ seawater. From Meech and Mackie (Meech and Mackie, 1993a). (E) Interaction between synaptic input from pacemaker system and Ca2+ spike electrogenesis at different points along the motor giant axon. The amplitude of the pacemaker EPSP (arrow) decreases exponentially as it spreads along the motor giant axon. Resting potential: −66 mV. Adapted from Meech and Mackie (Meech and Mackie, 1995).
Slow swimming, performed by Aglantha when ‘sink-fishing’ (Fig. 9A), is used to gain height in the water column. It occurs in bursts of activity of variable length. ‘During the early part of this phase, perhaps for three or four contractions, the tentacles are still extended, but then they shorten and curl inward. … Just before swimming stops the tentacles start to extend again’ (Mackie, 1980). Thus, during slow swimming, instead of shortening its tentacles before swimming gets started, Aglantha uses a strategy akin to that adopted by Chelophyes; its early swims are somewhat weaker than later ones (see Meech and Mackie, 1993a). Slow swims depend on low amplitude Ca2+ ‘spikes’ in the motor giant axons (Fig. 9B) (Mackie and Meech, 1985). These neuronal spikes are repolarized by a rapidly inactivating outward K+ current, and as in Chelophyes, this current recovers from inactivation only slowly (Meech and Mackie, 1993b). Consequently, during a burst of slow swim spikes, the availability of repolarizing K+ current decreases. This means that the slow swim spikes increase in duration, and as they do so, the contractile response in the myoepithelium becomes progressively stronger (Fig. 9D).
The length of the tentacles is also regulated via a slow tentacle system which is under the control of the relay system (see Fig. 5). The relay system is, in turn, excited by the pacemaker system so that during slow swimming the tentacles go through a series of graded tonic contractions (rather than the twitches produced by the tentacle giant). The relay system also excites the carrier system. When the pacemaker, relay and carrier systems fire together, their summating EPSPs may cause the ring giant axon to spike (Mackie and Meech, 1995b). If it does so, it fires the tentacle giant so that a stronger tentacle contraction may be interposed within the sequence of slow swims.
Interaction between slow and fast swimming in Aglantha
In Aglantha digitale the swim motor neurons have become fused into a set of eight multinucleate giant axons (see Mackie, 1989) plus a subsidiary system of ‘lateral neurons’ (Weber et al., 1982), none of which are repetitively active. Rhythmic swimming is initiated by a specialized module, which comprises a separate set of pacemaker neurons. Aglantha swims either as a means of raising its position in the water column (‘slow swimming’) or for protection (high-speed ‘escape swimming’). This dual form of swimming appears unique among single-belled jellyfish.
During an escape swim, ‘hair cells’ at the bell margin (Arkett et al., 1988) sense vibrations and trigger action potentials in the ring giant axon. Action potentials in the ring giant generate sharply rising synaptic events in each of the motor giant axons that travel radially in the subumbrella. The synaptic depolarization exceeds the threshold for the Na+ action potential (Fig. 9B), which then propagates the length of each axon traveling at about 4 m s−1. Neuromuscular synapses all along the axon carry the excitation to the myoepithelium and lateral neurons electrically coupled to the motor giant ensure that excitation spreads rapidly (about 39 cm s−1) across the muscle sheet between the giant motor axons (see Fig. 9C). The end result is that the entire myoepithelium is excited at once, the bell margin closing down to form a nozzle through which the water that fills the bell is forced (see Fig. 8A) (Donaldson et al., 1980). It is the high velocity of the ejected water that produces the substantial thrust to jet the animal forward by 5–10 body lengths. As Fig. 8C shows, during the refilling process the margin widens in advance of the bell so that a significant reverse thrust is avoided.
The square relationship between the kinetic energy supplied by the muscle epithelium and the velocity of the water leaving the bell means that each escape swim consumes a large amount of energy (Vogel, 1994), which may account for the fact that Aglantha uses fast jet propulsion relatively rarely. When collecting food, it enters an energy-saving slow swim mode (Mackie, 1980). Examination of the bell during a slow swim (see Fig. 8B) reveals that most of the contraction takes place in the upper half, the base of the bell remaining open with a wide aperture so that the velocity of the water ejected stays low and relatively little energy is consumed. This poses a physiological problem because the cells of the muscle epithelium are electrically coupled (Kerfoot et al., 1985) and as such would be expected to operate as a single unit producing uniform contractions in response to stimulation.
Surprisingly, the two forms of swim arise from the single set of ‘giant’ motor axons that synapse directly with the muscle epithelium (Singla, 1978; Kerfoot et al., 1985). The different patterns of contraction are elicited by two different forms of propagating signal (Fig. 9B); a fast overshooting Na+-dependent action potential and a slower low-amplitude ‘spike’ based on Ca2+ influx through T-type channels (Mackie and Meech, 1985; Meech and Mackie, 1993a). The motor axons have two thresholds, one for the high-threshold Na+-dependent action potential, and one for the low-threshold, low-amplitude slow swim Ca2+ spike. The difference in threshold allows the Ca2+ spike to propagate independently, below the threshold for escape swimming. Although apparently unique in the invertebrate kingdom, these low-amplitude Ca2+ spikes resemble those recorded from dendritic fields in the vertebrate brain (Llinás and Yarom, 1981; Pinato and Midtgaard, 2005).
The muscle sheet not only produces different strengths of contraction depending on the kind of impulse in the motor axon but, as we have seen, the strength of contraction is distinctly regionalized during slow swimming. The increased strength of contraction as excitation passes from the base to the apex of the bell is correlated with a change in the waveform of the Ca2+ spike recorded intracellularly in the giant motor axon (see Fig. 9E). During slow swimming, each low-amplitude Ca2+ spike in the motor axon develops from a slowly rising synaptic potential set off by pacemaker cells in the nerve ring at the base of the bell (Meech and Mackie, 1995). Near the pacemaker cell synapses, the high conductance EPSP short-circuits the Ca2+ spike, but this effect fades as the synaptic potential spreads electrotonically along the axon. As a consequence, the full Ca2+ spike only develops at distances from the base greater than about 4 mm (in a full-grown animal, 2 cm in length). The presence of a fully regenerative Ca2+ spike in the axon is correlated with an increase in the amplitude of the excitatory potential in the muscle and an increase in the strength of the local contraction.
The cells of the muscle epithelium appear capable of generating regenerative Ca2+ events even though there is no evidence that such events can propagate independently through the sheet. Use of the loose patch clamp technique has shown that the muscle cells, like the motor axons, have two separate thresholds that come into play during fast and slow swims (Meech and Mackie, 2006) (R.W.M. and G. O. Mackie, unpublished results). A series of voltage commands close to the holding potential of −80 mV have revealed a long-lasting inward current even with depolarizing commands as low as 10 mV. This low-threshold current has a peak amplitude at approximately −40 mV. Currents recorded in response to steps in the range −40 to +20 mV show a second inward current that reaches a peak value at +20 mV. The activation curve showing the voltage dependence of these two components can account for the different muscle spike thresholds seen during slow and fast swimming. The escape swim action potential in the motor giant axon generates a fast PSP in the myoepithelium, which is large enough to activate the higher threshold channels; the associated Ca2+ influx is large enough to generate a strong contraction. The smaller amplitude PSPs generated by the slow swim spike activate only the low-threshold channels associated with weaker contractions
The base to apex variation in the strength of contraction of the myoepithelium during a slow swim appears to depend upon the amplitude of the synaptic depolarization arising from the slow swim spike. Towards the base of the bell, the average amplitude of the synaptic event is 21.8±2.0 mV, less than half that measured towards the apex (49.2±1.5 mV). An explanation for the abruptness of the transition is that the thin muscle epithelium behaves like a two-dimensional sheet of conducting tissue. Potentials from a point source, such as a synapse, would be expected to decrease rapidly over distance (see Jack et al., 1975). In Aglantha, the epithelium is anisometric with the two-dimensional space constant of 770 μm for steady state current flow in a circular direction and 177 μm for radial flow towards the bell apex (Fig. 7D) (Kerfoot et al., 1985). Thus, although current spreads a long distance around the bell from one motor axon towards the next, it spreads only a short distance up the bell so that the basal and apical regions are electrically semi-isolated. An explanation for the anisometric nature of the myoepithelium is presented in Fig. 7C,D. There is no evidence that the lateral neurons play any part in spreading excitation in the myoepithelium during slow swimming.
Comparison with Scyphozoa and Cubozoa
Jellyfish swim rhythmically in such a way that energy stored by the mesoglea during contraction of the bell is largely regained as it refills with water. In the hydrozoa, swim contractions are synchronized by electrically coupled pacemaker neurons distributed around the bell margin. In the Scyphozoa and Cubozoa, however, the swim pacemakers are gathered together in ganglion-like rhopalia. Other differences between the three classes of jellyfish include the apparent absence of electrical coupling in scyphozoans and cubozoans (Mackie et al., 1984) and whether or not there is an inherent rhythm in their sensory integrating center.
Scyphomedusae
In the Scyphozoa, intermittent bursts of swimming such as exhibited by sink-fishing Hydrozoa, are rarely, if ever, seen. Instead, there are longer periods of continuous activity albeit with a highly variable inter-swim interval (Horridge, 1959). Swimming consists of a wave of contraction originating from the rhopalia (Romanes, 1877) with the fastest pacemaker determining the overall swim frequency (Horridge, 1959; Passano, 1965). This slowly propagated contraction is in contrast to the almost synchronized contraction seen in hydrozoan jellyfish.
The motor nerve net that transmits excitation from pacemaker neurons to the swim musculature and from one rhopalium to another (Anderson and Schwab, 1981; Anderson and Schwab, 1983) is a two-dimensional network of large neurons covering the subumbrella surface, and able to transmit activity in any direction. In Cyanea, communication between neurons depends upon bi-directional synapses with either side of the synaptic junction being able to release chemical transmitter (Anderson, 1985). Interneuronal facilitation between members of this nerve net is absent and in fact the EPSP amplitude declines with repeated stimuli.
Activity in a second nerve net, called the ‘diffuse nerve net’ (Horridge, 1956) sets off tentacle contraction (Romanes, 1877; Romanes, 1885), triggers slow manubrial reflexes (Passano, 1982) and can initiate swim impulses (Passano, 1965). As with the hydrozoan B system, there is a significant delay between its excitation and the appearance of swim impulses in the motor nerve net. The duration of this delay can be used to monitor the pacemaker state (Romanes, 1877; Passano, 1965). In Cassiopea, the normal swim interval in an undisturbed animal lasts 4–10 s, but this can be significantly reduced by a single stimulus to the diffuse nerve net, so long as it comes more than 1–2 s after a swim impulse. Passano (Passano, 1965) found that the precise timing depended on the interval between the impulse in the diffuse nerve net and the preceding swim. It ranged from 4 s or more if the diffuse nerve net was activated immediately after the pacemaker to less than 1 s if activation occurred 2–3 s later. As with the hydrozoan B system, paired diffuse nerve net stimuli were even more effective, with the delay being reduced to 0.5 s or less.
The diffuse nerve net in Cassiopea and the B system in Sarsia behave so similarly that it is tempting to use the same model to account for each of them: i.e. to assume that pacemaker impulses are spaced out by a fast outward potassium current (KA) that tends to delay the rise of the membrane to threshold. Such currents normally recover from inactivation rather slowly. Immediately after a swim the pacemaker will be unresponsive because of the refractory period. This may allow the recovery of much of the KA current, but it is likely that some inactivation will remain. If so a depolarizing input from the diffuse nerve net will be more effective than normal, any delay representing the rise time of the EPSP to threshold. At longer intervals, after the pacemaker impulse, we may suppose that the KA current will have fully recovered from inactivation. Now the delay represents the (slightly longer) time taken for the maintained depolarizing EPSP itself to inactivate KA so that the swim impulse can be fired.
A similar model was proposed (above) for the effect of the B system on pacemaker neurons in Polyorchis. In both cases, it is necessary to account for the striking delay between the stimulus and the motor response. In Polyorchis, the presence of long EPSPs in the swim motorneurons is well established, but they are attributed to the known electrical coupling there, which is thought to be absent in the Scyphozoa (Mackie et al., 1984). Could a prolonged input be generated by chemical synapses? According to Anderson (Anderson, 1985), the bidirectional synapses of the motor nerve net produce brief (about 20 ms) EPSPs only because they have an unusually high threshold for transmission (+20 mV or more). In a network with a lower transmission threshold, low-amplitude depolarizations would be sufficient to evoke transmitter release in a post-synaptic cell; this would, in turn, depolarize the pre-synaptic cell and evoke further transmitter release there, and so on. Perhaps this is another example of the way in which natural selection generates alternate electrophysiological applications to achieve a successful behavioural response.
Cubomedusae
Cubomedusae are capable of a wide range of complex behaviour including obstacle avoidance, courtship and mating (Lewis and Long, 2005). In addition to being the site of pacemaker activity, each rhopalium has two large eyes equipped with lenses and two pairs of ocelli. When stimulated, the different types of eye are able to influence the output of the associated pacemaker (Garm and Mori, 2009) making steering possible. Satterlie and Nolen (Satterlie and Nolen, 2001) have suggested that the reason cubomedusae have only four pacemaking rhopalia is that this provides an ideal means of enabling directional swimming. The normal swim frequency is somewhat faster than the natural rhythm of individual pacemakers because each cycle is driven by the first to reach threshold. Such a system has the advantage that a synaptic input can produce both upward and downward adjustments in frequency. Were the linkage between pacemakers to be absolute, however, there would be no possibility of asymmetric swimming such as may be called for when the animal is tracking prey. Modelling trials suggest that semi-independent coupling produces the best outcome (Satterlie and Nolen, 2001).
Impulses that originate in the rhopalia act on other rhopalia to suppress their activity. They also spread to the subumbrella muscle, generating graded muscle potentials: the larger the muscle potential, the stronger the force of contraction (Satterlie, 1979). Neither myoepthelial cells (Satterlie, 2011) nor the nerve net neurons are coupled electrically and the synaptic events have a relatively short time course. Satterlie (Satterlie, 1979) suggests that the different amplitudes arise from variations in the synaptic input to the polyinnervated muscle cell. The muscle potential amplitude depends on the preceding inter-pulse interval with maximum facilitation being associated with intervals of 0.5 s (Satterlie, 1979). From what we know of the ionic basis of electrogenicity in other cnidarian muscle cells (see Inoue et al., 2005), it is likely that impulse repolarization depends on a rapidly inactivating K+ current that requires several seconds to recover fully from inactivation. With shorter intervals, recovery from inactivation would be incomplete: less repolarizing current would be available and so the muscle potential amplitude would be larger.
Conclusion
In ‘The Integrative Action of the Nervous System’ Sherrington (Sherrington, 1906) referred to the work of Romanes, Nagel and Bethe and noted that the refractory period ensured coordination during jellyfish swimming by preventing contraction from taking place when the bell was refilling. Fifty-nine years later, Bullock and Horridge (Bullock and Horridge, 1965) categorized the integrative properties of neurons by itemizing the different forms of responsiveness that could be recorded intracellularly. These included: all-or-nothing action potentials, pacemaker potentials, local potentials and synaptic potentials. In each case, passive and active factors combine to tailor each neuronal structure to its integrative role. Passive factors include the decremental spread of current as determined by cellular anatomy while active factors include the voltage-dependent characteristics of ion channels in nerve and muscle membranes. A key active factor is the role of inactivating K+ channels in determining the amount of Ca2+ that enters the cell during electrical activity. This will determine the strength of muscle contraction or the amount of neurotransmitter released.
In the present review, I have focused on examples of the way in which active and passive properties of neurons interact to produce integrated behaviour. Along the way we have noted that not all ‘all-or-nothing’ action potentials are identical and that many invertebrate axons exhibit trains of action potentials that change their characteristics sequentially. This property may be involved in a variety of coordinated actions such as the rather precise way in which the manubrium in Aglantha is directed towards a source of food. Efficient locomotion in jellyfish is based on the timing afforded by pacemaker neurons, but swimming must be coordinated with feeding and the control of tentacle length and this relies on interaction between systems. We note that in Polyorchis the passive properties of the electrically coupled pacemaker system enhances the duration of all synaptic inputs, thereby increasing their influence over voltage-gated components. Finally, we have examined how the interactions between active and passive properties of the giant motor neurons in Aglantha combine to generate graded responses in the electrically coupled myoepithelium. The muscle sheet produces different strengths of contraction depending on the kind of active impulse in the motor giant axon. It also produces regionalized contractions during slow swimming as a consequence of the anisotropic spread of current and the interaction between synaptic input and slow swim electrogenicity at the base of the motor giant axon.
Different species of Cnidaria exhibit a wide range of mechanisms when swimming, controlling their tentacles, escaping or feeding. These successful behavioural strategies have arisen as a result of trial and error during the course of evolution, but changes in the different neuronal modules concerned are constrained by holistic factors such as the properties of an animal's skeleton, the arrangement of its muscles and the availability of electrical coupling. For example, in Aglantha ‘crumpling’ behaviour, which is common in many jellyfish (Hyman, 1940), is not a defensive option because the bell has evolved to accommodate escape swimming and the radial muscles required for crumpling are confined to the manubrium. However, Aglantha also operates in a more ‘classical’ fashion and the inhibition of swimming that takes place when it feeds is an example of this. Similarities between jellyfish species have given us the chance to ‘cherry pick’ them for experimental preparations that are likely to provide data applicable to all; species differences provide the means to explore the holistic constraints that apply to the molecular components of differently expressed neuronal modules.
Acknowledgements
This manuscript has benefitted greatly from the many discussions I have had with George Mackie, my friend and collaborator. I thank him for all the support, encouragement, advice and inspiration that he has freely given me. Work on Cnidaria was performed at the Friday Harbor Laboratories, Washington, USA and I thank the Director and staff for their skilled help and warm hospitality. I particularly thank Claudia Mills for her friendship, unfailing interest and help. The ctenophore research was carried out at the Station Zoologique, Villefranche-sur-Mer, France and at the University of Nice. It was carried out in collaboration with Marie-Luz Hernandez-Nicaise and Andre Bilbaut and I thank them for happy times and for permission to include unpublished data here. Micropipette studies on the glass sponge Rhabdocalyptus were carried out at Bamfield Marine Station, British Columbia, Canada, with support from the Director (Andrew Spencer) and would have been impossible without the enthusiastic encouragement of Sally Leys. The University of Bristol, UK has for many years provided a secure base for my research and I am truly grateful to my Bristol colleagues for their friendship and support. This work was presented at the ‘Evolution of the First Nervous Systems II’ meeting, which was supported by the National Science Foundation (NSF).
FOOTNOTES
Competing interests
The author declares no competing or financial interests.
Funding
Research funding was provided by the Natural Sciences and Engineering Research Council of Canada (Grant no. OGP0001427 01). Earlier work was supported by grants from The Royal Society and The Wellcome Trust.
References
- Anderson P. A. V. (1984). The electrophysiology of single smooth muscle cells from the ctenophore Mnemiopsis. J. Comp. Physiol. B 154, 257-268. [Google Scholar]
- Anderson P. A. V. (1985). Physiology of a bidirectional, excitatory, chemical synapse. J. Neurophysiol. 53, 821-835. [DOI] [PubMed] [Google Scholar]
- Anderson P. A. V., Mackie G. O. (1977). Electrically coupled, photosensitive neurons control swimming in a jellyfish. Science 197, 186-188. [DOI] [PubMed] [Google Scholar]
- Anderson P. A. V., Schwab W. E. (1981). The organization and structure of nerve and muscle in the jellyfish Cyanea capillata (Coelenterata; Scyphozoa). J. Morphol. 170, 383-399. [DOI] [PubMed] [Google Scholar]
- Anderson P. A. V., Schwab W. E. (1983). Action potential in neurons of motor nerve net of Cyanea (Coelenterata). J. Neurophysiol. 50, 671-683. [DOI] [PubMed] [Google Scholar]
- Arkett S. A., Mackie G. O., Meech R. W. (1988). Hair cell mechanoreception in the jellyfish Aglantha digitale. J. Exp. Biol. 135, 329-342. [Google Scholar]
- Armstrong C. M., Bezanilla F. (1974). Charge movement associated with the opening and closing of the activation gates of the Na channels. J. Gen. Physiol. 63, 533-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V. L. (1966). Physiology of electrotonic junctions. Ann. N. Y. Acad. Sci. 137, 509-539. [DOI] [PubMed] [Google Scholar]
- Bennett M. V. L., Zukin R. S. (2004). Electrical coupling and neuronal synchronization in the mammalian brain. Neuron 41, 495-511. [DOI] [PubMed] [Google Scholar]
- Bilbaut A., Meech R. W., Hernandez-Nicaise M.-L. (1988a). Isolated giant smooth muscle fibres in Beroe ovata. J. Exp. Biol. 135, 343-362. [Google Scholar]
- Bilbaut A., Hernandez-Nicaise M.-L., Leech C. A., Meech R. W. (1988b). Membrane currents that govern smooth muscle contraction in a ctenophore. Nature 331, 533-535. [DOI] [PubMed] [Google Scholar]
- Bone Q. (1981). The relation between the form of the action potential and contractions in the subumbrellar myoepithelium of Chelophyes (Coelenterata: Siphonophora). J. Comp. Physiol. 144, 555-558. [Google Scholar]
- Bullock T. H., Horridge G. A. (1965). Structure and Function in the Nervous Systems of Invertebrates, Vol. I San Francisco, CA; London: W. H. Freeman and Company. [Google Scholar]
- Chain B. M., Bone Q., Anderson P. A. V. (1981). Electrophysiology of a myoid epithelium in Chelophyes (Coelenterata: Siphonophora). J. Comp. Physiol. 143, 329-338. [Google Scholar]
- Clune J., Mouret J.-B., Lipson H. (2013). The evolutionary origins of modularity. Proc. Roy. Soc. B 280, 20122863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. (1971). Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J. Physiol. 213, 31-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S., Mackie G. O., Roberts A. (1980). Preliminary observations on escape swimming and giant neurons in Aglantha digitale (Hydromedusae: Trachylina). Can. J. Zool. 58, 549-552. [Google Scholar]
- Garm A., Mori S. (2009). Multiple photoreceptor systems control the swim pacemaker activity in box jellyfish. J. Exp. Biol. 212, 3951-3960. [DOI] [PubMed] [Google Scholar]
- Grigoriev N. G., Spafford J. D., Przysiezniak J., Spencer A. N. (1996). A cardiac-like sodium current in motor neurons of a jellyfish. J. Neurophysiol. 76, 2240-2249. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Kusano K., Saito N. (1961). Membrane changes of Onchidium nerve cell in potassium-rich media. J. Physiol. 155, 470-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Yoshida S., Yoshii M. (1981). Transient and delayed potassium currents in the egg cell membrane of the coelenterate, Renilla koellikeri. J. Physiol. 318, 123-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Nicaise M.-L., Passano L. M. (1967). A physiological analysis of the feeding behavior of Sarsia tubulosa (M. Sars), a hydrozoan jellyfish. Am. Zool. 7, 727. [Google Scholar]
- Hernandez-Nicaise M.-L. (1973). The nervous system of ctenophores. III. Ultrastructure of synapses. J. Neurocytol. 2, 249-263. [DOI] [PubMed] [Google Scholar]
- Hernandez-Nicaise M.-L. (1991). Ctenophora. In Microscopic Anatomy of Invertebrates: Placozoa, Porifera, Cnidaria, and Ctenophora, Vol. 2 (ed. Harrison F. W., Westfall J. A.), pp. 359-418 New York, NY: Wiley-Liss. [Google Scholar]
- Hernandez-Nicaise M.-L., Mackie G. O., Meech R. W. (1980). Giant smooth muscle cells of Beroë. Ultrastructure, innervation, and electrical properties. J. Gen. Physiol. 75, 79-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horridge G. A. (1955a). The nerves and muscles of medusae. II. Geryonia proboscidalis Eschscholtz. J. Exp. Biol. 32, 555-568. [Google Scholar]
- Horridge G. A. (1955b). The nerves and muscles of medusae. IV. Inhibition in Aequorea forskalea. J. Exp. Biol. 32, 642-648. [Google Scholar]
- Horridge G. A. (1956). The nerves and muscles of medusae V. Double innervation in Scyphozoa. J. Exp. Biol. 33, 366-383. [Google Scholar]
- Horridge G. A. (1959). The nerves and muscles of medusae. VI. The rhythm. J. Exp. Biol. 36, 72-91. [Google Scholar]
- Hyman L. H. (1940). Observations and experiments on the physiology of medusae. Biol. Bull. 79, 282-296. [Google Scholar]
- Inoue I., Tsutsui I., Bone Q. (2005). Long-lasting potassium channel inactivation in myoepithelial fibres is related to characteristics of swimming in diphyid siphonophores. J. Exp. Biol. 208, 4577-4584. [DOI] [PubMed] [Google Scholar]
- Jack J. J. B., Noble D., Tsien R. W. (1975). Electric Current Flow in Excitable Cells. Oxford: Clarendon Press. [Google Scholar]
- Jager M., Chiori R., Alié A., Dayraud C., Quéinnec E., Manuel M. (2011). New insights on ctenophore neural anatomy: immunofluorescence study in Pleurobrachia pileus (Müller, 1776). J. Exp. Zool. (Mol. Dev. Evol.) 316, 171-187. [DOI] [PubMed] [Google Scholar]
- Kerfoot P. A. H., Mackie G. O., Meech R. W., Roberts A., Singla C. L. (1985). Neuromuscular transmission in the jellyfish Aglantha digitale. J. Exp. Biol. 116, 1-25. [DOI] [PubMed] [Google Scholar]
- Laughlin S. B., Sejnowski T. J. (2003). Communication in neuronal networks. Science 301, 1870-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn I. D., Mackie G. O., Silver G. (1981). Conduction system in a sponge. Science 211, 1169-1171. [DOI] [PubMed] [Google Scholar]
- Lewis C., Long T. A. F. (2005). Courtship and reproduction in Carybdea sivickisi (Cnidaria: Cubozoa). Mar. Biol. 147, 477-483. [Google Scholar]
- Leys S. P., Mackie G. O. (1997). Electrical recording from a glass sponge. Nature 387, 29-30. [Google Scholar]
- Leys S. P., Mackie G. O., Meech R. W. (1999). Impulse conduction in a sponge. J. Exp. Biol. 202, 1139-1150. [DOI] [PubMed] [Google Scholar]
- Leys S. P., Meech R. W. (2006). Physiology of coordination in sponges. Can. J. Zool. 84, 288-306. [Google Scholar]
- Llinás R., Yarom Y. (1981). Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J. Physiol. 315, 549-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. O. (1964). Analysis of locomotion in a siphonophore colony. Proc. R. Soc. B 159, 366-391. [Google Scholar]
- Mackie G. O. (1965). Conduction in the nerve-free epithelia of Siphonophores. Am. Zool. 5, 439-453. [DOI] [PubMed] [Google Scholar]
- Mackie G. O. (1975). Neurobiology of Stomotoca. II. Pacemakers and conduction pathways. J. Neurobiol. 6, 357-378. [DOI] [PubMed] [Google Scholar]
- Mackie G. O. (1976). Propagated spikes and secretion in a coelenterate glandular epithelium. J. Gen. Physiol. 68, 313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. O. (1980). Slow swimming and cyclical ‘fishing’ behavior in Aglantha digitale (Hydromedusae, Trachylina). Can. J. Fish. Aquat. Sci. 37, 1550-1556. [Google Scholar]
- Mackie G. O. (1989). Evolution of cnidarian giant axons. In Evolution of the First Nervous Systems (ed. Anderson P. A. V.), pp. 395-407 New York, NY: Plenum Press. [Google Scholar]
- Mackie G. O. (1984). Fast pathways and escape behavior in Cnidaria. In Neural Mechanisms of Startle Behavior (ed. Eaton R. C.), pp. 15-42 New York, NY: Plenum Press. [Google Scholar]
- Mackie G. O., Mackie G. V. (1963). Systematic and biological notes on living hydromedusae from Puget Sound. Natl. Mus. Can. Bull. 199, 63-84. [Google Scholar]
- Mackie G. O., Marx R. M., Meech R. W. (2003). Central circuitry in the jellyfish Aglantha digitale IV. Pathways coordinating feeding behaviour. J. Exp. Biol. 206, 2487-2505. [DOI] [PubMed] [Google Scholar]
- Mackie G. O., Meech R. W. (1985). Separate sodium and calcium spikes in the same axon. Nature 313, 791-793. [DOI] [PubMed] [Google Scholar]
- Mackie G. O., Meech R. W. (1995a). Central circuitry in the jellyfish Aglantha digitale. I: The relay system. J. Exp. Biol. 198, 2261-2270. [DOI] [PubMed] [Google Scholar]
- Mackie G. O., Meech R. W. (1995b). Central circuitry in the jellyfish Aglantha digitale. II: The ring giant and carrier systems. J. Exp. Biol. 198, 2271-2278. [DOI] [PubMed] [Google Scholar]
- Mackie G. O., Meech R. W. (2000). Central circuitry in the jellyfish Aglantha digitale. III. The rootlet and pacemaker systems. J. Exp. Biol. 203, 1797-1807. [DOI] [PubMed] [Google Scholar]
- Mackie G. O., Meech R. W. (2008). Nerves in the endodermal canals of hydromedusae and their role in swimming inhibition. Invert. Neurosci. 8, 199-209. [DOI] [PubMed] [Google Scholar]
- Mackie G. O., Anderson P. A. V., Singla C. L. (1984). Apparent absence of gap junctions in two classes of cnidaria. Biol. Bull. 167, 120-123. [Google Scholar]
- Mackie G. O., Lawn I. D., Pavans de Ceccatty M. (1983). Studies on hexactinellid sponges II. Excitability, conduction and coordination of responses in Rhabdocalyptus dawsoni (Lambe, 1873). Philos. Trans. R. Soc. B 301, 401-418. [Google Scholar]
- Mackie G. O., Meech R. W., Spencer A. N. (2012). A new inhibitory pathway in the jellyfish Polyorchis penicillatus. Can. J. Zool. 90, 172-181. [Google Scholar]
- Mackie G. O., Passano L. M. (1968). Epithelial conduction in hydromedusae. J. Gen. Physiol. 52, 600-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. O., Singla C. L. (1997). The role of epithelial conduction in the behaviour of Aglantha digitale (O. F. Müller, 1776) (Hydromedusae: Rhopalonematidae). In Proceedings of the 6th International Conference on Coelenterate Biology, pp. 307-313 Leiden: National Natuurhistorisch Museum. [Google Scholar]
- Meech R. W. (2015). The evolution of neurons. In Handbook of Evolutionary Neuroscience (ed. Shepherd S. V.). Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- Meech R. W., Mackie G. O. (1993a). Ionic currents in giant motor axons of the jellyfish, Aglantha digitale. J. Neurophysiol. 69, 884-893. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Mackie G. O. (1993b). Potassium channel family in giant motor axons of Aglantha digitale. J. Neurophysiol. 69, 894-901. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Mackie G. O. (1995). Synaptic potentials and threshold currents underlying spike production in motor giant axons of Aglantha digitale. J. Neurophysiol. 74, 1662-1670. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Mackie G. O. (2006). Ionic currents in the myoepithelium of Aglantha digitale. FASEB J. 20, A826. [Google Scholar]
- Meech R. W., Mackie G. O. (2007). Evolution of excitability in lower metazoans. In Invertebrate Neurobiology (ed. North G., Greenspan R. J.), pp. 581-616 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Moroz L. L. (2015). Convergent evolution of neural systems in ctenophores. J. Exp. Biol. 218, 598-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz L. L., Kocot K. M., Citarella M. R., Dosung S., Norekian T. P., Povolotskaya I. S., Grigorenko A. P., Dailey C., Berezikov E., Buckley K. M., et al. (2014). The ctenophore genome and the evolutionary origins of neural systems. Nature 510, 109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz L. L., Meech R. W., Sweedler J. V., Mackie G. O. (2004). Nitric oxide regulates swimming in the jellyfish Aglantha digitale. J. Comp. Neurol. 471, 26-36. [DOI] [PubMed] [Google Scholar]
- Naitoh Y., Eckert R. (1969). Ionic mechanisms controlling behavioral responses of Paramecium to mechanical stimulation. Science 164, 963-965. [DOI] [PubMed] [Google Scholar]
- Ohtsu K., Yoshida M. (1973). Electrical activities of the anthomedusan Spirocodon saltatrix (Tilesius). Biol. Bull. 145, 532-547. [Google Scholar]
- Passano L. M. (1965). Pacemakers and activity patterns in medusae: homage to Romanes. Am. Zool. 5, 465-481. [DOI] [PubMed] [Google Scholar]
- Passano L. M. (1973). Behavioural control systems in medusae; a comparison between hydro- and schyphomedusae. Publications of the Seto Marine Biological Laboratory 20, 615-645. [Google Scholar]
- Passano L. M. (1982). Scyphozoa and cubozoa. In Electrical Conduction and Behaviour in ‘Simple’ Invertebrates (ed. Shelton G. A. B.), Oxford: Clarendon Press. [Google Scholar]
- Passano L. M., Mackie G. O., de Ceccatty M. P. (1967). Physiologie du comportement de l'hydromeduse Sarsia tubulosa Sars. Les systems des activites spontanees. C. R. Acad. Sci. Paris 264, 614-617. [Google Scholar]
- Pinato G., Midtgaard J. (2005). Dendritic sodium spikelets and low-threshold calcium spikes in turtle olfactory bulb granule cells. J. Neurophysiol. 93, 1285-1294. [DOI] [PubMed] [Google Scholar]
- Przysiezniak J. (1993). Voltage-activated Currents in Identified Motor Neurons from the Hydromedusa Polyorchis penicillatus. PhD thesis, University of Alberta, Edmonton, AL, Canada. [Google Scholar]
- Przysiezniak J., Spencer A. N. (1989). Primary culture of identified neurones from a cnidarian. J. Exp. Biol. 142, 97-113. [Google Scholar]
- Przysiezniak J., Spencer A. N. (1992). Voltage-activated calcium currents in identified neurons from a hydrozoan jellyfish, Polyorchis penicillatus. J. Neurosci. 12, 2065-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przysiezniak J., Spencer A. N. (1994). Voltage-activated potassium currents in isolated motor neurons from the jellyfish Polyorchis penicillatus. J. Neurophysiol. 72, 1010-1019. [DOI] [PubMed] [Google Scholar]
- Roberts A., Mackie G. O. (1980). The giant axon escape system of a hydrozoan medusa, Aglantha digitale. J. Exp. Biol. 84, 303-318. [DOI] [PubMed] [Google Scholar]
- Romanes G. J. (1877). Further observations on the locomotor system of medusae. Philos. Trans. R. Soc. 166, 659-752. [Google Scholar]
- Romanes G. J. (1885). Jelly-fish, Star-fish and Sea-Urchins. Being a Research on Primitive Nervous Systems (International Scientific Series), Vol. 49 New York, NY: D. Appleton & Company. [Google Scholar]
- Russell F. S. (1953). The Medusae of the British Isles. Anthomedusae, Leptomedusae, Limnomedusae, Trachymedusae and Narcomedusae. London: Cambridge University Press. [Google Scholar]
- Ryan J. F., Pang K., Schnitzler C. E., Nguyen A.-D., Moreland R. T., Simmons D. K., Koch B. J., Francis W. R., Havlak P., Smith S. A., et al. NISC Comparative Sequencing Program (2013). The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science 342, 1242592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlie R. A. (1979). Central control of swimming in the cubomedusan jellyfish Carybdea rastonii. J. Comp. Physiol. 133, 357-367. [Google Scholar]
- Satterlie R. A. (2011). Do jellyfish have central nervous systems? J. Exp. Biol. 214, 1215-1223. [DOI] [PubMed] [Google Scholar]
- Satterlie R. A., Nolen T. G. (2001). Why do cubomedusae have only four swim pacemakers? J. Exp. Biol. 204, 1413-1419. [DOI] [PubMed] [Google Scholar]
- Satterlie R. A., Spencer A. N. (1983). Neuronal control of locomotion in hydrozoan medusae. J. Comp. Physiol. 150, 195-206. [Google Scholar]
- Schlosser G., Wagner G. P. (2004). Modularity in Development and Evolution. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Schwab W. E., Josephson R. K. (1982). Lability of conduction velocity during repetitive activation of an excitable epithelium. J. Exp. Biol. 98, 175-193. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. 1906. The Integrative Action of the Nervous System. New York, NY: Charles Scribner's Sons. [Google Scholar]
- Simon H. A. (1962). The architecture of complexity. Proc. Am. Philos. Soc. 106, 467-482. [Google Scholar]
- Spencer A. N. (1975). Behavior and electrical activity in the hydrozoan Proboscidactyla flavicirrata (Brandt). II. The medusa. Biol. Bull. 149, 236-250. [DOI] [PubMed] [Google Scholar]
- Spencer A. N. (1981). The parameters and properties of a group of electrically coupled neurones in the central nervous system of a hydrozoan jellyfish. J. Exp. Biol. 93, 33-50. [Google Scholar]
- Spencer A. N., Arkett S. A. (1984). Radial symmetry and the organization of central neurones in a hydrozoan jellyfish. J. Exp. Biol. 110, 69-90. [Google Scholar]
- Singla C. L. (1978). Locomotion and neuromuscular system of Aglantha digitale. Cell Tissue Res. 188, 317-327. [DOI] [PubMed] [Google Scholar]
- Stein P. G., Anderson P. A. V. (1984). Maintenance of isolated smooth muscle cells of the ctenophore Mnemiopsis. J. Exp. Biol. 110, 329-334. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Roberts W. M., Almers W. (1983). The loose patch clamp. In Single Channel Recording (ed. Sakmann B., Neher E.), pp 123-132 New York, NY; London: Plenum Press. [Google Scholar]
- Tamm S. L. (2014). Cilia and the life of ctenophores. Invertebr. Biol. 133, 1-46. [Google Scholar]
- Weber C., Singla C. L., Kerfoot P. A. H. (1982). Microanatomy of the subumbrellar motor innervation in Aglantha digitale (Hydromedusae: Trachylina). Cell Tissue Res. 223, 305-312. [DOI] [PubMed] [Google Scholar]
- Vogel S. (1994). Life in Moving Fluids: The Physical Biology of Flow, 2nd edn. Princeton, NJ: Princeton University Press. [Google Scholar]