Abstract
Purpose.
The purpose of this study was to compare the accommodative performance of the amblyopic eye of children with unilateral amblyopia to that of their nonamblyopic eye, and also to that of children without amblyopia, during both monocular and binocular viewing.
Methods.
Modified Nott retinoscopy was used to measure accommodative performance of 38 subjects with unilateral amblyopia and 25 subjects with typical vision from 3 to 13 years of age during monocular and binocular viewing at target distances of 50, 33, and 25 cm. The relationship between accommodative demand and interocular difference (IOD) in accommodative error was assessed in each group.
Results.
The mean IOD in monocular accommodative error for amblyopic subjects across all three viewing distances was 0.49 diopters (D) (95% confidence interval [CI], ±1.12 D) in the 180° meridian and 0.54 D (95% CI, ±1.27 D) in the 90° meridian, with the amblyopic eye exhibiting greater accommodative errors on average. Interocular difference in monocular accommodative error increased significantly with increasing accommodative demand; 5%, 47%, and 58% of amblyopic subjects had monocular errors in the amblyopic eye that fell outside the upper 95% confidence limit for the better eye of control subjects at viewing distances of 50, 33, and 25 cm, respectively.
Conclusions.
When viewing monocularly, children with unilateral amblyopia had greater mean accommodative errors in their amblyopic eyes than in their nonamblyopic eyes, and when compared with control subjects. This could lead to unintended retinal image defocus during patching therapy for amblyopia.
Keywords: accommodation, amblyopia, children's vision
This study of amblyopic children shows an increased accommodative error during monocular viewing of naturalistic targets with the amblyopic eye compared to nonamblyopic and control eyes. These errors could degrade retinal image quality in the amblyopic eye during patching therapy.
Introduction
Unilateral amblyopia is a monocular reduction in best-corrected visual acuity in the absence of or in addition to the direct effect of vision-limiting ocular pathology. It is associated with one or more amblyogenic factors known to interfere with maturation of the visual cortex (strabismus, anisometropia, and/or visual deprivation). Standard treatment is refractive correction, if indicated, followed by patching or atropine penalization of the nonamblyopic eye. Unfortunately, 15% to 50% of amblyopic children do not achieve equal visual acuity in the two eyes despite treatment.1–3 Although many factors that could limit the effectiveness of current amblyopia treatment have been suggested, the reason for residual amblyopia is still poorly understood.4
Treatment of amblyopia is dependent on adequate retinal image quality driving improvements in cortical synaptic function. The importance of retinal image quality in amblyopia treatment is indicated by the improvements in amblyopic eye visual acuity achieved with refractive correction alone in children with strabismic, anisometropic, or mixed mechanism amblyopia. This includes complete resolution of amblyopia in 27% to 30% of previously untreated children aged 3 to 8 years after 16 to 22 weeks of refractive correction.5–7 Other factors, including accommodation, pupil size, higher-order monochromatic aberrations, and chromatic aberration, also contribute to retinal image quality, but among these, the accuracy of accommodative responses is likely to have the greatest contribution.8
It is generally thought that a minimum level of accommodative effort is exerted to place the image of an object of regard within the depth of focus (DOF) of the visual system, which has been found to be ±0.10 to ±0.50 diopter (D) in typical adults.9–11 Thus, the accommodative response is generally less than the accommodative demand; when this occurs, the difference is an accommodative error termed “accommodative lag.” Children have been shown to demonstrate an average accommodative lag of 0.41 D during binocular viewing of near stimuli (25–50 cm), with an average upper 95% confidence limit of 0.76 D across several studies.12–16 Accommodative lag generally increases with increasing accommodative demand15 and with optically corrected myopia relative to emmetropia and hyperopia17,18 but changes minimally with age during childhood.15,19,20 Because poor visual acuity has been found to be associated with an increased DOF,10,21 amblyopic eyes may have increased accommodative lags during monocular viewing, leading to degraded retinal image quality during patching treatment.
While studies have demonstrated poor accommodative performance in the amblyopic eye of adults with unilateral amblyopia,22,23 little is known about accommodation in amblyopic children. Ukai et al.24 report that amblyopic children have shallow accommodative stimulus-response function slopes in their amblyopic eyes compared to fellow eyes or formerly amblyopic eyes; however, subjects were tested without refractive correction, so it is possible that interocular differences in accommodative demand could have confounded the results.24
The purpose of this study was to compare the accommodative performance of the amblyopic eye of children with unilateral amblyopia to that of their nonamblyopic eye, and also to that observed in children without amblyopia, during both monocular and binocular viewing.
Methods
The study was conducted according to the tenets of the Declaration of Helsinki at Indiana University School of Optometry (IU) and Southern California College of Optometry at Marshall B. Ketchum University (SCCO). The protocol and Health Insurance Portability and Accountability Act (HIPAA)–compliant informed consent forms were approved by the institutional review boards at both sites, and the parent or legal guardian of each subject gave written informed consent. Assent was obtained from those subjects who were old enough to provide assent.
The study cohort consisted of children from 3 to 13 years of age. Subjects with unilateral amblyopia had an interocular difference (IOD) in best-corrected distance visual acuity of ≥2 lines when using the Amblyopia Treatment Study (ATS)–HOTV protocol,25 in the presence of an amblyogenic factor: strabismus (or documented history of strabismus before spectacle correction) and/or amblyogenic anisometropia (spherical equivalent [SE] anisometropic hyperopia of ≥1.00 D, SE anisometropic myopia of ≥3.00 D, or anisometropic astigmatism of ≥1.50 D). Some subjects had received previous treatment for amblyopia, while others had no prior treatment beyond spectacle correction. Control subjects had age-normal best-corrected visual acuities in both eyes (20/20 or better if >8 years, 20/30 or better if ≥6 to ≤8 years, 20/40 or better if 3 to <6 years of age),26 IOD in acuity of ≤1 line, and no strabismus or amblyogenic anisometropia. Nine (of 25) control subjects had an IOD in acuity of 1 line and their eyes were denoted “better eye” and “worse eye” accordingly. For control subjects with <1 line IOD in acuity, the “better eye” and “worse eye” were alternately assigned. No subjects had coexisting ocular pathology and no subjects had systemic conditions or were taking medications known to affect accommodation.
Study Procedures
Measurements were performed by the same examiner at each clinical site (VM at IU and AMC at SCCO). The study was conducted at each site with a written protocol that was established before the study was initiated. Study procedures were performed with subjects wearing their habitual refractive correction in order to elicit habitual accommodative performance. Any residual uncorrected refractive error was adjusted for in the data analysis. Monocular distance visual acuity was measured by using the ATS-HOTV protocol25 on an electronic visual acuity test system.27 Stereoacuity was measured at 40 cm by using the Randot Preschool Stereoacuity Test (Stereo Optical, Inc., Chicago, IL, USA).28
Accommodative responses were measured by using Nott dynamic retinoscopy,29 which is a commonly used clinical method to evaluate accommodative performance. Studies have suggested that when used in similar viewing conditions, Nott retinoscopy and objective autorefraction measurements of accommodative response are comparable at a range of target distances.30–32 Examiners used a retinoscope to determine the refractive state of the eye while the subject viewed an animated cartoon movie on a 15 cm × 8.5 cm LCD screen mounted on a motorized track. The subject's viewing distance was stabilized by using a forehead rest. The movie was used as the target to mimic naturalistic daily visual experience (approximately 1/f spatial amplitude spectra), and the subjects were simply instructed to watch the movie in an attempt to elicit their habitual viewing effort. The examiner adjusted her working distance from the subject to neutralize the retinoscopic reflex, and the resulting retinoscope-to-cornea dioptric distance was recorded as the subject's accommodative response. In this study, the Nott dynamic retinoscopy technique was modified with the use of a beam-splitter, allowing the examiner to perform retinoscopy to the side of the subject (Fig. 1), while a linear potentiometer attached to the retinoscope allowed the distance between the examiner's retinoscope and the subjects' corneas to be recorded and stored automatically with the press of a trigger button. The range of measurable dioptric distances (corresponding to measurable accommodative responses) was between 0.68 D at the farthest measurable distance and 4.69 D at the closest measurable distance. The instruments used at the two sites were designed, manufactured, and calibrated at Indiana University School of Optometry.
Figure 1.
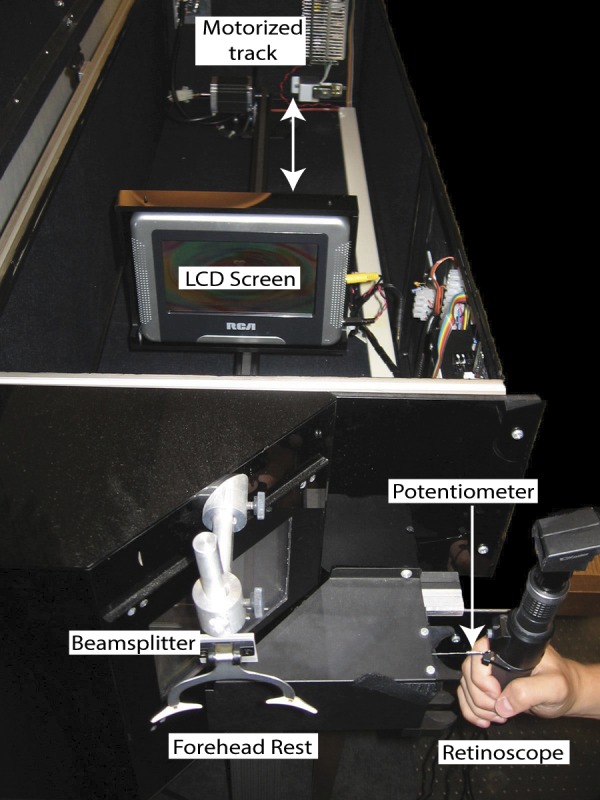
The automated Nott retinoscopy system. The limits of the linear potentiometer were 0.68 D at the far distance and 4.69 D at the close distance. Thus, refractive states beyond these points could not be measured.
Nott dynamic retinoscopy was performed for the 180° and 90° meridia of each eye, at target distances of 50 cm (2.00 D), 33 cm (3.00 D), and 25 cm (4.00 D), in both binocular and monocular viewing conditions (two meridia of two eyes at three distances generated 12 measurements for each viewing condition). The right eye and the 180° meridian of each eye were always tested first. The accommodative demand either increased (2.00 D, 3.00 D, 4.00 D) or decreased (4.00 D, 3.00 D, 2.00 D) for each subject, assigned in a random fashion by the investigator. The results were pooled across sequence of testing (increasing or decreasing target distance), as there have been no observed effects of the order of stimulus presentation on accommodative response.14 The examiner monitored the stability of accommodation by noting fluctuations of the retinoscopic reflex and asked subjects questions about the movie to encourage interest and steady fixation when necessary. Measurements were only taken when the retinoscopic reflex appeared stable. For subjects who sustained attentive fixation after the primary study measurements were completed, measurements were repeated for one eye in either binocular or monocular viewing to assess the intra-examiner, intra-instrument, and intrasession repeatability of the Nott retinoscopy approach. Intra-examiner, interinstrument, and intersession repeatability data were collected from one pre-presbyopic adult, as were another set of interexaminer, intra-instrument, and intrasession data.
The subjects' clinical charts were reviewed after completing the study procedures to determine medical history, surgical history, habitual spectacle prescription, cycloplegic refraction, duration of spectacle wear, duration of patching treatment (if any), and eye alignment measurements with distance and near fixation.
Data Analysis
Residual uncorrected refractive error was calculated as spectacle lens power subtracted from cycloplegic refraction. This was added to the accommodative demand (dioptric distance of the target) and measured accommodative response to derive the total accommodative demand and total accommodative response, respectively. Accommodative error was defined as the total accommodative response subtracted from the total accommodative demand. If the accommodative error was positive (i.e., underaccommodation), an accommodative lag was present; if the accommodative error was negative (i.e., overaccommodation), an accommodative lead was present. The IOD in accommodative error was calculated as better/nonamblyopic eye error subtracted from worse/amblyopic eye error, with positive values indicating a larger lag or smaller lead in the worse/amblyopic eye than in the better/nonamblyopic eye.
The IOD in accommodative error during binocular viewing was corrected by subtracting any residual uncorrected anisometropia, as illustrated in the following example: a cycloplegic refractive error of +1.00 D might be found in the nonamblyopic eye and +5.00 D in the amblyopic eye. On lensometry, the subject's habitual spectacle lenses might be +1.00 D in the nonamblyopic eye and +4.50 D in the amblyopic eye. The residual uncorrected anisometropia (amblyopic eye − nonamblyopic eye) would then be +0.50 D. The accommodative error during binocular viewing with the habitual spectacle correction might be +1.00 D in the nonamblyopic eye and +1.50 D in the amblyopic eye. The unadjusted IOD in accommodative error during binocular viewing (amblyopic eye − nonamblyopic eye) would then be +0.50 D. This IOD could, however, be attributed to residual uncorrected anisometropia. To control for this, the residual uncorrected anisometropia is subtracted from the IOD in accommodative error for binocular viewing, yielding an adjusted IOD for binocular viewing of zero in this example.
Error in variables (EIV) regression analysis (STATA [StataCorp LP, College Station, TX, USA] function designed to incorporate variability in the predictor variable) was used to describe total accommodative response as a function of total accommodative demand. Four regressions were performed for each eye in each group (amblyopic and control): binocular viewing/90° meridian, binocular viewing/180° meridian, monocular viewing/90° meridian, and monocular viewing/180° meridian. Error in variables regression analysis was also used to assess the relationship between the IOD in accommodative error and total accommodative demand, and to compare better eye accommodative responses between amblyopic and control subjects.
A stepwise multiple linear regression analysis was performed to evaluate whether amblyopic eye logMAR visual acuity, presence or absence of stereoacuity (with a criterion of 800 seconds of arc on the Randot Preschool Stereoacuity Test), or duration of patching treatment (in months), was associated with amblyopic eye accommodative error during monocular viewing at the highest accommodative demand of 4.00 D. A P value of 0.007 was used to determine significance for each factor (Bonferroni correction for multiple comparisons based on seven models).
To assess intra-examiner repeatability in amblyopic and control subjects as well as interinstrument and interexaminer repeatability of Nott retinoscopy, the mean difference between initial and repeated accommodative response measurements was derived with the associated 95% limits of agreement. A Bland-Altman plot, where the difference between initial and repeated measurements of accommodative response was plotted against the average of the two measurements,33 was also generated to evaluate whether repeatability varied systematically with average accommodative response.
All calculations and statistical testing were performed with Microsoft Excel (Microsoft, Redmond, WA, USA) or STATA.
Results
Thirty-eight subjects with unilateral amblyopia and 28 typically developing subjects were recruited. For children who were enrolled into the study, if it was determined during poststudy review of their clinical records that they had worn their refractive correction for less than 4 weeks at the time of data collection, they were excluded from the data analysis (three control subjects). This yielded a total of 38 amblyopic subjects (mean age = 6.89 years, SD = ±1.94 years; 11 IU, 27 SCCO) and 25 control subjects (mean age = 6.84, SD = ±2.83 years; 19 IU, 6 SCCO) who were included in the data analysis.
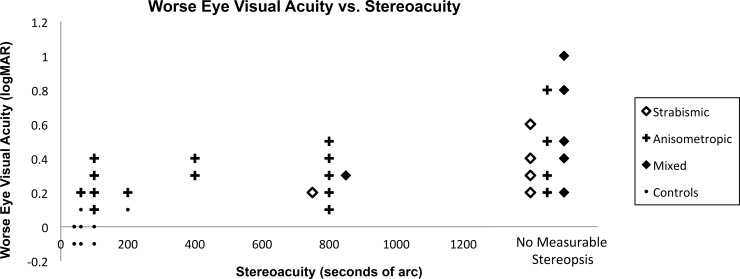
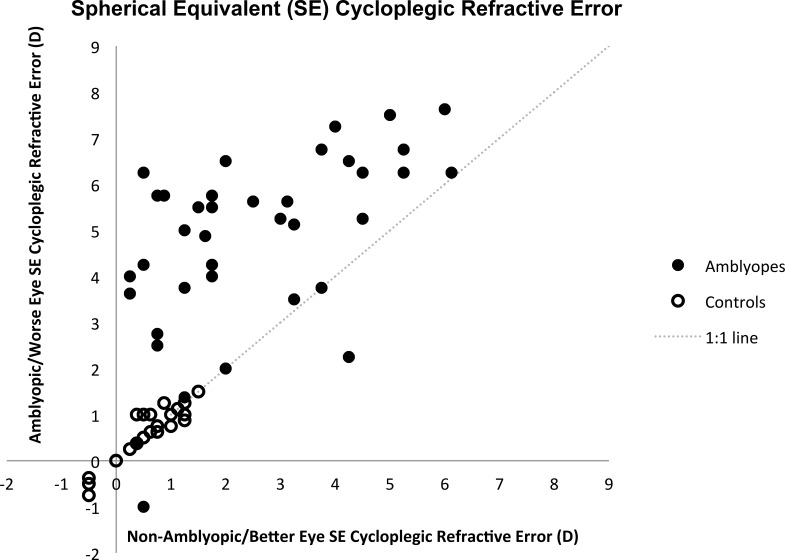
In the control group, median visual acuity in the worse eye was 0 logMAR (interquartile range [IQR]: −0.1 to 0 logMAR) and median stereoacuity was 40″ (IQR: 40″ to 60″). In the amblyopic group, visual acuity in the amblyopic eye ranged from 0.1 logMAR to 1.0 logMAR with a median of 0.3 logMAR (IQR: 0.2–0.4 logMAR). Of the 38 amblyopic subjects, 20 (52.6%) had no measurable stereopsis (i.e., worse than 800″). Twenty-two (57.9%) had anisometropic amblyopia, 7 (18.4%) had strabismic amblyopia, and 9 (23.7%) had combined-mechanism amblyopia (Fig. 2). All amblyopic subjects and no control subjects wore optical correction. The median SE cycloplegic refractive error was +0.75 D (IQR: 0.38–1.00 D) in both the better and worse eyes of control subjects and +1.88 D (IQR: 0.97–3.94 D) in the nonamblyopic eye and +5.25 D (IQR: 3.75–6.25 D) in the amblyopic eye of amblyopic subjects (Fig. 3). The median astigmatism was 0 D (IQR: 0–0.25 D) in both the better and worse eyes of control subjects and 0.50 D (IQR: 0–0.5 D) in the nonamblyopic eye and 0.75 D (IQR: 0.5–1.5 D) in the amblyopic eye of amblyopic subjects.
Figure 2.
The relationship between amblyopic eye/worse eye logMAR visual acuity and stereoacuity (seconds of arc or ″) for amblyopic and control subjects. Amblyopic subjects are grouped by the associated amblyogenic factor: strabismus, anisometropia, or mixed (i.e., strabismus and anisometropia). No measurable stereopsis indicates stereoacuity worse than 800”, which is the coarsest stereoacuity level that can be assessed with the Randot Preschool Stereoacuity Test. The symbol groupings that represent the associated amblyogenic risk factors are shifted horizontally at stereoacuity levels of 800” and “no measurable stereopsis” to allow for ease of viewing.
Figure 3.
Amblyopic/worse eye spherical equivalent cycloplegic refractive error plotted against nonamblyopic/better eye in amblyopic and control subjects.
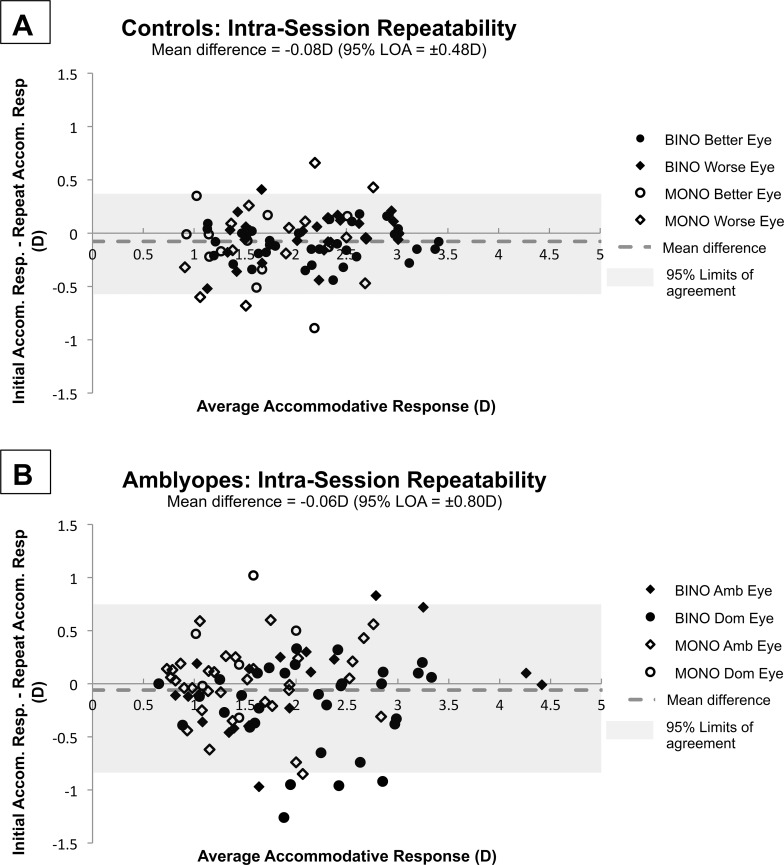
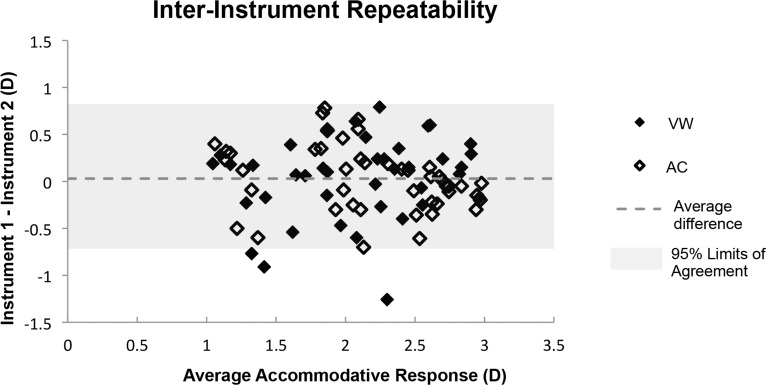
Interinstrument repeatability of Nott dynamic retinoscopy for one pre-presbyopic adult, assessed by both of the two study examiners on two different days (one instrument on one day and the other instrument on another day), revealed a mean signed difference of +0.03 D (95% Limits of Agreement [LOA]: ±0.76 D; Fig, 4) and a mean unsigned difference of 0.30 D. Interexaminer repeatability for one pre-presbyopic adult, performed on the same instrument within 1 hour, revealed a mean signed difference of +0.02 D (95% LOA: ±0.42 D) and a mean unsigned difference of 0.16 D. Intra-examiner, intra-instrument, and intrasession repeatability of Nott dynamic retinoscopy were assessed in a subset of subjects who sustained attentive fixation after the primary study measurements were completed. No significant differences were found between the characteristics of the subjects who participated in the repeatability analysis and those who did not. Neither age (amblyopes, P = 0.34; controls, P = 0.97) nor presence of strabismus (amblyopes, P = 0.85) was predictive of the ability to participate in collection of repeatability data. There was a mean signed difference of −0.06 D (95% LOA: ±0.80 D) and a mean unsigned difference of 0.30 D for the 15 amblyopic subjects, and a mean signed difference of −0.08 D (95% LOA: ±0.48 D) and a mean unsigned difference of 0.19 D for the 16 control subjects (Figs. 5A, 5B). Neither intra-examiner, nor interexaminer, nor interinstrument repeatability varied with average total accommodative response.
Figure 5.
Bland-Altman style plots,33 where the difference between the initial and repeated intrasubject and intrasession Nott retinoscopy measurements are plotted against the average of the two measurements. Each subject provided repeatability data from one eye in either monocular or binocular viewing at 2D, 3D, and 4D demands. (A) Intrasession repeatability measurements from 16 control subjects. Mean difference was −0.08 D (LOA: ±0.48 D). (B) Intrasession repeatability measurements from 15 amblyopic subjects. Mean difference was −0.06 D (95% LOA: ±0.80 D).
Figure 4.
Bland-Altman style plot33 of the difference between Nott retinoscopy measurements from two instruments, performed by the same examiner as a function of the average of the two measurements. Two examiners (AMC, VM) obtained these measurements from one pre-presbyopic, visually normal adult subject on different days. The mean intraexaminer, interinstrument, intersession repeatability was 0.03 (95% LOA: ±0.76 D).
All of the subjects attended to the target well during the data collection. A total of 960 accommodative response measurements were collected from amblyopic subjects and 600 from control subjects. Thirty-nine data points from the amblyopic group and 32 data points from the control group could not be collected, primarily due to the limited range of the potentiometer (39/39 cases in amblyopes and 31/32 in controls). In one case, the trigger button was not pressed to record the measurement. In amblyopic subjects, most missing data were due to accommodative responses < 0.68 D, while in controls, missing data were typically due to responses > 4.69 D.
Residual Uncorrected Refractive Error
No subject had residual oblique astigmatism (axis > 15° from 90° or 180°) with his/her habitual correction and 25 (65.8%) amblyopic subjects and 18 (72%) controls had no residual regular astigmatism. Those with residual regular astigmatism were undercorrected by ≤1.00 D. The median residual uncorrected astigmatism in amblyopic subjects was 0 D (IQR: 0–0 D) in both amblyopic and nonamblyopic eyes. The median residual uncorrected astigmatism in control subjects was 0 D (IQR: 0–0.25 D) in both the worse and better eyes.
The median residual uncorrected SE refractive error in amblyopic subjects was +1.00 D (IQR: +0.25 to +1.50 D) in amblyopic eyes and +1.00 D (IQR: +0.25 to +1.50 D) in nonamblyopic eyes. The median residual uncorrected SE refractive error in control subjects was +0.75 D (IQR: +0.5 to +1.00 D) in the worse eyes and +0.75 D (IQR: +0.25 to +1.00 D) in the better eyes. There were no significant differences in residual uncorrected SE refractive error between the amblyopic and control subjects in any meridian of either eye, using the Wilcoxon signed-rank test (P > 0.24).
The median signed residual uncorrected SE anisometropia (defined as the nonamblyopic eye/better eye subtracted from the amblyopic eye/worse eye residual uncorrected SE refractive error) in amblyopic and control subjects was 0 D (IQR: 0–0 D). Twenty-five (65.8%) amblyopic and 15 (60%) control subjects had zero residual uncorrected anisometropia. There was no significant difference in residual uncorrected SE anisometropia between the groups, using a Wilcoxon signed-rank test (P > 0.84). The median uncorrected astigmatic anisometropia was 0 D (IQR: 0–0 D) in both amblyopic and control groups.
Accommodative Stimulus-Response Functions
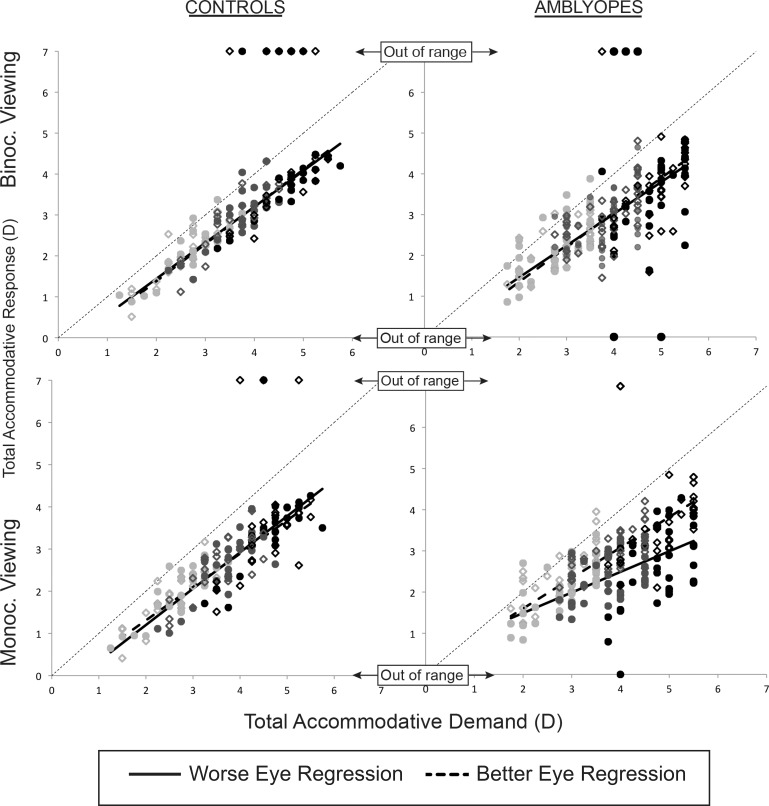
An EIV regression analysis was used to assess the accommodative stimulus-response relationship within each group. The residuals around the fitted function in all conditions did not differ significantly from a normal distribution and had no systematic trend in variance, indicating that the regression approach was appropriate. The coefficients (slopes), constants, and R2 values for each of the four regression analyses performed for each eye (monocular and binocular viewing for each meridian) for amblyopic and control subjects are listed in Table 1. Data from monocular and binocular viewing for the 90° meridian, including the out-of-range data points (accommodative responses > 4.69 D or < 0.68 D) and linear regression, for both amblyopic and control groups are shown in Figure 6.
Table 1.
Summary of EIV Regressions Performed on Total Accommodative Response as a Function of Total Accommodative Demand
|
Viewing Condition |
Group |
Meridian |
Eye Condition |
EIV Regression Model |
||
|
Coefficient |
Constant |
R2 |
||||
| Binocular | Controls | 90° | Better eye | 0.91 | −0.44 | 0.93 |
| Worse eye | 0.88 | −0.32 | 0.91 | |||
| 180° | Better eye | 0.89 | −0.46 | 0.93 | ||
| Worse eye | 0.93 | −0.56 | 0.96 | |||
| Amblyopes | 90° | Nonamblyopic eye | 0.85 | −0.35 | 0.77 | |
| Amblyopic eye | 0.78 | −0.10 | 0.65 | |||
| 180° | Nonamblyopic eye | 0.75 | −0.26 | 0.76 | ||
| Amblyopic eye | 0.70 | −0.06 | 0.66 | |||
| Monocular | Controls | 90° | Better eye | 0.79 | −0.27 | 0.83 |
| Worse eye | 0.86 | −0.52 | 0.86 | |||
| 180° | Better eye | 0.80 | −0.36 | 0.84 | ||
| Worse eye | 0.85 | −0.51 | 0.84 | |||
| Amblyopes | 90° | Nonamblyopic eye | 0.74 | 0.12 | 0.80 | |
| Amblyopic eye | 0.50 | 0.49 | 0.41 | |||
| 180° | Nonamblyopic eye | 0.68 | 0.05 | 0.78 | ||
| Amblyopic eye | 0.54 | 0.17 | 0.49 | |||
The coefficient, constant, and R2 values derived for each group (amblyopic or control), meridian (90° or 180°), and eye (better/nonamblyopic or worse/amblyopic) are listed.
Figure 6.
Graph of total accommodative response (measured accommodative response of the eye + residual uncorrected ametropia) as a function of total accommodative demand (target demand + residual uncorrected ametropia) for control and amblyopic subjects under binocular and monocular viewing as measured in the 90° meridian. The “out of range” points plotted above the graphs indicate the measured accommodative positions of >4.69 D and those plotted below the graphs indicate measured positions of <0.68 D. Closed circles, worse eye; open diamonds, better eye. Pale gray symbols, 2-D target position; gray symbols, 3-D target position; and black symbols, 4-D target position.
There was no apparent difference between the nonamblyopic/better eye and amblyopic/worse eye slopes or intercepts during binocular viewing in either subject group, or during monocular viewing in controls, for either meridian. However, during monocular viewing in amblyopic subjects, the slope for the amblyopic eye was shallower (90° meridian = 0.50, 180° meridian = 0.54) than that of the nonamblyopic eye (90° meridian = 0.74, 180° meridian = 0.68), with the amblyopic eye exhibiting poorer accommodative responses with increasing total accommodative demands. The greatest variance in accommodative responses other than that attributable to variation in accommodative demand was exhibited by the amblyopic eyes during monocular viewing conditions (average R2 value of 0.45 across the two meridia).
Accommodative Error
The mean IOD in accommodative error was minimal (<±0.10 D) in control subjects viewing monocularly, and in both amblyopic and control subjects viewing binocularly (correcting for residual uncorrected anisometropia in binocular measures) for both meridia (Table 2). A larger mean IOD in accommodative error existed in amblyopic subjects for monocular viewing. On average they demonstrated higher accommodative errors in the amblyopic eye relative to the nonamblyopic eye for both meridia (Table 2). These values likely underestimate the true mean IOD in accommodative error, as responses less than 0.68 D could not be measured (28/39 missing data points).
Table 2.
Summary of Interocular Differences in Accommodative Lag (Better Eye/Nonamblyopic Eye Subtracted From the Worse Eye/Amblyopic Eye) in Each Viewing Condition
|
Viewing Condition |
Meridian |
Accommodative Demand, D |
Amblyopes, n
= 38 |
Controls, n
= 25 |
||
|
Mean IOD, D |
95% CI, D |
Mean IOD, D |
95% CI, D |
|||
| Monocular | 180° | 2 | 0.24 | ±0.96 | −0.01 | ±0.60 |
| 3 | 0.52 | ±0.94 | ||||
| 4 | 0.70 | ±1.28 | ||||
| 90° | 2 | 0.29 | ±1.02 | 0.02 | ±0.55 | |
| 3 | 0.50 | ±0.99 | ||||
| 4 | 0.84 | ±1.54 | ||||
| Binocular | 180° | 0.04 | ±1.19 | −0.04 | ±0.60 | |
| 90° | 0.07 | ±1.28 | 0.04 | ±0.59 | ||
Amblyopic subjects demonstrate an increasing interocular difference in monocular viewing conditions in both meridia that is not observable in binocular viewing or in control subjects in either viewing condition. Therefore, mean interocular difference in accommodative lags are reported for each accommodative demand for that condition.
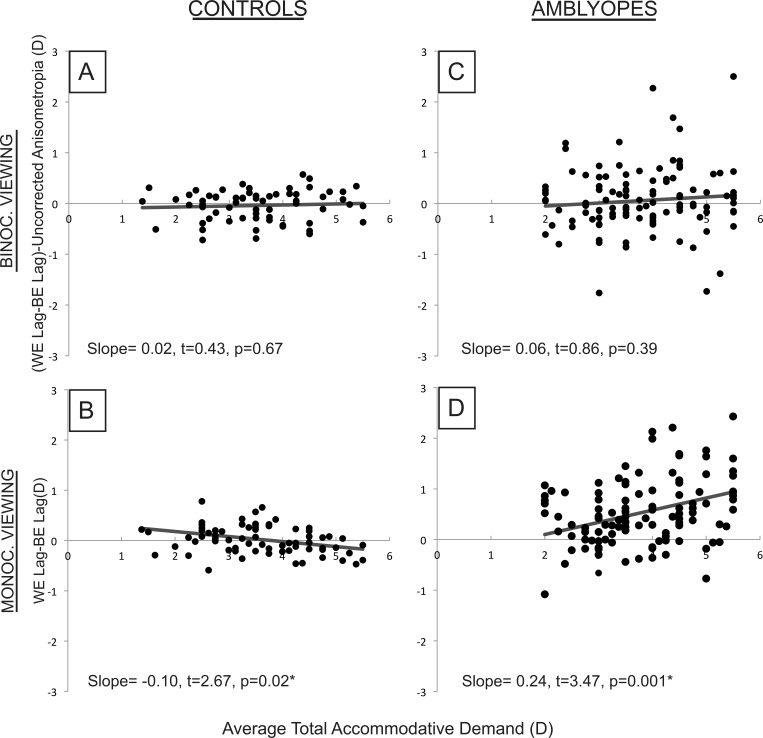
The mean IOD in accommodative error in amblyopic subjects viewing monocularly increased significantly with increasing total accommodative demand (P = 0.001 and P = 0.02 for 90° and 180° meridia, respectively; Fig. 7). In control subjects viewing monocularly, for the 90° meridian only, the IOD in accommodative error decreased significantly (P = 0.02) with increasing accommodative demand, but this relationship was clinically insignificant (0.10-D decrease in IOD in error per 1.00-D increase in demand). Control and amblyopic subjects viewing binocularly showed no significant association between IOD in accommodative error (corrected for residual anisometropia) and total accommodative demand (Fig. 7).
Figure 7.
Graph of interocular difference in accommodative lag (better eye/nonamblyopic eye subtracted from worse eye/amblyopic eye) plotted against the average demand for the two eyes for control (A, B) and amblyopic (C, D) subjects in binocular and monocular viewing, as measured in the 90° meridian.
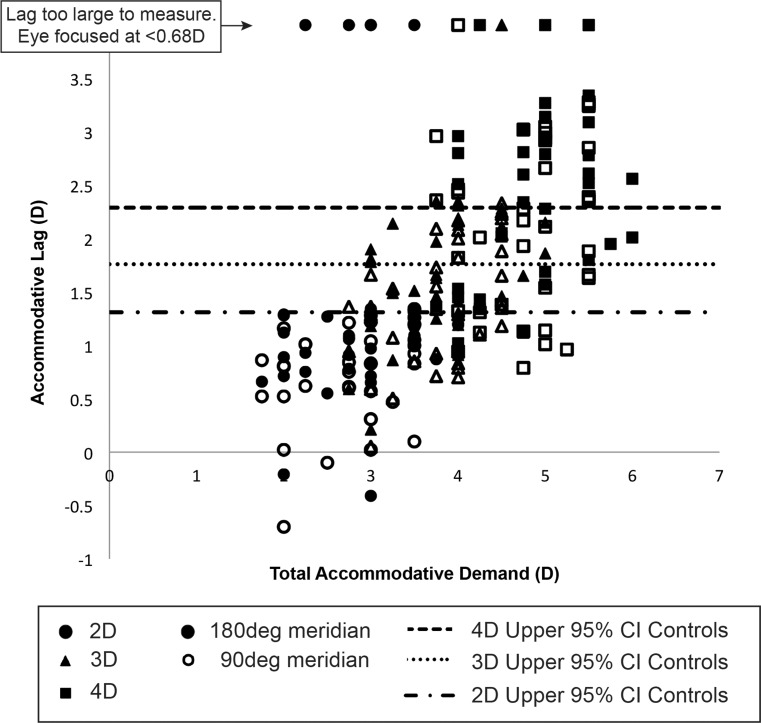
The better eyes of control subjects when viewing monocularly exhibited upper 95% confidence limits for accommodative error (lags) of 1.31 D, 1.76 D, and 2.29 D for 2.00 D, 3.00 D, and 4.00 D accommodative demands, respectively. For corresponding accommodative demands, 5%, 47%, and 58% of amblyopic eyes had accommodative errors outside this “normal” upper limit in either meridian during monocular viewing (including out-of-range responses < 0.68 D) (Fig. 8).
Figure 8.
Graph of amblyopic eye monocular accommodative lag plotted against total accommodative demand at 2 D, 3 D, and 4 D for both 90° and 180° meridia. The upper limits of the 95% confidence interval for the better eyes of control subjects are plotted as horizontal lines.
Multiple linear regression analysis revealed no significant association between amblyopic eye accommodative error for a 4.00-D demand during monocular viewing and amblyopic eye visual acuity, presence or absence of stereopsis, or duration of patching treatment, although visual acuity and duration of patching appeared to have more impact than the presence or absence of stereopsis in the models (Table 3).
Table 3.
Summary of Findings From a Stepwise Regression Testing Amblyopic Eye Visual Acuity, Presence or Absence of Stereopsis, and Duration of Patching Treatment as Predictors of Monocular Accommodative Lag at a Accommodative Demand of 4 D
|
Predicting Monocular Accommodative Lag, Slope, and
P
Values for Each Factor |
||||||||
|
Meridian |
Models |
I |
II |
III |
IV |
V |
VI |
VII |
| 90° | Amblyopic eye visual acuity, logMAR | 1.01, P = 0.11 | 1.33, P = 0.06 | 0.79, P = 0.21 | 1.03, P = 0.15 | |||
| Stereopsis, presence or absence | 0.10, P = 0.70 | 0.32, P = 0.25 | 0.03, P = 0.92 | 0.21, P = 0.45 | ||||
| Duration of patching, mo | −0.03, P = 0.06 | −0.03, P = 0.07 | −0.02, P = 0.11 | −0.02, P = 0.17 | ||||
| 180° | AE visual acuity, logMAR | 1.24, P = 0.07 | 1.66, P = 0.03 | 0.90, P = 0.17 | 1.18, P = 0.12 | |||
| Stereopsis, presence or absence | 0.13, P = 0.59 | 0.37, P = 0.15 | 0.01, P = 0.96 | 0.21, P = 0.43 | ||||
| Duration of patching, mo | −0.03, P = 0.01 | −0.03, P = 0.02 | −0.03, P = 0.03 | −0.03, P = 0.06 | ||||
A P value of 0.007 was necessary to claim significance to correct for multiple comparisons (based on seven models and a Bonferroni correction).
Nonamblyopic Eye Versus Control Eye
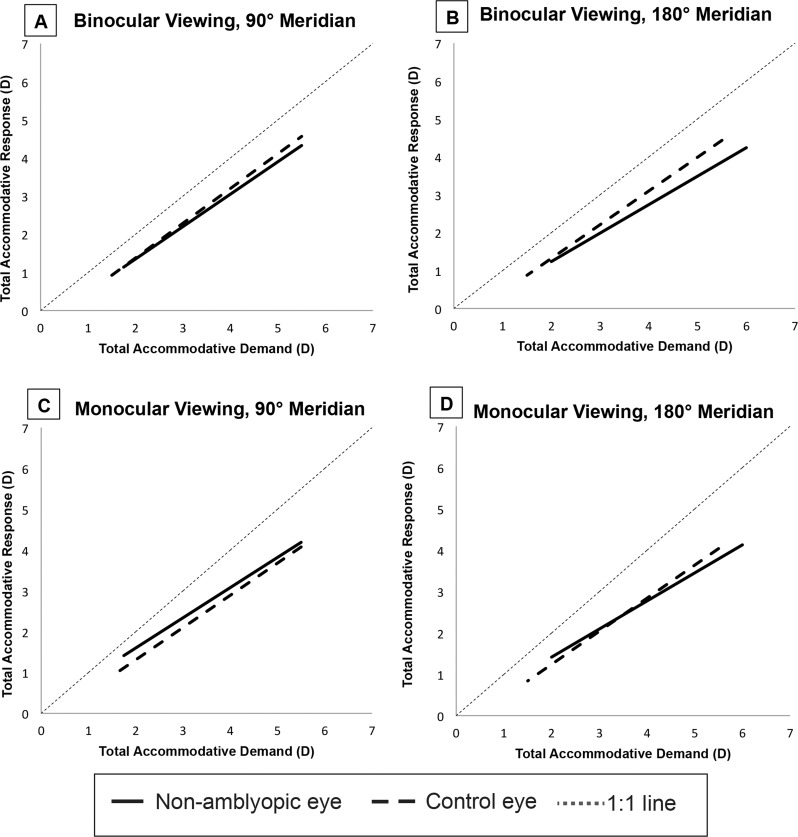
The accommodative performance of the nonamblyopic eye of amblyopic subjects was compared with the better eye of control subjects. In binocular viewing, EIV regression analysis showed a significant difference between the two groups in both meridia (90°: slope = 0.18, P = 0.001; 180°: slope = 0.33, P < 0.001). Accommodative errors were greater in the nonamblyopic eyes of amblyopic subjects than in the better eyes of control subjects (Table 4). The differences increased with increasing accommodative demands (Figs. 9A, 9B). In monocular viewing, EIV regression analysis showed a significant difference between the two groups in the 90° meridian only (slope, −0.18; P < 0.001). During monocular viewing in the 90° meridian, accommodative errors were greater in the better eyes of control subjects than in the nonamblyopic eyes of amblyopic subjects (Table 4), especially with lower accommodative demands (Fig. 9C).
Table 4.
Mean Accommodative Error of the Nonamblyopic Eye of Amblyopic Subjects and Better Eye of Control Subjects in Each Viewing Condition and Accommodative Demand (2 D, 3 D, 4 D)
|
Viewing Condition |
Meridian |
Accommodative Demand, D |
Amblyopes, n
= 38 |
Controls, n
= 25 |
||
|
Mean Accommodative Error, D |
±95% CI, D |
Mean Accommodative Error, D |
±95% CI, D |
|||
| Binocular | 180° | 2 | 0.76 | 0.70 | 0.63 | 0.63 |
| 3 | 1.25 | 0.94 | 0.82 | 0.72 | ||
| 4 | 1.62 | 1.23 | 1.12 | 0.39 | ||
| 90° | 2 | 0.65 | 0.80 | 0.55 | 0.62 | |
| 3 | 0.92 | 1.03 | 0.72 | 0.69 | ||
| 4 | 1.35 | 1.17 | 1.02 | 0.50 | ||
| Monocular | 180° | 2 | 0.74 | 0.81 | 0.74 | 0.56 |
| 3 | 1.11 | 0.85 | 1.08 | 0.65 | ||
| 4 | 1.63 | 1.04 | 1.46 | 0.89 | ||
| 90° | 2 | 0.45 | 0.91 | 0.70 | 0.62 | |
| 3 | 0.89 | 0.85 | 0.97 | 0.81 | ||
| 4 | 1.33 | 0.87 | 1.41 | 0.89 | ||
Figure 9.
Error in variables regression functions fit to the total accommodative response as a function of total accommodative demand. Functions for the better eye of control subjects and the nonamblyopic eye of amblyopes are shown for binocular viewing in the 90° (A) and 180° (B) meridia and for monocular viewing in 90° (C) and 180° (D) meridia.
Discussion
In this study, the amblyopic eyes of children with unilateral amblyopia were found to have greater accommodative errors than nonamblyopic eyes during monocular viewing, and the difference increased with greater accommodative demands. At demands of 3.00 D and greater, approximately half the amblyopic children showed monocular accommodative errors (lags) falling outside the upper 95% confidence interval for control subjects. These results are consistent with previous studies evaluating accommodative performance of amblyopic adults.22 In addition, we found that the nonamblyopic eyes of amblyopic subjects showed greater accommodative error than the better eyes of control subjects during binocular viewing. This may reflect the reduced binocular function commonly associated with unilateral amblyopia, resulting in reduced retinal disparity cues available to drive a vergence-accommodation response, and is consistent with previous literature.34,35 On the other hand, the nonamblyopic eyes of amblyopic subjects showed a somewhat smaller error than the better eyes of control subjects during monocular viewing conditions, suggesting that the amblyopes were less affected by the removal of binocular cues.
The 95% limits of agreement found for repeated Nott dynamic retinoscopy estimates in the control group in this study are consistent with those reported previously for normal individuals,31,32,36,37 despite differences in age (Table 5). Amblyopic subjects demonstrated greater variability of repeated accommodative response measurements than the control group and previous studies of normal subjects. However, given that repeated measurements were not systematically biased toward better or worse performance than initial measurements in our repeatability analysis, it is unlikely that the worse mean accommodative error found in amblyopic eyes when viewing monocularly is related to the increased variability of repeated measurements.
Table 5.
Summary of Studies Evaluating the Repeatability of Retinoscopy in Visually Normal Individuals
|
Study |
N |
Age, y |
Type of Repeatability |
Accommodative Demand, D |
Mean Difference, D ± 95% LOA, D |
| Locke and Somers, 1989 | 10 | 24–30 | Interexaminer | 2.5 | −0.08 ± 0.37 |
| (2 examiners) | |||||
| Intrasession | |||||
| Zadnik et al., 1992 | 40 | 20–43 | Intra-examiner | Infinity | −0.006 ± 0.78 |
| (2 examiners) | |||||
| Intersession | |||||
| McClelland and Saunders, 2003 | 41 | 6–43 | Intra-examiner | 10 | +0.02 ± 1.34 |
| (1 examiner) | 6 | −0.14 ± 1.09 | |||
| Intersession | 4 | +0.04 ± 0.56 | |||
| Goss et al., 2005 | 50 | 20–35 | Interexaminer | 2.5 | +0.03 ± 0.17 |
| (2 examiners) | |||||
| Intrasession | |||||
| Antona et al., 2009 | 61 | 18–32 | Intra-examiner | 2.5 | −0.10 ± 0.66 |
| (1 examiner) | |||||
| Intersession |
Two hypotheses have been suggested regarding the etiology of poor monocular accommodative performance in the amblyopic eye.22,24,38 The motor hypothesis, which predicts an inefficient efferent pathway and output of the accommodative system, is not supported by studies of amblyopic adults who show normal consensual accommodative responses in amblyopic eyes during binocular viewing.39 In this study, we also observed normal consensual accommodative responses in amblyopic eyes, as evidenced by the absence of IOD in accommodative error in amblyopic subjects viewing binocularly. There is greater support in the literature for the sensory DOF hypothesis: internal and external factors related to the stimulus and optics of the eye have been found to increase the sensory DOF in adult amblyopes,40 which can result in an increased accommodative error. Several studies have directly shown that the DOF of an amblyopic eye is larger than that of its fellow nonamblyopic eye.22,41,42
The sensory DOF hypothesis would predict that accommodative performance should be correlated with the magnitude of vision deficits. Studies have found that adults with unilateral amblyopia have both reduced contrast sensitivity and higher accommodative lags over the entire spatial frequency spectrum when viewing with their amblyopic eye.41,43–46 The loss of sensitivity to high spatial frequencies (reduced resolution acuity) in amblyopic eyes has also been found to be significantly correlated with accommodative performance in monkeys.45 However, in visually normal adults, the accommodative response appears to be relatively robust to reduced high spatial frequency content when visual acuity is better than 20/300 (although it drops rapidly toward the tonic level beyond this visual acuity limit47). In the present study, visual acuity was 20/200 or better for all amblyopic eyes, and a broadband spatial cartoon presented on a back-lit screen was used to ensure that eyes with varying levels of amblyopia were provided with adequate stimulus content. Under these conditions, we found that amblyopic eye visual acuity was not a significant predictor of monocular accommodative error in the amblyopic eye at a demand of 4.00 D. Thus, our data do not provide direct evidence for the sensory DOF hypothesis. On the other hand, the absence of a relationship with visual acuity may also be attributed to the small sample size and limited range of amblyopic eye visual acuities in this study.
Gaze instabilities,48–52 nystagmus, and eccentric fixation35,42,53 could also contribute to increased steady-state accommodative error in amblyopic eyes, by causing the stimulus to fall on less sensitive retina,54 or by inducing a smearing effect in the retinal image with subsequent blurring of moderate and high spatial frequency components.41,48,55 Several studies have shown decreased accommodative lags and increased slopes of the accommodative stimulus-response function22,35,54 with a reduction of eccentric fixation in amblyopic subjects. Fixational eye movements were not assessed in this study and so their impact on the findings is unknown.
Our findings indicate that children with unilateral amblyopia may experience increased defocus while undergoing patching of their nonamblyopic eye, particularly when asked to perform visual activities with substantial accommodative demands. The mean habitual reading distance of children from kindergarten to sixth grade (ages 4 to 12 years) is reported to be 25 cm (SD: ±5 cm).12 Many clinicians commonly prescribe partial hyperopic refractive error corrections to amblyopic children without esotropia to facilitate better spectacle adaptation,56 but this undercorrection further increases the accommodative demand. If one assumes that the habitual working distance of a child with unilateral amblyopia is 25 cm (4.00-D demand) and that he/she is wearing a hyperopic spectacle correction that is undercorrecting hyperopia by 1.00 D, the resultant total accommodative demand at near is 5.00 D. Based on the results of this study, it is likely that a sizeable proportion of young amblyopes will experience a significantly defocussed retinal image at this high accommodative demand when they view naturalistic images (1/f) during patching therapy. The defocus may be even more significant if they are using handheld electronic devices at even closer distances. On the other hand, it is conceivable that accommodative performance in amblyopes may be better than suggested here if children are provided with more detailed and spatially challenging targets than those used in this study.
The larger amblyopic eye accommodative lags that we observed at higher demands compared to lower demands might lead one to predict that amblyopic eye visual acuities at near would be worse than at distance. However, when the Pediatric Eye Disease Investigator Group looked at differences between distance and near visual acuities in children from 2 to 6 years of age with unilateral amblyopia, they found no significant difference beyond test–retest repeatability.57 This may be because optotype targets stimulate better accommodation than naturalistic ones or because amblyopic children can briefly generate a normal accommodative response during visual acuity testing that may not be sustained for longer periods of time.
Prescribing bifocals for near viewing,58,59 full cycloplegic hyperopic spectacle prescriptions for distance viewing,60 and vision therapy61–64 have all been suggested as approaches to supplement conventional patching treatment for amblyopia in light of the accommodative deficits that exist in amblyopic eyes. Several case studies11,61–63 have reported improvements in accommodative performance concurrent with improvement in visual acuity, contrast sensitivity, and eye movements after accommodative vision therapy, in children as well as some adult amblyopes. The results of the current study showed that accommodation tends to be more accurate in the amblyopic eye under binocular viewing conditions than monocular viewing conditions in children with unilateral amblyopia. This finding suggests that an amblyopia treatment incorporating accurate accommodation driven by the nonamblyopic eye may have advantages over occlusion treatment in terms of retinal image quality, and may lend further theoretical support to efforts in developing biocular (monocular training in a binocular field) or antisuppression training as an effective means of improving visual acuity in adults65–67 and children with unilateral amblyopia.68
To the extent that poor accommodation in amblyopic eyes might be a direct consequence of decreased vision (as suggested by the sensory DOF hypothesis), it might be argued that as vision starts to improve with amblyopia therapy (e.g., patching), monocular accommodative performance during patching would naturally improve in the course of treatment, reinforcing treatment efficacy and eliminating the need to specifically address the accommodative deficit in the treatment plan. Our finding of accommodative deficits in amblyopia cannot predict whether specific interventions to enhance retinal image quality during amblyopia therapy would have any impact on the magnitude or time course of visual acuity improvement.
Limitations
The limits of the current instrumentation prevented the measurement of accommodative responses of >4.69 D or <0.68 D, and approximately 5% of data points could not be obtained in this study. In control subjects, most (91%) of the missing points were due to accommodative responses of >4.69 D, especially when the stimulus was placed at an accommodative demand of 4.00 D. Subjects with normal vision and only spherical refractive error do not normally exhibit accommodative leads, especially to a 4.00-D stimulus. The leads we observed might have been secondary to the presence of astigmatism (≤1.00 D in all subjects) or subjects looking at their own reflections in the beam-splitter. Conversely, in amblyopic subjects, most (72%) of the missing data points were due to accommodative responses of <0.68 D, especially when the stimulus was placed at a target demand of 2.00 D, further reinforcing the conclusion that amblyopic subjects have reduced accommodative responses.
Off-axis observation can affect retinoscopy measurements. For a fixing eye, the maximum possible off-axis fixation while the subject was viewing the LCD screen was 16.7° horizontally and 9.6° vertically. Off-axis retinoscopy errors of 1.08 D and 0.35 D have been reported for 20° horizontal and vertical eccentricities,69 while induced astigmatism at 20° eccentricity may be up to 1 D.70,71 However, off-axis fixation of 20° was evident to the trained examiners, and subjects were prompted to refixate when this occurred. Therefore, errors from off-axis retinoscopy in fixing eyes are presumed to be less than these values and unlikely to have significantly impacted our results. In the case of strabismic subjects viewing binocularly, the nonfixing eye could have been up to 14.5° (≤25 PD) deviated from the path of the retinoscope beam when the subject was viewing the center of the screen with the fixing eye. However, the finding of minimal IOD in accommodative error between amblyopic and nonamblyopic eyes under binocular viewing conditions suggests a negligible effect of off-axis measurements even for strabismic subjects viewing binocularly.
Variability of accommodative responses was greater in amblyopic subjects than controls, as evidenced by repeatability testing. Assuming this is due to increased variability of accommodative performance itself, a corresponding short-term variability of accommodative responses would be expected in amblyopic eyes during the period of observation for any single measurement. If examiners were biased toward preferentially sampling the worst accommodative performance occurring during the period of observation, rather than the average performance during the period of observation, this might bias our findings toward larger differences between amblyopic and nonamblyopic eyes. In fact, however, examiners were cognizant that transiently larger lags of accommodation might be an artifact of a temporary lapse of attention on the part of the child, and consequently were biased, if at all, toward preferentially sampling the best accommodative performance in any period of observation. Therefore, we do not believe that the lower repeatability of amblyopic eye accommodative performance has biased our results toward finding larger differences between amblyopic and nonamblyopic eyes.
Finally, the amblyopic subjects represented a wide range of clinical states; some subjects had been treated, while others had not. The amount of monocular visual experience during therapy may impact accommodative performance, but we did not find duration of patching to be a significant predictor of monocular accommodative lag in the amblyopic eyes at a 4.00-D demand. In addition, some subjects were fully corrected optically while others were not. Adaptation to different levels of optical correction may also impact accommodative responses, but this was not tested in the data analysis owing to the small sample size.
Conclusions
This study determined that in amblyopic children aged 3 to 13 years, accommodative errors were significantly larger in their amblyopic eyes under monocular viewing conditions than in their nonamblyopic eyes or the eyes of control subjects. For demands of 3.00 D or greater, at least half of amblyopic eyes had monocular accommodative errors (lags) outside the normal range. These high accommodative errors exhibited by amblyopes during monocular viewing of naturalistic targets may be associated with retinal image defocus in the amblyopic eye during patching therapy.
Acknowledgments
Supported by National Eye Institute R01 EY014460 (TRC), K23 EY016699 (KTH), and P30 EY019008 (Indiana University).
Disclosure: V. Manh, None; A.M. Chen, None; K. Tarczy-Hornoch, None; S.A. Cotter, None; T.R. Candy, None
References
- 1. Woodruff G, Hiscox F, Thompson JR, Smith LK. Factors affecting the outcome of children treated for amblyopia. Eye (Lond). 1994; 8 (pt 6): 627–631. [DOI] [PubMed] [Google Scholar]
- 2. Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci. 2004; 45: 3048–3054. [DOI] [PubMed] [Google Scholar]
- 3. Repka MX, Wallace DK, Beck RW, et al. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2005; 123: 149–157. [DOI] [PubMed] [Google Scholar]
- 4. Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013; 33: 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cotter SA, Edwards AR, Wallace DK, et al. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006; 113: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cotter SA, Foster NC, Holmes JM, et al. Optical treatment of strabismic and combined strabismic-anisometropic amblyopia. Ophthalmology. 2012; 119: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart CE, Moseley MJ, Fielder AR. Amblyopia therapy: an update. Strabismus. 2011; 19: 91–98. [DOI] [PubMed] [Google Scholar]
- 8. Candy TR, Wang J, Ravikumar S. Retinal image quality and postnatal visual experience during infancy. Optom Vis Sci. 2009; 86: E556–E571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell FW. The depth of focus of the human eye. J Physiol. 1954; 125: 29P–30P. [PubMed] [Google Scholar]
- 10. Green DG, Powers MK, Banks MS. Depth of focus, eye size and visual acuity. Vision Res. 1980; 20: 827–835. [DOI] [PubMed] [Google Scholar]
- 11. Ciuffreda K, Kenyon RV. Accommodative vergence and accommodation in normals, amblyopia, and strabismics. In: Schor C. ed Vergence Eye Movements: Basic and Clinical Aspects. Boston: Butterworth; 1983: 101–173. [Google Scholar]
- 12. Rouse MW, Hutter RF, Shiftlett R. A normative study of the accommodative lag in elementary school children. Am J Optom Physiol Opt. 1984; 61: 693–697. [DOI] [PubMed] [Google Scholar]
- 13. Jackson TW, Goss DA. Variation and correlation of clinical tests of accommodative function in a sample of school-age children. J Am Optom Assoc. 1991; 62: 857–866. [PubMed] [Google Scholar]
- 14. Leat SJ, Gargon JL. Accommodative response in children and young adults using dynamic retinoscopy. Ophthalmic Physiol Opt. 1996; 16: 375–384. [PubMed] [Google Scholar]
- 15. McClelland JF, Saunders KJ. Accommodative lag using dynamic retinoscopy: age norms for school-age children. Optom Vis Sci. 2004; 81: 929–933. [PubMed] [Google Scholar]
- 16. Manny RE, Chandler DL, Scheiman MM, et al. Accommodative lag by autorefraction and two dynamic retinoscopy methods. Optom Vis Sci. 2009; 86: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993; 34: 690–694. [PubMed] [Google Scholar]
- 18. McBrien NA, Millodot M. The effect of refractive error on the accommodative response gradient. Ophthalmic Physiol Opt. 1986; 6: 145–149. [PubMed] [Google Scholar]
- 19. Chen AH, O'Leary DJ. Are there age differences in the accommodative response curve between 3 and 14 years of age? Ophthalmic Physiol Opt. 2002; 22: 119–125. [DOI] [PubMed] [Google Scholar]
- 20. Anderson HA, Glasser A, Stuebing KK, Manny RE. Minus lens stimulated accommodative lag as a function of age. Optom Vis Sci. 2009; 86: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Legge GE, Mullen KT, Woo GC, Campbell FW. Tolerance to visual defocus. J Opt Soc Am A. 1987; 4: 851–863. [DOI] [PubMed] [Google Scholar]
- 22. Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: Basic and Clinical Aspects. Boston: Butterworth-Heinemann; 1991. [Google Scholar]
- 23. Otto J, Safra D. Methods and results of quantitative determination of accommodation in amblyopia and strabismus. In: Moore S, Mein J, Stockbridge L. eds Orthoptics: Past, Present, Future. New York: Stratton Intercontinental Medical Book Corp; 1976: 45–67. [Google Scholar]
- 24. Ukai K, Ishii M, Ishikawa S. A quasi-static study of accommodation in amblyopia. Ophthalmic Physiol Opt. 1986; 6: 287–295. [PubMed] [Google Scholar]
- 25. Holmes JM, Beck RW, Repka MX, et al. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001; 119: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 26. Drover JR, Felius J, Cheng CS, Morale SE, Wyatt L, Birch EE. Normative pediatric visual acuity using single surrounded HOTV optotypes on the Electronic Visual Acuity Tester following the Amblyopia Treatment Study protocol. J AAPOS. 2008; 12: 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthalmol. 2001; 132: 903–909. [DOI] [PubMed] [Google Scholar]
- 28. Birch E, Williams C, Drover J, et al. Randot Preschool Stereoacuity Test: normative data and validity. J AAPOS. 2008; 12: 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nott I. Dynamic skiametry, accommodation, and convergence. Am J Optom Physiol Opt. 1925; 6: 490–503. [Google Scholar]
- 30. Rosenfield M, Portello JK, Blustein GH, Jang C. Comparison of clinical techniques to assess the near accommodative response. Optom Vis Sci. 1996; 73: 382–388. [DOI] [PubMed] [Google Scholar]
- 31. Antona B, Sanchez I, Barrio A, Barra F, Gonzalez E. Intra-examiner repeatability and agreement in accommodative response measurements. Ophthalmic Physiol Opt. 2009; 29: 606–614. [DOI] [PubMed] [Google Scholar]
- 32. McClelland JF, Saunders KJ. The repeatability and validity of dynamic retinoscopy in assessing the accommodative response. Ophthalmic Physiol Opt. 2003; 23: 243–250. [DOI] [PubMed] [Google Scholar]
- 33. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1: 307–310. [PubMed] [Google Scholar]
- 34. Kandel GL, Grattan PE, Bedell HE. Are the dominant eyes of amblyopes normal? Am J Optom Physiol Opt. 1980; 57: 1–6. [DOI] [PubMed] [Google Scholar]
- 35. Kirschen DG, Kendall JH, Riesen KS. An evaluation of accommodation response in amblyopic eyes. Am J Optom Physiol Opt. 1981; 58: 597–602. [PubMed] [Google Scholar]
- 36. Zadnik K, Mutti DO, Adams AJ. The repeatability of measurement of the ocular components. Invest Ophthalmol Vis Sci. 1992; 33: 2325–2333. [PubMed] [Google Scholar]
- 37. Goss D, Groppel P, Dominguez L. Comparison of MEM retinoscopy and Nott retinoscopy and their inter-examiner repeatabilities. J Behav Optom. 2005; 16: 149–155. [Google Scholar]
- 38. France L. Dull faces: exoteric and esoteric facts and theories relative to amblyopia. Am Orthop J. 2003; 53: 60–74. [DOI] [PubMed] [Google Scholar]
- 39. Hokoda SC, Ciuffreda KJ. Measurement of accommodative amplitude in amblyopia. Ophthalmic Physiol Opt. 1982; 2: 205–212. [PubMed] [Google Scholar]
- 40. Wang B, Ciuffreda KJ. Depth-of-focus of the human eye: theory and clinical implications. Surv Ophthalmol. 2006; 51: 75–85. [DOI] [PubMed] [Google Scholar]
- 41. Ciuffreda KJ, Hokoda SC. Spatial frequency dependence of accommodative responses in amblyopic eyes. Vision Res. 1983; 23: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 42. Ciuffreda KJ, Hokoda SC, Hung GK, Semmlow JL. Accommodative stimulus/response function in human amblyopia. Doc Ophthalmol. 1984; 56: 303–326. [DOI] [PubMed] [Google Scholar]
- 43. Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Res. 1977; 17: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 44. Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1981; 21: 467–476. [PubMed] [Google Scholar]
- 45. Kiorpes L, Boothe RG. Accommodative range in amblyopic monkeys (Macaca nemestrina). Vision Res. 1984; 24: 1829–1834. [DOI] [PubMed] [Google Scholar]
- 46. Ciuffreda KJ, Rumpf D. Contrast and accommodation in amblyopia. Vision Res. 1985; 25: 1445–1457. [DOI] [PubMed] [Google Scholar]
- 47. Heath GG. The influence of visual acuity on accommodative responses of the eye. Am J Optom Arch Am Acad Optom. 1956; 33: 513–524. [DOI] [PubMed] [Google Scholar]
- 48. Ciuffreda KJ, Kenyon RV, Stark L. Fixational eye movements in amblyopia and strabismus. J Am Optom Assoc. 1979; 50: 1251–1258. [PubMed] [Google Scholar]
- 49. Subramanian V, Jost RM, Birch EE. A quantitative study of fixation stability in amblyopia. Invest Ophthalmol Vis Sci. 2013; 54: 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Regan D, Giaschi DE, Kraft SP, Kothe AC. Method for identifying amblyopes whose reduced line acuity is caused by defective selection and/or control of gaze. Ophthalmic Physiol Opt. 1992; 12: 425–432. [PubMed] [Google Scholar]
- 51. Gonzalez EG, Wong AM, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ. Eye position stability in amblyopia and in normal binocular vision. Invest Ophthalmol Vis Sci. 2012; 53: 5386–5394. [DOI] [PubMed] [Google Scholar]
- 52. Carpineto P, Ciancaglini M, Nubile M, et al. Fixation patterns evaluation by means of MP-1 microperimeter in microstrabismic children treated for unilateral amblyopia. Eur J Ophthalmol. 2007; 17: 885–890. [DOI] [PubMed] [Google Scholar]
- 53. Brock FW, Givner I. Fixation anomalies in amblyopia. AMA Arch Ophthalmol. 1952; 47: 775–786. [DOI] [PubMed] [Google Scholar]
- 54. Bullimore MA, Gilmartin B. Retinal eccentricity and the accommodative response. Am J Optom Physiol Opt. 1987; 64: 644–645. [PubMed] [Google Scholar]
- 55. Ciuffreda KJ, Kenyon RV, Stark L. Increased drift in amblyopic eyes. Br J Ophthalmol. 1980; 64: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moore B, Augsburger AR, Cockrell DA, Fern KD, Harb E. Care for the Patient With Hyperopia. St. Louis: American Optometric Association; 2008. [Google Scholar]
- 57. Christoff A, Repka MX, Kaminski BM, Holmes JM. Distance versus near visual acuity in amblyopia. J AAPOS. 2011; 15: 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guyton DL, O'Connor GM. Dynamic retinoscopy. Curr Opin Ophthalmol. 1991; 2: 78–80. [DOI] [PubMed] [Google Scholar]
- 59. Singh V, Agrawal S. Visual functions in amblyopia as determinants of response to treatment. J Pediatr Ophthalmol Strabismus. 2013; 50: 348–354. [DOI] [PubMed] [Google Scholar]
- 60. Campos EC, Inzillo G. Functional differences between free alternators and non-alternators successfully treated for strabismic amblyopia. Int Ophthalmol. 1986; 9: 191–194. [DOI] [PubMed] [Google Scholar]
- 61. Selenow A, Ciuffreda KJ. Vision function recovery during orthoptic therapy in an exotropic amblyope with high unilateral myopia. Am J Optom Physiol Opt. 1983; 60: 659–666. [DOI] [PubMed] [Google Scholar]
- 62. Hokoda SC, Ciuffreda KJ. Different rates and amounts of vision function recovery during orthoptic therapy in an older strabismic amblyope. Ophthalmic Physiol Opt. 1986; 6: 213–220. [PubMed] [Google Scholar]
- 63. Selenow A, Ciuffreda KJ. Vision function recovery during orthoptic therapy in an adult esotropic amblyope. J Am Optom Assoc. 1986; 57: 132–140. [PubMed] [Google Scholar]
- 64. Ciuffreda KJ, Kenyon RV, Stark L. Different rates of functional recovery of eye movements during orthoptics treatment in an adult amblyope. Invest Ophthalmol Vis Sci. 1979; 18: 213–219. [PubMed] [Google Scholar]
- 65. Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restor Neurol Neurosci. 2010; 28: 793–802. [DOI] [PubMed] [Google Scholar]
- 66. Hess RF, Thompson B, Black JM, et al. An iPod treatment of amblyopia: an updated binocular approach. Optometry. 2012; 83: 87–94. [PubMed] [Google Scholar]
- 67. Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol. 2013; 23: R308–R309. [DOI] [PubMed] [Google Scholar]
- 68. Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci. 2012; 53: 817–824. [DOI] [PubMed] [Google Scholar]
- 69. Ferree C, Rand G, Hardy C. Refraction for the peripheral field of vision. Arch Ophthalmol. 1931; 5: 717–731. [Google Scholar]
- 70. Rempt F, Hoogerheide J, Hoogenboom WP. Peripheral retinoscopy and the skiagram. Ophthalmologica. 1971; 162: 1–10. [DOI] [PubMed] [Google Scholar]
- 71. Millodot M. Effect of ametropia on peripheral refraction. Am J Optom Physiol Opt. 1981; 58: 691–695. [DOI] [PubMed] [Google Scholar]