Abstract
As bodies grow and change throughout early development and across the lifespan, animals must develop, refine, and maintain accurate sensorimotor maps. Here we review evidence that myoclonic twitches—brief and discrete contractions of the muscles, occurring exclusively during REM (or active) sleep, that result in jerks of the limbs—help animals map their ever-changing bodies by activating skeletal muscles to produce corresponding sensory feedback, or reafference. First, we highlight the spatiotemporal characteristics of twitches. Second, we review findings in infant rats regarding the multitude of brain areas that are activated by twitches during sleep. Third, we discuss evidence demonstrating that the sensorimotor processing of twitches is different from that of wake movements; this state-related difference in sensorimotor processing provides perhaps the strongest evidence yet that twitches are uniquely suited to drive certain aspects of sensorimotor development. Finally, we suggest that twitching may help inform our understanding of neurodevelopmental disorders, perhaps even providing opportunities for their early detection and treatment.
Keywords: sleep, REM sleep, myoclonic twitching, spinal circuitry, sensorimotor integration, cerebellum, activity-dependent development, corollary discharge, sensory feedback
Introduction
The adult animal possesses a fine-tuned sensorimotor system. Fast and efficient integration of motor commands and their resulting movements with associated sensory consequences is necessary for virtually every action an animal makes. The act of grasping a pencil, for example, requires a constant, bidirectional flow of information between the sensory and motor systems. And yet, despite the obvious importance of this sensorimotor integration, we know relatively little about where it comes from. A fine-tuned sensorimotor system cannot simply be endowed or preprogrammed; it must develop, and it must do so within the context of a continually and often rapidly changing body, with limb sizes and biomechanics changing from one day to the next [1]. How, then, do sensorimotor networks adapt to these changes? What developing animals do while awake certainly matters. Here we explore the ways in which movements during sleep might matter, too.
Young animals spend the majority of their time asleep. For example, human newborns typically sleep for 14-16 hours a day, of which half is spent in REM (or active) sleep [2-4]. Conventionally, sleep is thought of as a time of behavioral quiescence, but this could not be further from the truth, especially during early development. Indeed, skeletal muscles throughout the body twitch frequently during active sleep [5]. As muscles twitch and limbs move, tactile receptors and proprioceptors transmit signals to the brain (e.g., [6,7]). Recent research suggests that this sensorimotor experience helps to drive activity-dependent development of the sensorimotor system [8,9].
Here we review three aspects of twitching that provide insight into their role in sensorimotor development. We begin with the behavioral expression of twitches and their spatiotemporal structure. Next, we describe how twitches activate the central nervous system; we also describe how sensory feedback is processed differently during sleep than during wakefulness and how this difference may be necessary for twitches to contribute to sensorimotor development. Lastly, we suggest that studying twitching may help us understand better the etiology of certain neurodevelopmental disorders.
An Ideal Movement
Twitches of the limbs are first expressed in the late fetal period in rats [10,11] and can still be observed in adults [12], albeit at a reduced frequency. In rats, the highest amount of twitching can be observed during the first two postnatal weeks [13], which coincides with a period of rapid growth [1] and the emergence of adult-like motor skills [14]. Twitches occur in all skeletal muscles that have been investigated thus far, including those that control the limbs and digits [5], eyes [15], and whiskers [16], and it has been estimated that newborn rats produce hundreds of thousands of twitches each day [17]. Because motor learning requires copious amounts of experience [18], the sheer quantity of twitches suggests that they serve an important role.
Quantity matters, but so does quality. Twitches are produced in a state that allows them to be uniquely discrete and salient. Produced only during active sleep, twitches occur when all skeletal muscles are atonic [19]. Also, although twitches can occur in rapid succession, they rarely happen simultaneously [5], further reinforcing their discrete nature. In contrast, wake movements are typically composed of continuous and simultaneous muscle activations. If twitches are to provide information about the body to develop, refine, and maintain sensorimotor maps [9,17,20], then the singular event of a discrete twitch would seem to be well suited to contributing to those processes. It is much easier to pay attention to a single event when all else is quiet than when that single event is drowned out by a sea of extraneous activity.
Twitches occur so rapidly and abruptly, and at such low amplitudes compared to wake movements, that, to the naked eye, they appear to be randomly generated movements with no observable structure. High-speed videography combined with three-dimensional motion tracking has proven to be a powerful tool to reveal the intricate kinematics of twitches and how they change across early development [5]. When captured at 250 frames per second, what initially looks like a single twitch of the forelimb amidst a bout of twitching reveals itself as just one piece of a complex, sequential activation of twitches at multiple joints within and between limbs. Indeed, as early as two days of age, rats exhibit such synergistic patterns of twitching.
Furthermore, in the same way that synapses compete amongst themselves for access to postsynaptic sites, patterns of twitching appear to compete for retention and expression during the early postnatal period [5]. That the structure of twitches can be shaped by developmental experience suggests an underlying change in the spinal and brainstem mechanisms that control them. More broadly, they suggest that twitching contributes to the fine-tuning of the sensorimotor system as a whole, thus influencing the developmental and expression of movements during wake.
The value of twitching is not limited to biological systems. Despite incredible and rapid advances in modern robotics, we are still unable to create a robot that can learn and adapt in the real world. Some roboticists see this problem as a limitation inherent in the way that robots come into being: In short, they don’t develop and therefore never experience—and never learn to adapt to—the variability and vicissitudes of a growing body [21]. In taking this idea seriously, developmental roboticists created a model of the musculoskeletal system, complete with neural circuitry, and provided it with the ability to “twitch” and update its circuitry based on sensory feedback [22,23]. Equipped in this way, the system was able to self-organize its neural circuitry, including circuits that resemble those underlying the most basic spinal reflexes. This mimicking of biological processes within a robotic environment holds great promise for testing hypotheses regarding the control and functions of twitching in animals.
The above-described features of twitches are indicative of an autonomous mechanism that is capable of providing the animal with a sizeable amount of experience to learn about its body throughout development. Additionally, this experience is organized and structured in a way that can change from one day to the next as the animal develops its repertoire of movements. However, for the organism to benefit from the experience provided by twitches, the brain must receive this information and process it in a meaningful way.
An Abundance of Sensory Feedback
Despite their discrete nature and low amplitude, twitches readily activate the brain. In fact, in response to twitches, bouts of neural activity are triggered throughout the brain, including the brainstem [24], thalamus [16], hippocampus [6], and primary somatosensory and motor cortices [6-8]. Arguably, twitches are a primary activator of neural activity in the developing brain. This neural activity is phasic, displaying short bursts of action potentials that follow the onset of a twitch, indicative of sensory feedback. Furthermore, neural responses exhibit different latencies from twitch onset, depending on the distance that the feedback signal must travel and the number of interposed synapses.
The short bursts of action potentials that follow each twitch result in strong correlations between twitching and neural activity [6,8,16]. Such strong correlations result from the discrete nature of twitches within a low-noise environment. It has been suggested that correlation-based learning associated with twitching contributes to the self-organization of spinal circuits underlying the withdrawal reflex [9]. This form of learning could also be important for the development and maintenance of somatotopic maps.
To further explore the role of twitching in sensorimotor development, we turned our attention to the cerebellum, a structure that plays a critical role in sensorimotor integration [25] and undergoes tremendous growth in rats over the first few weeks of postnatal life [26,27]. Postnatal changes in organization and synaptic connectivity in the cerebellum have been proposed to be activity dependent [28-30]. Therefore, if twitches activate cells within the cerebellar circuitry, as observed in other brain areas, they could provide precise information and thereby facilitate the postnatal development and refinement of this sensorimotor structure.
We focused on Purkinje cells, the sole output of the cerebellar cortex. At birth the Purkinje cell layer is already established [26] and neural activity can be measured by the third postnatal day in rats [31,32]. Purkinje cells receive two primary inputs, climbing fibers and mossy fibers. Climbing fibers arise from the inferior olive and mossy fibers arise from a number of brainstem nuclei, including the lateral reticular nucleus and pontine gray [33]. In adults, each Purkinje cell receives input from only one climbing fiber; in contrast, each Purkinje cell receives multiple mossy fiber inputs via the granule cell-parallel fiber pathway. Early in the postnatal period, however, each Purkinje cell is innervated by multiple climbing fibers and the granule cell-parallel fiber pathway is undeveloped, with mossy fibers forming direct, transient connections with Purkinje cells [28,34,35]. Because development of the cerebellar system continues into the third postnatal week, it is a potentially powerful model system for assessing the role of twitching on activity-dependent developmental processes.
Purkinje cells show two distinct firing patterns based on input from either climbing fibers or mossy fibers: complex spikes and simple spikes, respectively. Although both complex spikes and simple spikes are produced in response to peripheral stimulation in young rats, the majority of Purkinje cell activity in the developing cerebellum has been thought to be spontaneous and random [32,36]. However, in week-old rats, we found that 60% of Purkinje cells exhibited state dependency. Of these Purkinje cells, 65% exhibited more activity during active sleep than during wakefulness [37]. Moreover, we found strong correlations between the onset of a twitch and the onset of complex and simple spikes; twitches preceded both complex and simple spikes. These observations promise to help us understand the contributions of twitching to the development of the cerebellum and its complex interactions with other structures, including the neocortex [38], as well as the role of the cerebellum in the development of motor synergies.
The Sleeping Brain Processes Sensory Information in a Unique Way
In retrospect, it is perhaps not surprising that twitches readily activate the cerebellum, thalamus, cerebral cortex, and hippocampus. But it was surprising that, time and time again, we noted considerably less neural activity in these same structures when pups were awake and vigorously moving their limbs. What could explain such puzzling observations? Could there be differences in sensorimotor processing during sleep and wake? To answer this question, we must first review some basic features of sensorimotor processing.
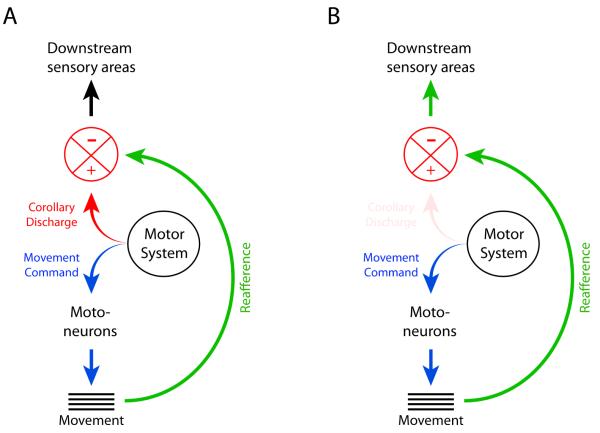
The generation of movements begins with the transmission of motor commands from motor areas in the brain and spinal cord to the muscles (Figure 1A). When a movement occurs, sensory receptors in the moving limb transmit information back to the brain. Also, at the same time that the motor command is generated, a motor copy, or corollary discharge, is produced and transmitted to non-motor areas [39]. This corollary discharge signal is then compared to the sensory feedback produced by the associated movement. If the movement happens as expected, and no unknown forces disturb the planned movement (i.e., the limb does not collide unexpectedly with an external object), the sensory feedback will match the corollary discharge signal, informing the brain that no unexpected outcome occurred. On the other hand, if the limb does collide unexpectedly with an external object, the resulting sensory feedback cannot be matched to the corollary discharge signal. Because of this mismatch, feedback is relayed to downstream sensory areas, informing the brain about the unexpected outcome. Using this mechanism, organisms are able to understand that the movements they make are indeed their own [40].
Figure 1.
Hypothetical pathways depicting the sensorimotor neural networks of wake-related and sleep-related movements. (A) Conventional model representing wake-related self-generated movements. Motor systems generate motor commands that activate muscles, thereby eliciting sensory reafference (green arrow). At the same time, motor systems generate a corollary discharge (red arrow). This corollary discharge is then compared to reafference, which can result in modulation of the neural feedback before it reaches other downstream sensory areas (black arrow). (B) Proposed model for the neural circuitry involved in the generation and processing of twitches. Similar to other self-generated movements, motor systems generate motor commands that activate muscles, thereby eliciting sensory reafference (curved green arrow). Unique to twitches however, the motor systems do not generate a simultaneously produced corollary discharge, or alternatively, the corollary discharge is generated but its effects are inhibited. Because of this, reafference is not compared to corollary discharge and is therefore minimally modulated on its way to downstream sensory areas (short green arrow). Adapted from Tiriac et al., Current Biology, 2014.
So after repeatedly observing very little neural activity when wake movements were produced, we hypothesized that corollary discharge mechanisms might be cancelling or gating the sensory feedback [37]. We further hypothesized that twitches, although self-generated, are processed by the brain as if they are unexpected (Figure 1B). To test these hypotheses, we contrived various experimental paradigms in which infant rats produced expected (i.e., in which corollary discharge is presumably recruited) and unexpected (i.e., in which corollary discharge is presumably not recruited) self-generated movements. If the mechanisms of corollary discharge filter out sensory feedback arising from expected movements, we predicted that only the unexpected self-generated movements would trigger neural activity, thereby mimicking what was observed with twitches.
To produce unexpected self-generated movements, we activated motorneurons directly in the spinal cord while recording from primary motor cortex (M1). We chose to record from M1 because, contrary to its name, it processes both sensory [41] and motor activity during early development [42]. By directly activating the motorneurons, we bypassed motor circuitry in the brain and spinal cord where corollary discharge signals are likely generated [43,44]. Initially, we used the serotonin agonist quipazine, a drug known to directly activate spinal motorneurons to induce stepping [45]. As we predicted, animals injected with quipazine (but not saline) exhibited vigorous movements of the hindlimbs that were reminiscent of waking movements but, in contrast with waking movements, sensory feedback from the movements was robustly relayed to M1.
Next, we designed two methods to evoke expected and unexpected self-generated movements in the same subjects, thereby allowing us to directly compare the effects of the two types of movements on M1 activity. To produce an unexpected self-generated movement, we flicked the tail of the animal, thereby triggering a spinal reflex that in turn elicited a hindlimb movement. We assumed that this tail-to-hindlimb spinal reflex circuitry would not recruit corollary discharge mechanisms. To produce an expected self-generated movement, we applied a cold stimulus to the snout of the pup, thereby causing global arousal and vigorous hindlimb movements. Because this method activated motor networks in the brain, we assumed that it would recruit corollary discharge mechanisms. Once again, as predicted, only the unexpected movements caused by flicks of the tail triggered robust activity in M1.
All together, these results led us to infer that twitching is a self-generated movement that is processed as if it is unexpected. To our knowledge, this is the first such behavior to exhibit this characteristic. Importantly, this finding has intriguing functional implications. Specifically, under normal waking conditions, the filtering of sensory feedback by corollary discharge mechanisms makes functional sense. But for a developing infant that relies on activity-dependent mechanisms for the development of neural networks, the engagement of corollary discharge mechanisms and the resulting filtering of sensory feedback would be counterproductive—such filtering would deprive the pup of important sensory information about the structure and biomechanical properties of its limbs. Therefore, in showing that twitches differ from wake movements on this critical dimension, we have provided additional support for the notion that they participate in a unique way in the development of the sensorimotor system.
Sensorimotor Deficiencies and Neurodevelopmental Disorders
If we think of our various sensorimotor, cognitive, and emotional systems as modular and encapsulated, then there is no harm in studying them separately. But such modular thinking, we believe, is misguided. Cognition is embodied [46]; therefore, we should not be surprised by the fact that people with severe mental illness (e.g., schizophrenia, autism) typically exhibit pronounced sensorimotor deficits. The studies described in this review address the role that sensorimotor processes during sleep play in the activation of the infant brain. In that context, it seems reasonable to suggest that any genetic or environmental factor that disrupts twitching and its effects on brain activity could, in turn, disrupt downstream developmental processes that depend on sensorimotor processing. In other words, sensorimotor dysfunction in early development could drive later-emerging cognitive and emotional problems. It follows that a thorough understanding of sleep and twitching in early development may prove useful for the early detection and treatment of mental illness.
For example, our recent findings regarding corollary discharge mechanisms in early infancy [7] open new avenues for research into the mechanisms of state-dependent modulation of the sensorimotor system in health and disease. Of particular interest here is accumulating evidence that people with schizophrenia exhibit deficits in the functioning of their corollary discharge system, potentially explaining such positive symptoms as hallucinations and delusions of control [47]. Our methods and concepts could easily be exported to investigations of sensorimotor processing in children at risk for schizophrenia.
As another example, consider the well-established links between early cerebellar damage and autism, as well as “evidence that the cerebellum may guide the maturation of remote nonmotor neural circuitry and influence cognitive development” [48]. Indeed, the cerebellum has been implicated in a variety of cognitive processes and mental disorders [49]. In light of our recent demonstration of cerebellar activation by sleep-related twitching [37], it seems plausible that active sleep is an important moderator of cerebellar development and the downstream consequences of alterations in that development. For example, as shown many years ago in infant rodents, twitching is very sensitive to changes in the thermal environment [50], which may help to explain the link between extreme environmental deprivation and the development of autism-like characteristics [51].
Conclusions
Twitches appear ideally suited to instruct the brain about the rapidly developing body. On the motor side, twitches are produced in a non-random, organized fashion, and complex patterns of twitches are selectively enhanced or eliminated throughout development. In other words, the structure and pattern of twitching is organized but flexible. Additionally, unlike the continuous movements occurring during wakefulness, twitches are produced discretely against a background of muscle atonia, providing a high signal-to-noise ratio. On the sensory side, early in development, twitches are a primary activator of neural networks throughout the central nervous system. The sheer quantity of neural activity resulting from twitches alone provides a sizeable amount of sensory experience at ages when activity-dependent development is so important [52]. Lastly, it is imperative that sensory information arriving from the periphery is transmitted with high fidelity, which is made possible by the apparent lack of corollary discharge mechanisms during twitching.
The findings described here provide evidence that strongly implicates twitching as playing an important and unique role in sensorimotor development. The fact that twitches occur so prevalently during developmental periods associated with accelerated growth [1], reflex integration [9], and locomotor development [14] seems unlikely to be a mere coincidence. On the contrary, the evidence seems to be pointing the other way, in favor of the notion that active sleep and its associated twitching during early development were selected over long periods of evolution for their ability to provide animals with an effective system for learning about their bodies.
Footnotes
Conflict of Interest Alexandre Tiriac, Greta Sokoloff, and Mark S. Blumberg declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Seelke A, Dooley JC, Krubitzer LA. The emergence of somatotopic maps of the body in S1 in rats: the correspondence between functional and anatomical organization. PloS one. 2012;7(2):e32322. doi: 10.1371/journal.pone.0032322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 3.Bruni O, Baumgartner E, Sette S, Ancona M, Caso G, Di Cosimo ME, et al. Longitudinal Study of Sleep Behavior in Normal Infants during the First Year of Life. JCSM. 2013;10(10):1119–1127. doi: 10.5664/jcsm.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152(3722):604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Current Biology. 2013;23(21):2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in infant rats. The Journal of Neuroscience. 2010;30(9):3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiriac A, Del Rio C. Self-Generated Movements with “Unexpected” Sensory Consequences. Current Biology. 2014;24(18):2136–2141. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432(7018):758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 9.Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424(3):72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- 10.Narayanan CH, Fox MW, Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus albinus) Behaviour. 1971;40(1/2):100–134. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- 11.Robinson SR, Blumberg MS, Lane MS, Kreber LA. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behavioral neuroscience. 2000;114(2):328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- 12.Chase MH, Morales FR. Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science. 1983;221(4616):1195–1198. doi: 10.1126/science.6310749. [DOI] [PubMed] [Google Scholar]
- 13.Marcano-Reik AJ, Prasad T, Weiner JA, Blumberg MS. An abrupt developmental shift in callosal modulation of sleep-related spindle bursts coincides with the emergence of excitatory-inhibitory balance and a reduction of somatosensory cortical plasticity. Behavioral neuroscience. 2010;124(5):600–611. doi: 10.1037/a0020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Animal Behaviour. 1975;23(4):896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- 15.Seelke A, Karlsson K, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. European Journal of Neuroscience. 2005;22(4):911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Current Biology. 2012;22(21):2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Current Biology. 2013;23(12):R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends in cognitive sciences. 2001;5(11):487–494. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]
- 19.Blumberg MS, Seelke A, Lowen S, Karlsson KÆ. Dynamics of sleep-wake cyclicity in developing rats. PNAS. 2005;102(41):14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends in neurosciences. 2006;29(7):414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Lungarella M, Metta G, Pfeifer R, Sandini G. Developmental robotics: a survey. Connection Science. 2003;15(4):151–190. [Google Scholar]
- 22.Marques HG, Bharadwaj A, Iida F. From Spontaneous Motor Activity to Coordinated Behaviour: A Developmental Model. PLoS computational biology. 2014;10(7):e1003653. doi: 10.1371/journal.pcbi.1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques HG, Völk K, König S, Iida F. Self-organization of Spinal Reflexes Involving Homonymous, Antagonist and Synergistic Interactions. 2012 [Google Scholar]
- 24.Karlsson KÆ, Gall AJ, Mohns EJ, Seelke A, Blumberg MS. The neural substrates of infant sleep in rats. PLoS biology. 2005;3(5):e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nature Reviews Neuroscience. 2005;6(4):297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- 26.Altman J. Postnatal Development of the Cerebellar Cortex in the Rat II. Phases in the Maturation of Purkinje Cells and of the Molecular Layer. 1972;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 27.Altman J. Postnatal Development of the Cerebellar Cortex in the Rat I. The External Germinal Layer and the Transitional Molecular Layer. 1972;145(3):353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto K, Kano M. Synapse elimination in the developing cerebellum. Cellular and Molecular Life Sciences. 2013;70(24):4667–4680. doi: 10.1007/s00018-013-1405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odeh F, Ackerley R, Bjaalie JG, Apps R. Pontine maps linking somatosensory and cerebellar cortices are in register with climbing fiber somatotopy. The Journal of Neuroscience. 2005;25(24):5680–5690. doi: 10.1523/JNEUROSCI.0558-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolbert DL, Pittman T, Alisky JM, Clark BR. Chronic NMDA receptor blockade or muscimol inhibition of cerebellar cortical neuronal activity alters the development of spinocerebellar afferent topography. Developmental brain research. 1994;80(1):268–274. doi: 10.1016/0165-3806(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 31.Crepel F. Maturation of climbing fiber responses in the rat. Brain research. 1971;35(1):272–276. doi: 10.1016/0006-8993(71)90617-2. [DOI] [PubMed] [Google Scholar]
- 32.Puro DG, Woodward DJ. Maturation of evoked climbing fiber input to rat cerebellar Purkinje cells (I.) Experimental Brain Research. 1977;28(1-2):85–100. doi: 10.1007/BF00237088. [DOI] [PubMed] [Google Scholar]
- 33.Eccles JC. The cerebellum as a neuronal machine. 1967 [Google Scholar]
- 34.Shimono T, Nosaka S, Sasaki K. Electrophysiological study on the postnatal development of neuronal mechanisms in the rat cerebellar cortex. Brain research. 1976;108(2):279–294. doi: 10.1016/0006-8993(76)90186-4. [DOI] [PubMed] [Google Scholar]
- 35.Kalinovsky A, Boukhtouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, et al. Development of axon-target specificity of ponto-cerebellar afferents. PLoS biology. 2011;9(2):e1001013. doi: 10.1371/journal.pbio.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puro DG, Woodward DJ. Maturation of evoked mossy fiber input to rat cerebellar Purkinje cells (II) Experimental Brain Research. 1977;28(3-4):427–441. doi: 10.1007/BF00235721. [DOI] [PubMed] [Google Scholar]
- 37.Sokoloff G, Uitermarkt BD, Blumberg MS. REM sleep twitches rouse nascent cerebellar circuits: Implications for sensorimotor development. Developmental neurobiology. 2014 doi: 10.1002/dneu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roš H, Sachdev R, Yu Y, Šestan N, McCormick DA. Neocortical networks entrain neuronal circuits in cerebellar cortex. The Journal of Neuroscience. 2009;29(33):10309–10320. doi: 10.1523/JNEUROSCI.2327-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulet J, Hedwig B. The cellular basis of a corollary discharge. Science. 2006;311(5760):518–522. doi: 10.1126/science.1120847. [DOI] [PubMed] [Google Scholar]
- 40.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nature Reviews Neuroscience. 2008;9(8):587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatsopoulos NG, Suminski AJ. Sensing with the motor cortex. Neuron. 2011;72(3):477–487. doi: 10.1016/j.neuron.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An S, Kilb W, Luhmann HJ. Sensory-Evoked and Spontaneous Gamma and Spindle Bursts in Neonatal Rat Motor Cortex. The Journal of Neuroscience. 2014;34(33):10870–10883. doi: 10.1523/JNEUROSCI.4539-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hantman AW, Jessell TM. Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nature neuroscience. 2010;13(10):1233–1239. doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014;508(7496):357–363. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brumley MR, Robinson SR. The Serotonergic Agonists Quipazine, CGS-12066A, and α-Methylserotonin Alter Motor Activity and Induce Hindlimb Stepping in the Intact and Spinal Rat Fetus. Behavioral neuroscience. 2005;119(3):821–833. doi: 10.1037/0735-7044.119.3.821. [DOI] [PubMed] [Google Scholar]
- 46.Pfeifer R, Bongard J. How the body shapes the way we think: a new view of intelligence. 2007 [Google Scholar]
- 47.Ford JM, Mathalon DH. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? International Journal of Psychophysiology. 2005;58(2):179–189. doi: 10.1016/j.ijpsycho.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83(3):518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito M. Control of mental activities by internal models in the cerebellum. Nature Reviews Neuroscience. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 50.Blumberg MS, Stolba MA. Thermogenesis, myoclonic twitching, and ultrasonic vocalization in neonatal rats during moderate and extreme cold exposure. Behavioral neuroscience. 1996;110(2):305–314. doi: 10.1037//0735-7044.110.2.305. [DOI] [PubMed] [Google Scholar]
- 51.Nelson CA, Bos K, Gunnar MR, Sonuga-Barke EJSV. The neurobiological toll of early human deprivation. Monographs of the Society for Research in Child Development. 2011;76(4):127–146. doi: 10.1111/j.1540-5834.2011.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkby LA, Sack GS, Firl A, Feller MB. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron. 2013;80(5):1129–1144. doi: 10.1016/j.neuron.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]