The results of this cost-effectiveness study suggest that a decision rule based on assessment of cerebrovascular reserve is cost-effective compared with medical therapy or immediate revascularization strategies to treat asymptomatic patients with carotid stenosis.
Abstract
Purpose
To project and compare the lifetime health benefits, health care costs, and incremental cost-effectiveness of a decision rule based on assessment of cerebrovascular reserve (CVR) compared with medical therapy and immediate revascularization in asymptomatic patients with carotid artery stenosis for prevention of stroke.
Materials and Methods
The three strategies compared included immediate revascularization (carotid endarterectomy) and ongoing medical therapy (with antiplatelet, statin, and antihypertensive agents plus lifestyle modification), medical therapy-based treatment with revascularization only for patients who progressed, and use of a CVR-based decision rule for treatment in which patients with CVR impairment undergo immediate revascularization and all others receive medical therapy. A decision analytic model was developed to project lifetime quality-adjusted life years (QALYs) and costs for asymptomatic patients with carotid stenosis with 70%–89% carotid luminal narrowing at presentation. Risks of clinical events, costs, and quality-of-life values were estimated on the basis of those in published sources. The analysis was conducted from a health care system perspective, with health and cost outcomes discounted at 3%.
Results
Total costs per person and lifetime QALYs were lowest for the medical therapy-based strategy ($14 597, 9.848 QALYs), followed by CVR testing ($16 583, 9.934 QALYs) and immediate revascularization ($20 950, 9.940 QALYs). The incremental cost-effectiveness ratio for the CVR-based strategy compared with the medical therapy–based strategy was $23 000 per QALY and for the immediate revascularization versus the CVR-based strategy was $760 000 per QALY. Results were sensitive to variations in model inputs for revascularization costs and complication risks and baseline stroke risk.
Conclusion
CVR testing can be a cost-effective tool to identify asymptomatic patients with carotid stenosis who are most likely to benefit from revascularization.
© RSNA, 2014
Introduction
Stroke is a major cause of mortality, morbidity, and health care costs in the United States (1,2). Carotid artery stenosis is the primary cause of approximately 15%–20% of ischemic strokes, which account for approximately 85% of all strokes (3). Revascularization procedures such as carotid endarterectomy and endovascular stent placement are recommended by the American Heart Association for patients who have experienced a stroke or transient ischemic attack caused by symptomatic carotid artery stenosis (4).
There are estimated to be 400 000 patients aged 70 years and older in the United States with asymptomatic carotid artery stenosis, which is associated with a higher risk of stroke compared with that in the general population but not as high a risk as that in patients with symptomatic carotid stenosis (5). The American Heart Association and others have recognized that performing revascularization in asymptomatic patients with carotid stenosis for primary stroke prevention is controversial due to uncertainties about the risks and benefits of revascularization procedures compared with less invasive approaches, such as aggressive medical therapy (6–8). The American Heart Association guidelines recommend considering other patient factors such as life expectancy, other comorbid conditions that can affect stroke risk, and patient preferences when considering revascularization for these patients (6). Recommendations are particularly uncertain for asymptomatic patients with carotid luminal narrowing greater than 70% but less than 90% (6). There is substantial geographic variation in carotid artery revascularization rates for Medicare beneficiaries, which suggests that standardized guidelines could lead to more appropriate use of these procedures (9).
Neuroimaging techniques for this population have been focused traditionally on the degree of carotid luminal narrowing for assessment of disease progression. Alternatives are positron emission tomography (PET), nuclear medicine, computed tomography (CT), magnetic resonance (MR) perfusion, or transcranial Doppler ultrasonography (US). Transcranial Doppler US can be used to assess cerebrovascular reserve (CVR), which has been shown to help identify asymptomatic patients with carotid artery stenosis who are at especially high risk for future stroke (10). This suggests that providers could use a relatively low-cost imaging modality (transcranial Doppler US) to identify the patients for whom the benefits of revascularization are more likely to outweigh the risks of complications (patients at high risk) and the patients who would benefit most from medical therapy (patients at low risk). The economic value of using transcranial Doppler US to assess CVR in asymptomatic patients with carotid artery stenosis is not only dependent on long-term health outcomes, but also on the costs associated with neuroimaging, revascularization and its associated risks, acute stroke events, and chronic care (11–13).
Our objective was to project and compare the lifetime health benefits, health care costs, and incremental cost-effectiveness of three competing stroke prevention strategies for asymptomatic patients with carotid artery stenosis: (a) immediate revascularization (carotid endarterectomy) for all patients in addition to ongoing medical therapy (antiplatelet, statin, and antihypertensive agents and lifestyle modification), (b) medical therapy for all patients with subsequent revascularization only for patients who progress, and (c) use of a decision-making rule for treatment in which those with CVR impairment undergo immediate revascularization and all others receive medical therapy.
Materials and Methods
Model Overview
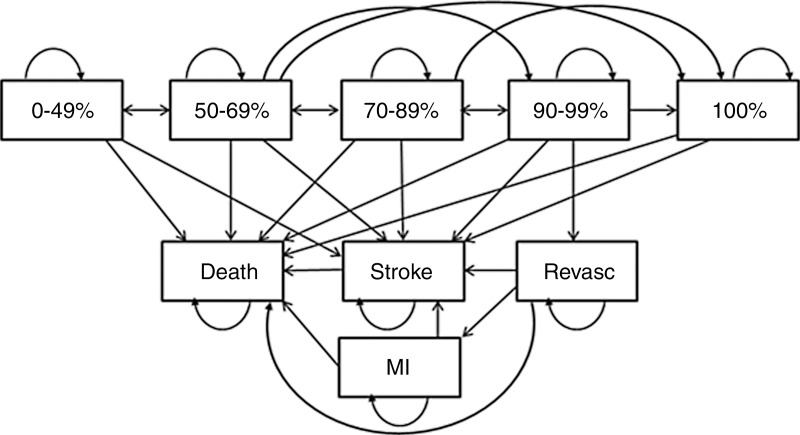
We developed a computer simulation state-transition model that projected stroke events, life expectancy, quality-adjusted life years (QALYs), and lifetime health care costs for a cohort of asymptomatic patients with carotid artery stenosis and 70%–89% carotid artery luminal narrowing at clinical presentation (Fig 1). The annual risk of stroke varied depending on CVR status, risk of complications from revascularization, and progression of luminal narrowing with time. Death could occur as a result of revascularization complications or stroke and nonstroke causes (ie, all-cause mortality without deaths from stroke). Depending on the clinical treatment strategy, patients in the model were chosen to undergo revascularization procedures immediately, later in life based on disease progression, or never. Base-case model inputs and sensitivity analysis ranges are reported in Table 1.
Figure 1:
Simplified illustration shows disease simulation model. Patients start in the 70%–89% luminal narrowing state and progress or regress to other narrowing states, experience a stroke, undergo revascularization (revasc), or die of stroke-related or nonstroke-related causes.
Table 1.
Model Variables with Base-Case Values and Ranges Used in One-Way Sensitivity Analysis
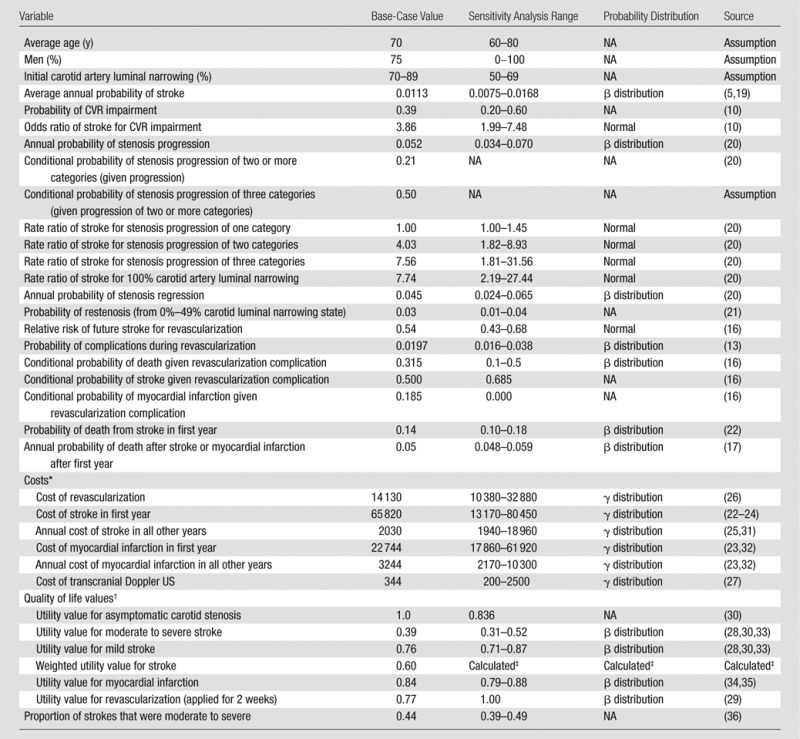
Note.—Death from nonstroke causes was determined on the basis of life tables (37). NA = not available.
All costs are in 2011 U.S. dollars.
Quality of life is represented by utility values between 0 (death) and 1 (perfect health).
Indicates a weighted average of utility values for mild and moderate to severe stroke calculated from values reported above with weight provided in last row of the Table.
The costs and QALYs projected for each strategy were used to calculate incremental cost-effectiveness ratios (ICERs) that were compared with a cost-effectiveness threshold of $100 000 per QALY, which represented the willingness of a health care system to pay for care (14). The analysis was conducted from a health care system perspective throughout a lifetime horizon, with all costs in 2011 U.S. dollars. Future health care costs and QALYs were discounted at 3% annually (15). The model was programmed by using software (TreeAge Pro 2012; TreeAge Software, Williamstown, Mass).
Clinical Strategies Evaluated
For the immediate revascularization strategy, the simulated patients received revascularization procedures at the time of diagnosis of carotid artery stenosis. Revascularization procedures had a 2% chance of complications in the base-case analysis, of which 31.5% were fatal, 18.5% resulted in nonfatal myocardial infarctions (with a subsequent increased risk of death in future years), and the remaining 50% resulted in nonfatal perioperative strokes (13,16,17). Revascularization without complications reduced the risk of future stroke by 46% (16). For the medical therapy strategy, patients did not undergo revascularization procedures immediately but did undergo subsequent revascularization if their luminal narrowing reached 90%–99%. Luminal narrowing was monitored every year by means of US of the carotid artery. Patients with 100% narrowing (occlusion) were assumed to have not received revascularization procedures on the basis of clinical guidelines (18). When the CVR strategy was used, patients with CVR impairment (as determined by using transcranial Doppler US) underwent immediate revascularization, while those without CVR impairment received medical therapy. CVR can be estimated by means of transcranial Doppler US measurement of cerebral blood flow velocity (typically in the middle cerebral artery) both before and after a vasodilatory stimulus such as increasing levels of carbon dioxide or a pharmacologic challenge with acetazolamide (10).
Stroke Risk and Disease Progression
The simulated patient population was assumed to be 75% male, with an average age of 70 years (varied between 60 years and 80 years in sensitivity analyses) and started with an average annual risk of stroke of 1.13% based on a meta-analysis of data from two studies (5,19). We modeled five degrees of luminal narrowing for patients with carotid artery stenosis: 0%–49%, 50%–69%, 70%–89%, 90%–99%, and 100%. All patients started with 70%–89% stenosis and could progress to an advanced category of luminal narrowing (90%–99% or 100%) with an annual probability of 5.2%; 79% of these progressions were by two categories and 21% of these progressions were by three categories (only relevant for patients in lower stenosis states of 0%–49% and 50%–69%) (20). Patients whose stenosis progressed by two or more categories in a given year were assigned a higher risk of stroke. A rate ratio of 4.03 was applied for patients who progressed two categories in a given year and a rate ratio of 7.56 was applied for patients who progressed three categories in a year (20). The annual regression rate, defined as the probability of regression by one luminal narrowing category, was 4.5% (20). Patients with 100% narrowing experienced an increased annual risk of stroke (rate ratio of 7.56) with no chance of regression (18,20). Successful revascularization moved patients to the lowest luminal narrowing category (0%–49%), but these patients could experience restenosis (ie, moving from 0%–49% narrowing to 50%–69% narrowing) with an annual probability of 3% (21).
We assumed that all patients for whom the CVR strategy was used were assigned a CVR status (impaired or not impaired) on the basis of transcranial Doppler US imaging. We stratified the risk of stroke according to binary CVR status by applying an odds ratio of 3.86 to patients with CVR impairment on the basis of data from a recent meta-analysis (10) in which a pooled random effects model of 13 studies was used to evaluate the risk of future stroke according to CVR status in patients with carotid artery stenosis. In the meta-analysis, the odds ratio estimate was robust to subset analyses for ischemic outcome measures, symptomatic or asymptomatic disease, stenosis or occlusion, or CVR testing methods. The diagnostic accuracy of the CVR test (ie, sensitivity and specificity) is shown in the odds ratio for stroke. Mortality in the absence of stroke was calculated on the basis of age- and sex-specific life tables for patients for whom all strategies were used. Stroke events increased the immediate risk of death (case-fatality rate of 14%) and the risk of death in subsequent years among stroke survivors (to 5%, unless mortality derived from age- and sex-specific life tables exceeded 5%) (17,22).
Costs and Health-related Quality of Life
Costs associated with stroke and myocardial infarction events were estimated on the basis of an analysis of a large managed-care population in the United States and other published sources (23–25). Costs of revascularization were from the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy trial conducted at 29 hospitals in the United States and included the costs of the procedure, hospital room and ancillary costs, and physician fees (26). Annual transcranial Doppler US costs were $344, and costs of medical therapy were not explicitly included (27); all patients were assumed to have received the same drug regimens whether or not they underwent a revascularization procedure. Similarly, costs of carotid sonography for monitoring of luminal narrowing were not modeled explicitly because we assumed that all patients received this test annually. Health-related quality of life was represented by utility values between 0 (equivalent to death) and 1 (perfect health) assigned to all health states in the model. Major and minor stroke events were assigned utility values of 0.39 and 0.77, respectively, which were varied in sensitivity analyses (28). Revascularization was assigned a utility value of 0.77 applied for 2 weeks (29). All other health states were assigned a utility value of 1.0 in the base case and 0.836 in sensitivity analyses (30).
Sensitivity Analyses
Parameters were varied individually in one-way sensitivity analyses to evaluate the sensitivity of results to plausible variations in model inputs (Table 1). A two-way sensitivity analysis was performed for baseline risk of stroke and probability of complications during revascularization because these parameters can vary according to patient, provider, and institution. Overall model uncertainty was evaluated in probabilistic sensitivity analysis by simultaneously conducting 10 000 random draws from probability distributions (Table 1) for each variable and recalculating the cost-effectiveness of each strategy. All analyses also were performed for an alternative scenario that used 50%–69% stenosis as the starting point for all patients to assess whether cost-effectiveness results differed for patients with this lower grade of stenosis.
Results
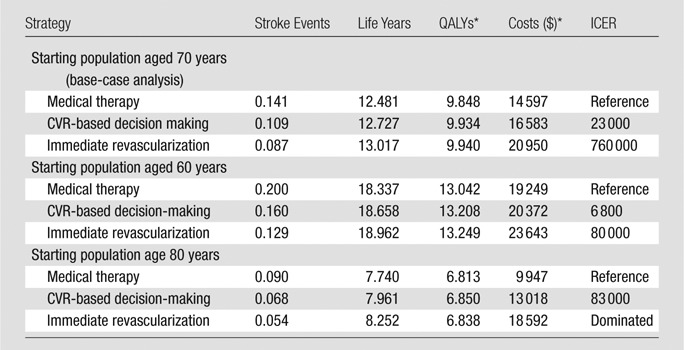
In the base-case analysis, 11% of patients who were chosen for the medical therapy strategy and 6% of patients chosen for the CVR strategy were assigned to undergo revascularization after disease progression, and 39% of patients in the CVR-based strategy were assigned to undergo immediate revascularization. The medical therapy strategy resulted in the highest lifetime expected probability of stroke events (14.1%) and acute stroke deaths (2.0%), followed by the CVR strategy (10.9% and 1.5%) and the immediate revascularization strategy (8.7% and 1.2%). The medical therapy strategy had the lowest total lifetime discounted QALYs and costs, followed by the CVR strategy and the immediate revascularization strategy.
Table 2 shows the cost-effectiveness results for populations starting at the age of 70 years (base-case) in addition to 60 and 80 years (sensitivity analyses). The CVR strategy had an ICER of $23 000 per QALY compared with the medical therapy strategy, and the immediate revascularization strategy had an ICER of $760 000 per QALY compared with the CVR strategy in the base-case incremental cost-effectiveness analysis. The CVR strategy was optimal (with a cost-effectiveness threshold of $100 000 per QALY) for all ages, except for in patients starting at age 60 years, when immediate revascularization was optimal. Table E1 (online) shows the intermediate outcomes (proportion of individuals receiving revascularization procedures, stroke events, and specific cost results) from the base-case analysis.
Table 2.
Lifetime Per-Person Cost-effectiveness Results for Patients Starting at 70%–89% Stenosis
Discounted at 3%.
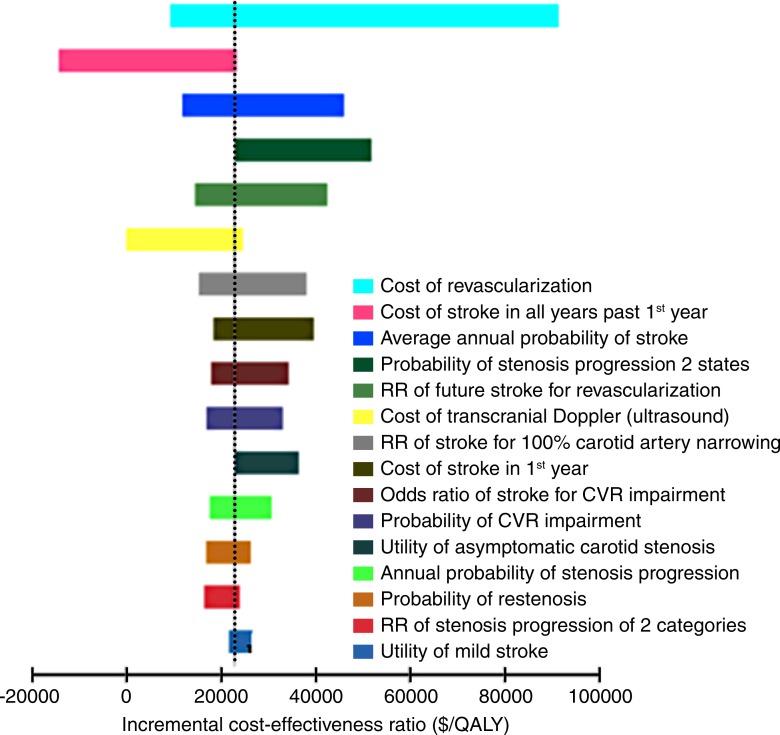
Figure 2 shows ICER results for the CVR strategy compared with the medical therapy strategy for the 15 most influential variables evaluated in one-way sensitivity analyses. The cost-effectiveness results were sensitive to uncertainty in the cost of revascularization, cost of chronic stroke, average stroke risk in this population, and the relative risk of stroke after revascularization. The ICER for the CVR strategy compared with the medical therapy strategy was below the $100 000 per QALY threshold in all one-way sensitivity analyses. The ICER for the immediate revascularization strategy compared with the CVR strategy was also below this threshold when the annual risk of stroke was greater than 0.018 (base-case value, 0.0113; range, 0.0075–0.0500), the cost of revascularization was less than $6250 ($14 130; range $10 380–32 880), or the relative risk of stroke after revascularization was lower than 0.18 (0.54; range, 0.43–0.68). The cost-effectiveness results were robust to plausible changes in carotid disease progression and regression parameters, acute and chronic stroke mortality parameters, and utility values. Table E2 (online) shows results of one-way sensitivity analysis.
Figure 2:
Tornado diagram summarizes one-way sensitivity analyses for the CVR strategy compared with the medical therapy strategy for the base-case analysis. Most ICERs were close to the base-case result ($20 000 per QALY) because model parameters were varied through plausible ranges, with the exceptions of the cost of revascularization and the cost of chronic stroke care. Parameters are shown in descending order of influence on model results. RR = risk ratio.
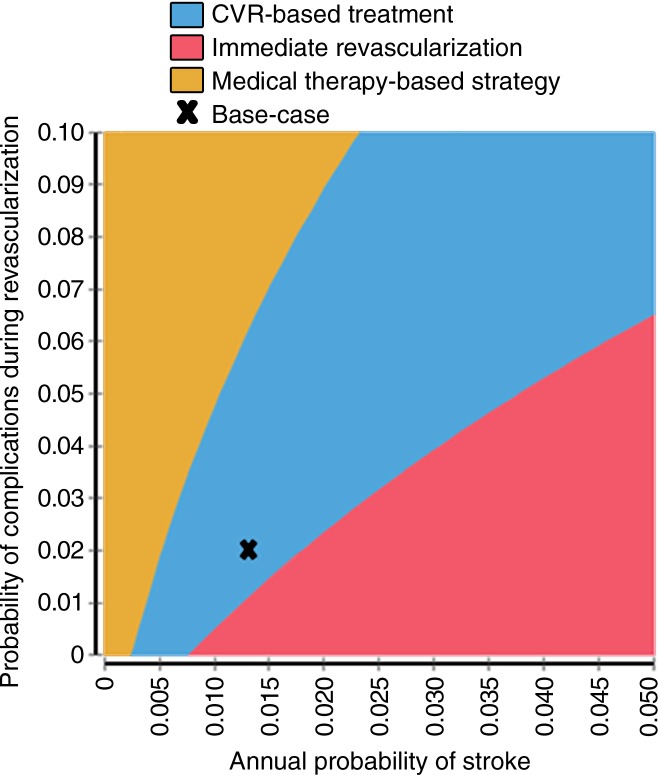
Figure 3 shows the two-way sensitivity analysis results varying the average annual risk of stroke and the probability of complications from revascularization procedures. Combinations of low stroke risk and high complication rates favored the medical therapy-based strategy, but high stroke risk and low complication rates favored the immediate revascularization strategy. For example, an annual baseline stroke risk of 0.5% and revascularization complication rate of 3% resulted in the medical therapy strategy being optimal, but values of 2.0% and 1.5%, respectively, resulted in the immediate revascularization strategy being optimal. Figure E1 (online) shows the cost-effectiveness acceptability curve results for the probabilistic sensitivity analysis. The CVR strategy was most likely to be optimal in the probabilistic sensitivity analysis when a cost-effectiveness threshold of $100 000 per QALY was used (CVR strategy optimal in 45% of probabilistic sensitivity analysis iterations; immediate revascularization optimal in 41% of probabilistic sensitivity analysis iterations). The variation in the probabilistic sensitivity analysis results was driven by the uncertainty in the costs of revascularization (mean ± standard deviation, $14 130 ± $18 750) (26); when revascularization costs were held constant, the CVR strategy was optimal in greater than 80% of probabilistic sensitivity analysis iterations (Fig E2 [online]).
Figure 3:
Graph of two-way sensitivity analysis shows optimal strategy for different combinations of baseline stroke risk and probability of complications during revascularization. CVR strategy is optimal in the blue region, which includes base-case result (marked by an x); other strategies could be optimal given other combinations of stroke risk and revascularization complication risks.
Table 3 shows results for the scenario analysis with 50%–69% stenosis as the starting point for all patients in the model. In this scenario, at a $100 000 per QALY threshold, the CVR strategy was preferred at ages 60 and 70, and medical therapy was preferred at age 80. Figure 3, Tables E3 and E4 (online), and Figure E4 (online) show full base-case and sensitivity analysis results for the scenario analysis with 50%–69% as the starting point for all patients in the model.
Table 3.
Lifetime Per-Person Cost-effectiveness Results for Patients Starting at 50%–69% Stenosis
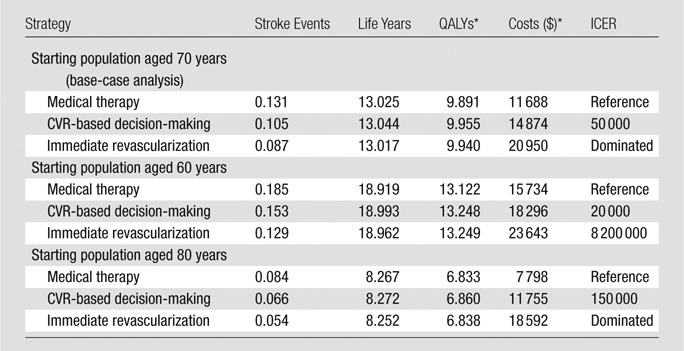
Note.—In cost-effectiveness analysis, a “dominated” strategy has higher costs and worst health outcomes compared with a competing strategy.
Discounted at 3%.
Discussion
We developed a decision analytic model to compare three clinical treatment strategies for stroke prevention in asymptomatic patients with carotid artery stenosis and found that revascularization decisions informed by CVR imaging assessments were optimal based on a cost-effectiveness threshold of $100 000 per QALY in the United States. The CVR-based strategy resulted in approximately two-fifths of the patient population undergoing immediate revascularization because of CVR impairment and approximately half of the population undergoing a revascularization in their lifetime. The medical therapy strategy resulted in not only the lowest lifetime costs and complications and the fewest revascularizations with time, but also the lowest life expectancy and lifetime QALYs. The immediate revascularization strategy yielded very small health gains compared with the CVR-based decision-making strategy, and these incremental benefits did not provide sufficient value when compared with the additional costs of revascularization (ICER of $8 800 000 per QALY).
We focused on CVR assessment with transcranial Doppler US, but other modalities such as CT perfusion could be used in its place. Our findings were robust to plausible changes in the performance and cost of transcranial Doppler US, but CT could carry additional costs and risks from radiation exposure that could affect the cost-effectiveness profile of CVR testing; future studies could confirm whether other modalities also could be efficient approaches to measuring CVR. In addition to transcranial Doppler US parameters, our cost-effectiveness results were also robust for variations in disease progression and regression assumptions, stroke mortality parameters, and utility estimates. Our cost-effectiveness results were most sensitive to model input values for the cost of revascularization and to plausible combinations of average stroke risk in this population and the probability of complications during revascularization. By using base-case model inputs, the CVR strategy was optimal for most age and stenosis combinations, except for patients 60 years old with 70%–89% stenosis (for whom immediate revascularization was favored) and patients 80 years old with 50%–69% stenosis (for whom medical therapy strategies were favored). These scenario results are intuitive; younger patients with stenosis closer to occlusion benefit most from successful revascularization, whereas the lifetime benefits of revascularization are less pronounced in older patients with lower degrees of stenosis.
Tholen et al (38) previously assessed the value of noninvasive diagnostic imaging tests (duplex US, CT angiography, and MR modalities) for determining the optimal use of revascularization in patients with carotid artery stenosis who had experienced a previous transient ischemic attack or minor stroke. They found that duplex US was a cost-effective initial test for patients suspected of having carotid artery stenosis, with CT angiography performed for positive initial tests and revascularization performed in those with 70%–99% luminal narrowing. In our study, which was focused on asymptomatic patients with established carotid artery stenosis, we also showed that imaging tests can be a cost-effective option for optimizing patient selection for revascularization procedures. Our results indicated that patients with most plausible combinations of stroke risk and revascularization complication risk would, on average, benefit from the CVR strategy, although these factors could vary widely among individual patients and providers. An optimized personalized approach based on a multifactorial model could enable more tailored decisions. For instance, providers might use our two-way sensitivity analysis findings to determine the optimal decision for a given combination of patient, surgeon, and institution, but this would require additional clinical or demographic information on stroke risk and predicted procedure complication rates that might not be known. From a health care system perspective, the optimal decision might also be affected by the expected cost of the procedure at the facility (5,39–41).
Our study had several other limitations. First, like all simulation model–based cost-effectiveness analyses, our study required combining model inputs from various sources. Despite this unavoidable limitation, our one-way sensitivity analyses showed that our cost-effectiveness results were robust for plausible changes in model inputs. Second, the clinical benefit assigned to revascularization was based on results of the Asymptomatic Carotid Surgery Trial-1, in which carotid endarterectomy was compared with medical therapy before recent improvements in medical therapy; therefore, the incremental benefits of revascularization might be lower if compared with improved medical treatments (7,19). Our sensitivity analysis showed that the relative risk of stroke after revascularization would need to be greater than the upper bound of uncertainty reported in the trial for the medical therapy strategy to be optimal under base-case conditions. Third, our study focused on carotid endarterectomy revascularization, but carotid artery stenting is being considered increasingly as an option for revascularization in this patient group (7,8). However, the clinical and cost-effectiveness findings for carotid endarterectomy versus stenting have been mixed, and stenting has not been recommended for use in asymptomatic patients with carotid artery stenosis (6,26,27,42,43). Fourth, our probabilistic sensitivity analysis results were affected by the uncertainty of the cost estimate for carotid endarterectomy. This parameter was estimated from a cost analysis of the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy trial, and the large variance in cost that affected our results was due to two outlier observations with very high ancillary procedure costs that might not have been related to the revascularization procedure (26). Fifth, the odds ratio for CVR impairment is from assessment with transcranial Doppler US, as opposed to a different measure of CVR impairment such as direct measurement of cerebral blood flow with PET or other flow sensitivity imaging techniques and could be described more accurately as the odds ratio of stroke for transcranial Doppler US–indicated CVR impairment.
The decision whether to perform revascularization in this asymptomatic patient population is uncertain given the benefits, risks, and costs associated with these procedures (7,8). CVR assessment can help identify patients with carotid artery stenosis who are at higher baseline risk for stroke, and thus, are better candidates for revascularization procedures. Our results suggest that a decision rule based on CVR assessment is cost-effective compared with medical therapy or immediate revascularization strategies. Authors of future studies should seek greater accuracy in determining the cost of revascularization and develop methods that would allow more individualized decision making on the basis of patient stroke risk factors and predicted procedure complication risks.
Advance in Knowledge
■ Assessment of cerebrovascular reserve by means of transcranial Doppler US can be a cost-effective tool for informing clinical decisions regarding immediate revascularization compared with medical therapy for treatment of asymptomatic patients with carotid stenosis, with an incremental cost-effectiveness ratio of $23 000 per quality-adjusted life year.
Implications for Patient Care
■ Assessment of cerebrovascular reserve with transcranial Doppler US can allow identification of patients with carotid artery stenosis who are at higher baseline risk for stroke, and thus, are better candidates for revascularization procedures.
■ The optimal decision on performance of revascularization procedures in asymptomatic patients with carotid artery stenosis depends on baseline risk of stroke and the likelihood of procedure complications, factors that can vary according to patient, provider, and institution.
SUPPLEMENTAL FIGURES
SUPPLEMENTAL TABLES
Received March 11, 2014; revision requested April 18; revision received May 1; accepted May 30; final version accepted June 18.
A.P. supported by the American Heart Association National Center (grant 14CRP18730014). A.G. supported by the Association of University Radiologists General Electric Radiology Research Academic Fellowship (GERRAF) grant.
Funding: This research was supported by the National Institutes of Health (grants 14CRP18730014, K23NS082367, KL2 TR000458-06, and 5K23NS058387).
Disclosures of Conflicts of Interest: A.P. disclosed no relevant relationships. A.G. disclosed no relevant relationships. H.K. disclosed no relevant relationships. B.B.N. disclosed no relevant relationships. P.C.S. disclosed no relevant relationships. B.R.S. disclosed no relevant relationships.
Abbreviations:
- CVR
- cerebrovascular reserve
- ICER
- incremental cost-effectiveness ratio
- QALY
- quality-adjusted life year
References
- 1.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke 2011;42(8):2351–2355. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127(1):143–152. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi S, Bruno A, Feasby T, et al. Carotid endarterectomy—an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65(6):794–801. [DOI] [PubMed] [Google Scholar]
- 4.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42(1):227–276. [DOI] [PubMed] [Google Scholar]
- 5.Wolff T, Guirguis-Blake J, Miller T, Gillespie M, Harris R. Screening for carotid artery stenosis: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2007;147(12):860–870. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42(2):517–584. [DOI] [PubMed] [Google Scholar]
- 7.Grotta JC. Clinical practice. Carotid stenosis. N Engl J Med 2013;369(12):1143–1150. [DOI] [PubMed] [Google Scholar]
- 8.Beckman JA. Management of asymptomatic internal carotid artery stenosis. JAMA 2013;310(15):1612–1618. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Greiner MA, DiMartino LD, et al. Geographic variation in carotid revascularization among Medicare beneficiaries, 2003-2006. Arch Intern Med 2010;170(14):1218–1225. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Chazen JL, Hartman M, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 2012;43(11):2884–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DL, Boden-Albala B, Langa KM, et al. Projected costs of ischemic stroke in the United States. Neurology 2006;67(8):1390–1395. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123(8):933–944. [DOI] [PubMed] [Google Scholar]
- 13.Wang FW, Esterbrooks D, Kuo YF, Mooss A, Mohiuddin SM, Uretsky BF. Outcomes after carotid artery stenting and endarterectomy in the Medicare population. Stroke 2011;42(7):2019–2025. [DOI] [PubMed] [Google Scholar]
- 14.Cutler DM, Rosen AB, Vijan S. The value of medical spending in the United States, 1960-2000. N Engl J Med 2006;355(9):920–927. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 16.Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010;376(9746):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law MR, Watt HC, Wald NJ. The underlying risk of death after myocardial infarction in the absence of treatment. Arch Intern Med 2002;162(21):2405–2410. [DOI] [PubMed] [Google Scholar]
- 18.Powers WJ, Clarke WR, Grubb RL, Jr, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 2011;306(18):1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raman G, Moorthy D, Hadar N, et al. Management strategies for asymptomatic carotid stenosis: a systematic review and meta-analysis. Ann Intern Med 2013;158(9):676–685. [DOI] [PubMed] [Google Scholar]
- 20.Hirt LS. Progression rate and ipsilateral neurological events in asymptomatic carotid stenosis. Stroke 2014;45(3):702–706. [DOI] [PubMed] [Google Scholar]
- 21.Lal BK, Beach KW, Roubin GS, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol 2012;11(9):755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KK, Cipriano LE, Owens DK, Go AS, Hlatky MA. Cost-effectiveness of using high-sensitivity C-reactive protein to identify intermediate- and low-cardiovascular-risk individuals for statin therapy. Circulation 2010;122(15):1478–1487. [DOI] [PubMed] [Google Scholar]
- 23.O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics 2011;29(8):693–704. [DOI] [PubMed] [Google Scholar]
- 24.Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke 1999;30(2):338–349. [DOI] [PubMed] [Google Scholar]
- 25.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med 2006;144(5):326–336. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney EM, Greenberg D, Lavelle TA, et al. Costs and cost-effectiveness of carotid stenting versus endarterectomy for patients at increased surgical risk: results from the SAPPHIRE trial. Catheter Cardiovasc Interv 2011;77(4):463–472. [DOI] [PubMed] [Google Scholar]
- 27.Kilaru S, Korn P, Kasirajan K, et al. Is carotid angioplasty and stenting more cost effective than carotid endarterectomy? J Vasc Surg 2003;37(2):331–339. [DOI] [PubMed] [Google Scholar]
- 28.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med 1996;156(16):1829–1836. [PubMed] [Google Scholar]
- 29.Cohen DJ, Lavelle TA, Van Hout B, et al. Economic outcomes of percutaneous coronary intervention with drug-eluting stents versus bypass surgery for patients with left main or three-vessel coronary artery disease: one-year results from the SYNTAX trial. Catheter Cardiovasc Interv 2012;79(2):198–209. [DOI] [PubMed] [Google Scholar]
- 30.Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics 2003;21(3):191–200. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Zhang Z, Ayala C, Dunet DO, Fang J, George MG. Costs of hospitalization for stroke patients aged 18-64 years in the United States. J Stroke Cerebrovasc Dis 2014;23(5):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauf TL, Velazquez EJ, Crosslin DR, et al. The cost of acute myocardial infarction in the new millennium: evidence from a multinational registry. Am Heart J 2006;151(1):206–212. [DOI] [PubMed] [Google Scholar]
- 33.Hallan S, Asberg A, Indredavik B, Widerøe TE. Quality of life after cerebrovascular stroke: a systematic study of patients’ preferences for different functional outcomes. J Intern Med 1999;246(3):309–316. [DOI] [PubMed] [Google Scholar]
- 34.Kamel H, Easton JD, Johnston SC, Kim AS. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology 2012;79(14):1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26(4):410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154(1):1–11. [DOI] [PubMed] [Google Scholar]
- 37.Heron M. Deaths: Leading causes for 2006. Natl Vital Stat Rep http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_14.pdf. Published March 31, 2010. Accessed August 7, 2014.
- 38.Tholen AT, de Monyé C, Genders TS, et al. Suspected carotid artery stenosis: cost-effectiveness of CT angiography in work-up of patients with recent TIA or minor ischemic stroke. Radiology 2010;256(2):585–597. [DOI] [PubMed] [Google Scholar]
- 39.Bineau S, Dufouil C, Helmer C, et al. Framingham stroke risk function in a large population-based cohort of elderly people: the 3C study. Stroke 2009;40(5):1564–1570. [DOI] [PubMed] [Google Scholar]
- 40.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2004;160(3):259–269. [DOI] [PubMed] [Google Scholar]
- 41.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22(3):312–318. [DOI] [PubMed] [Google Scholar]
- 42.Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 2006;355(16):1660–1671. [DOI] [PubMed] [Google Scholar]
- 43.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.