Abstract
Presilphiperfolanols constitute a family of biosynthetically important sesquiterpenes that can rearrange to diverse sesquiterpenoid skeletons. While the origin of these natural products can be traced to simple linear terpene precursors, the details of the enzymatic cyclization mechanism that form the stereochemically dense tricyclic skeleton have required extensive biochemical, computational, and synthetic investigation. Parallel efforts to prepare the unique and intriguing structures of these compounds by total synthesis have also inspired novel strategies, resulting in two synthetic approaches and two completed syntheses. While the biosynthesis and chemical synthesis studies performed to date have provided much insight into the role and properties of these molecules, new questions regarding the biosynthesis of newer members of the family and subtle details of the cyclization mechanism have yet to be explored.
Keywords: natural products, terpenoids, structure elucidation, biosynthesis, total synthesis
1. The Presilphiperfolanol Natural Products
The presilphiperfolane (or prebotrydial) skeleton serves as an important branch point for the biosynthesis of many sesquiterpene natural products. As inherently high-energy structures, presilphiperfolanyl cations are especially prone to skeletal rearrangement by C–C bond migrations. While these intermediates are crucial for the formation of various downstream sesquiterpenes, natural products possessing an unmodified presilphiperfolane framework are rare in nature.
1.1 Isolation and Structural Elucidation
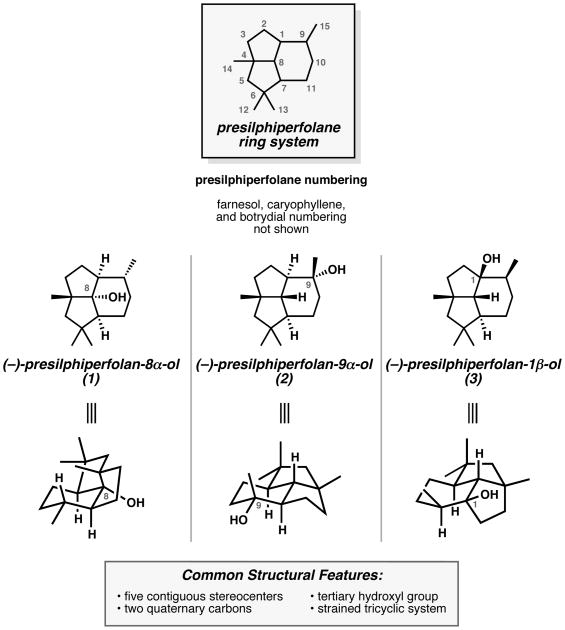
Currently, three presilphiperfolanols have been isolated and characterized: presilphiperfolan-8α-ol (1),[1] presilphiperfolan-9α-ol (2),[2] and presilphiperfolan-1β-ol (3)[3,4] (Figure 1). Each of these natural products corresponds to the hydration product of a presilphiperfolanyl cation involved in terpene cyclization pathways. To date, naturally occurring stereoisomers of structures 1–3 have not been reported. The structurally complex presilphiperfolanols are distinguished by their uncommon, compact tricyclo[5.3.1.04,11]undecane sesquiterpene skeleton, which bears five contiguous stereocenters, two all-carbon quaternary centers, and a tertiary hydroxyl group. In addition to these readily apparent structural features, considerable ring strain is present in the tricyclic system,[5,6] allowing these compounds to undergo thermodynamically favorable skeletal rearrangements that lead to structurally diverse polycyclic sesquiterpenes. Computational studies have shown that the heat of formation (ΔHf) of the presilphiperfolane skeleton is at least 7.1 kcal/mol greater than those for several isomeric sesquiterpene skeletons formed later in the biosynthetic sequence.[5]
Figure 1.
Presilphiperfolanol (Prebotrydial) Natural Products.
Presilphiperfolan-8α-ol (1) was the first member of the family to be identified.[1] Bohlmann and co-workers isolated the compound from the flowering plants Eriophyllum staechadifolium and Flourensia heterolepis in 1981. The tricyclic structure and stereochemistry were assigned based on detailed 1H NMR analysis employing chiral shift reagents. Subsequent work by Coates provided an X-ray crystal structure of the p-nitrobenzoate ester derivative.[7]
Presilphiperfolan-9α-ol (2)[2] was later discovered by Weyerstahl in the wormwood Artemisia lacinata in 1993, and subsequently by Marco in the related species Artemisia chamaemelifolia in 1996. The structure of 2 was determined based on NMR spectroscopic analysis and additionally confirmed by the total synthesis of (±)-2.[8]
In contrast to presilphiperfolanols 1 and 2, the structure of presilphiperfolan-1β-ol (3)[3,4] has been revised several times (Figure 2). Alcohol 3 was initially isolated by König in small quantities from the liverwort Conocephalum conicum in 1999,[3] but was incorrectly assigned structure 4 based on NMR data. The same compound was isolated by Leitão from the fern Anemia tomentosa var. anthriscifolia and reported as a unique natural product with initial structure 5 from the analysis of NMR spectra.[4a] Subsequent collaborations between Leitão and Joseph-Nathan unambiguously determined that the isolated compound possessed revised structure 3 by X-ray crystallography.[4b] Recently, the Stoltz group proposed that the compounds isolated by König and Leitão are in fact the same natural product 3 based on synthetic studies, spectroscopic data, and analysis of the likely biosynthetic pathway (see Section 2.5).
Figure 2.
Structural Reassignments of Presilphiperfolan-1β-ol (3).
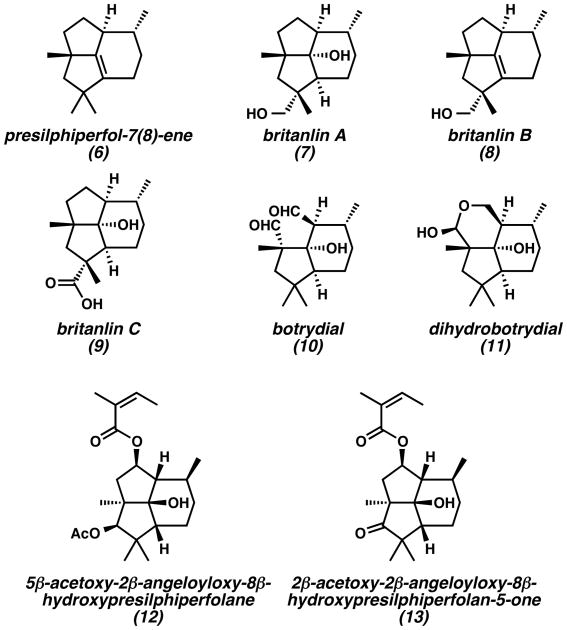
In addition to the parent presilphiperfolanols, natural products with dehydrated or oxidized tricyclic skeletons have also been reported (Figure 3). Presilphiperfol-7(8)-ene (6)[9] presumably arises from the deprotonation of presilphiperfolanyl cation intermediates. Natural products such as the britanlins (7–9)[10] display additional oxidation at primary carbons in the presilphiperfolane skeleton. Other isolated compounds, such as angelates 12 and 13, show oxidation at multiple secondary carbons in the tricyclic framework.[11] Oxidative ring cleavage is also possible as evidenced by the structures of botrydial (10)[12] and dihydrobotrydial (11).[12] All of these natural products arise from structural modification of the presilphiperfolanols, which exhibit a low level of oxidation.
Figure 3.
Natural Products with Dehydrated or Oxidized Presilphiperfolanol Skeletons.
1.2 Biosynthesis of the Presilphiperfolanols
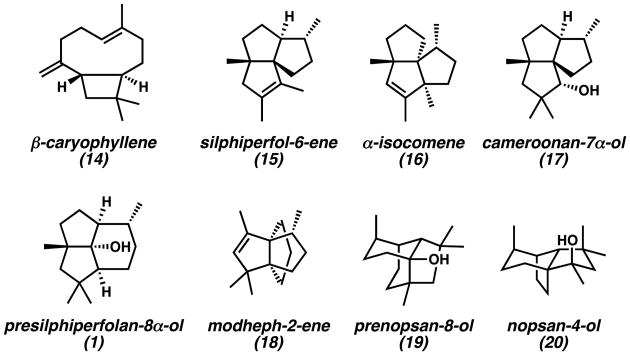
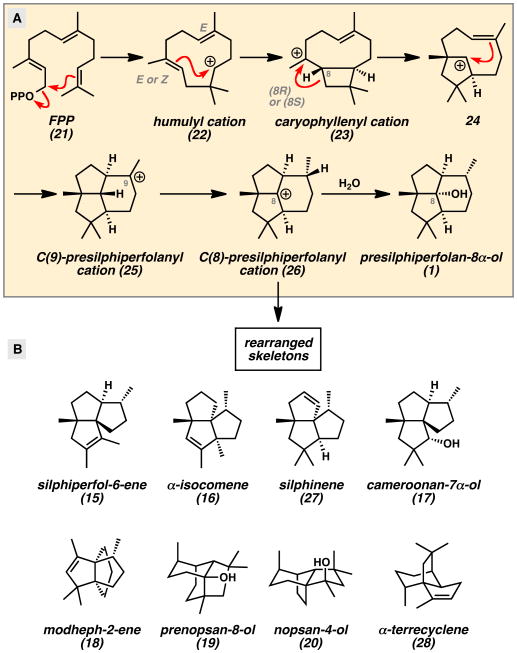
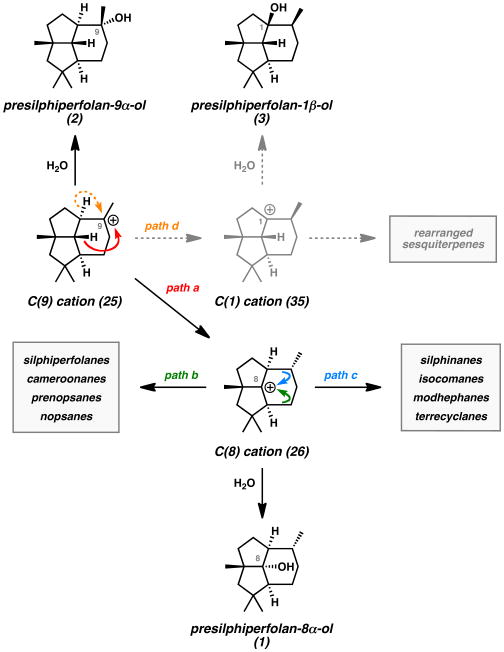
The co-isolation of the presilphiperfolanols with structurally related sesquiterpenes provided important clues for their biosynthetic origin. Bohlmann and co-workers observed that presilphiperfolan-8α-ol (1) was often found with various triquinane natural products.[1,13] Tricyclic alcohol 1 and β-caryophyllene (14) (Figure 4) were also isolated from the same natural sources in numerous reports.[9,14] These findings suggested that three classes of polycyclic sesquiterpenes were connected in a common biosynthetic pathway. In 1980, Bohlmann explained these results by proposing that farnesyl pyrophosphate (FPP) (21) undergoes enzymatic polycyclization to caryophyllenyl cation 23 (Scheme 1A).[13] Subsequent cyclobutane ring expansion and cation-alkene cyclization leads to the C(8)-presilphiperfolanyl cation (26). From this common intermediate, rearrangement of the carbon skeleton by Wagner–Meerwein shifts can lead to the observed triquinanes.
Figure 4.
Selected Co-isolated Sesquiterpenes from Rhizome Echinops giganteus var. lelyi.
Scheme 1.
Bohlmann Mechanism for Presilphiperfolane Biosynthesis (1A) and Diverse Rearranged Sesquiterpene Natural Products (1B).
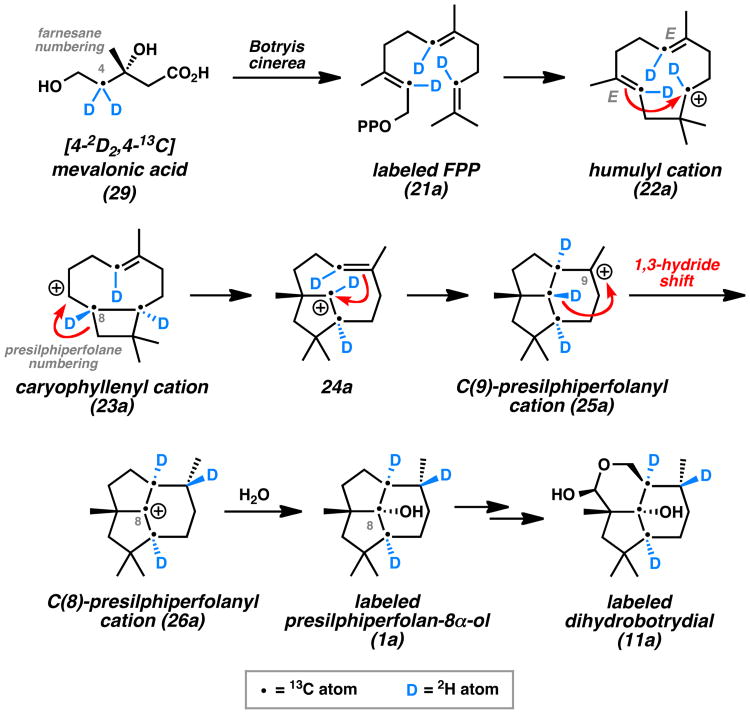
Concurrent studies by Hanson in 1981 helped to elucidate the presilphiperfolane biosynthetic pathway.[15] In an effort to understand the biogenesis of the downstream metabolite dihydrobotrydial (11) from simple terpene building blocks, his group performed NMR studies with isotopically labeled mevalonic acid (29) (Scheme 2). Linked 2H and 13C labels could be incorporated into this precursor, which was fed to the fungus Botryis cinerea. Subsequent analysis of the cyclized and oxidized dihydrobotrydial isolate (11a) revealed that three units of mevalonic acid (29) were incorporated into the molecule. Furthermore, the isotopic pair at C(8) (presilphiperfolane numbering) became separated during the biosynthetic transformations while the other two pairs remained intact. This provided the first evidence for an unusual 1,3-hydride shift linking the initially formed C(9)-presilphiperfolanyl cation (25) to the isomeric C(8)-cation (26). From this intermediate, Hanson reasoned that hydration and enzymatic oxidative cleavage of the less-substituted cyclopentane ring would lead to botrydial (10) and dihydrobotrydial (11) (Figure 3).
Scheme 2.
Hanson Mechanism for Presilphiperfolane Biosynthesis (Representative Isotope Labeling Study).
The Bohlmann–Hanson mechanism has been refined and expanded by numerous groups through biochemical, spectroscopic, and computational techniques in recent years. The groups of Collado, Cane, and Viaud worked together to identify the BcBOT gene cluster in B. cinerea responsible for the enzymatic conversion of FPP (15) to botrydial (10).[16] In these studies, it was demonstrated that the BcBOT2 gene encoded an essential sesquiterpene cyclase while other genes in the cluster expressed cytochrome P450 monooxygenases responsible for the oxidation of the presilphiperfolane skeleton to botrydial (10) and related derivatives (Scheme 1B).
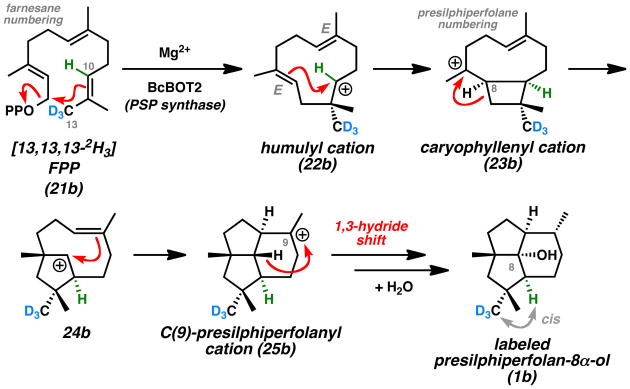
Subsequent work by Cane focused on the incubation of isotopically labelled FPP derivatives with the isolated BcBOT2 enzyme to further elucidate the stereochemical details of the cyclization mechanism (Scheme 3).[17] In total, Cane investigated four different FPP derivatives to probe the different cyclization steps, corroborating the earlier work of Bohlmann and Hanson. In a representative study, 2H labeling at the C(13) methyl group (farnesane numbering) translated to deuterium substitution at C(14) (presilphiperfolane numbering) of the presilphiperfolan-8α-ol isolate (1b). This study indicated that the cis relationship of the labeled C(13) methyl group and the alkene proton at C(10) is conserved throughout the terpene cyclization sequence, which led to the new proposal that a cis-caryophyllenyl cation (23) is a key intermediate. While β-caryophyllene (14, trans ring fusion) was co-isolated with presilphiperfolan-8α-ol (1) by Bohlmann, 2-epi-caryophyllene (48, cis ring fusion)[18] was not observed.
Scheme 3.
Cane Mechanism for Presilphiperfolane Biosynthesis (Representative Isotope Labeling Study).
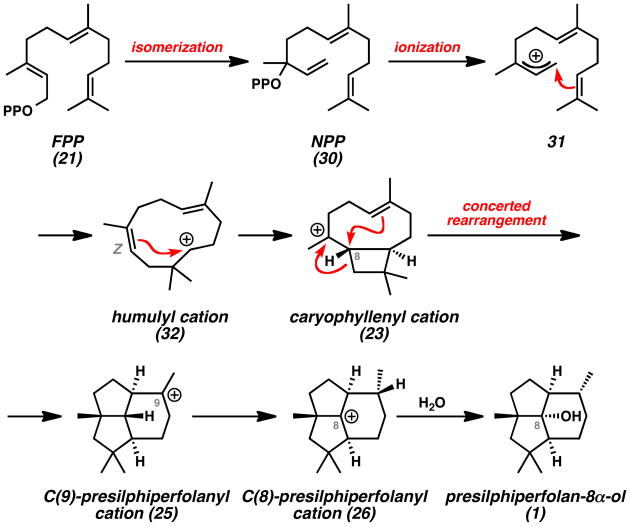
Computational studies by Tantillo also sought to understand the presilphiperfolanol biosynthetic pathway.[19] Numerous theoretical terpene cyclization pathways were evaluated and a different mechanism was proposed on the basis of these results (Scheme 4). The key findings were the proposed isomerization of FPP (21) to nerolidyl pyrophosphate (NPP, 30), the conformer of caryophyllenyl cation 23 responsible for cyclization, the highly synchronous nature of the cation-alkene cyclizations leading from 23 to 25, and the feasibility of the 1,3-hydride shift leading from C(9)-cation 25 to C(8)-cation 26. Barquera-Lozada used molecular mechanics calculations to evaluate a similar mechanism for the conversion of humulyl carbocation 22 to the terrecylenyl cation precursor to α -terrecyclene (28) (Scheme 1B).[20]
Scheme 4.
Tantillo Mechanism for Presilphiperfolane Biosynthesis (Computational Study).
1.3 Structural Rearrangements of Presilphiperfolanols
The importance of the presilphiperfolanols in sesquiterpene biosynthesis has prompted more detailed investigations of the rearrangements leading to other related natural products.[7,9] A report by Weyerstahl in 1998 described the constituents of the essential oil from the rhizome Echinops giganteus var. lelyi as containing a rich collection of biogenetically related sesquiterpenes (Figure 4).[9] Along with β-caryophyllene (14) and presilphiperfolan-8α-ol (1), 18 unique tricyclic natural products were discovered. All of the tricyclic compounds could be traced to common presilphiperfolanyl cation intermediates through reasonable Wagner–Meerwein shifts. The co-occurrence of these compounds further supports the findings of Bohlmann.[1,13]
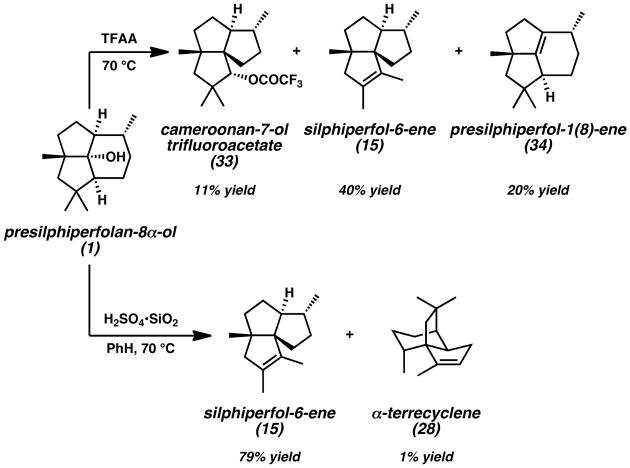
In conjunction with these natural product isolation studies, others have sought to understand the biosynthetic conversion of presilphiperfolane skeletons to those of other sesquiterpene natural products through chemical semi-synthesis. Coates successfully performed the rearrangement of presilphiperfolan-8α-ol (1) with TFAA at 70 °C to obtain cameroonan-7-ol trifluoroacetate (33) in 11% yield, silphiperfol-6-ene (15) in 40% yield, and presilphiperfol-1(8)-ene (34)[21] in 20% yield (Scheme 5).[5,7] Ionization of alcohol 1 with H2SO4·SiO2 in benzene at 70 °C provided silphiperfol-6-ene (15) in 79% yield and α-terrecyclene (28) in 1% yield. The different distribution of sesquiterpene products obtained under these reaction conditions highlights the strong influence of reaction parameters on competing rearrangement pathways.
Scheme 5.
Rearrangement of Presilphiperfolan-8α-ol to Other Sesquiterpene Skeletons. TFAA = trifluoroacetic anhydride.
Currently, the presilphiperfolane skeleton is believed to serve as the precursor to silphiperfolane, silphinane, isocomane, modhephane, terrecyclane, prenopsane, nopsane, and cameroonane skeletons (Scheme 1B and Scheme 6).[9] The structural diversity of polycyclic skeletons produced from the presilphiperfolane skeleton underscores their fundamental biosynthetic importance in sesquiterpene cyclase pathways.
Scheme 6.
Rearrangement of Presilphiperfolanols to Other Sesquiterpene Natural Products.
While past work has explored the formation of presilphiperfolan-9α-ol (2) and presilphiperfolan-8α-ol (1) in great detail, existing biosynthetic proposals have not accounted for the formation of presilphiperfolan-1β-ol (3), the newest discovered member of the family (Scheme 5). The understanding of the mechanistic pathway leading to this natural product could additionally provide new insight into the formation of downstream rearranged sesquiterpene natural products.
1.4 Biological Activity of the Presilphiperfolanols
While the presilphiperfolanols have proven to be important biosynthetic precursors to a number of polycyclic sesquiterpenes, they also exhibit modest biological activity. As a relatively nonpolar low molecular weight alcohol, presilphiperfolan-9α-ol (2) has pleasant olfactory properties and has attracted interest as a fragrance compound.[2, 22] The natural product (−)-2[2] has a pleasantly sweet and woody aroma with hints of coconut and celery. Synthetic (±)-2[8] possesses a slightly different olfactory profile with a strongly radiative, woody, resinous, and amber(gris) notes.
González-Coloma and co-workers discovered the insect antifeedant properties of presilphiperfolan-9α-ol (2) while screening a collection of polycyclic sesquiterpenoids.[23] The tricyclic alcohol displayed an EC50 of 19.5 nmol/cm2 against the Colorado potato beetle Leptinotarsa decemlineata and 47.5 nmol/cm2 against the aphid species Diuraphis noxia. Direct injection or oral dosing of this compound with L. decemlineata beetles led to 47% mortality after 72 hours. While the mode of action has not been fully elucidated, alcohol 2 is believed to be toxic to the insect's peripheral and central nervous system.
Leitão and co-workers have found that presilphiperfolan-1β-ol (3) possesses antimycobacterial properties.[24] The natural product is active against Mycobacterium tuberculosis (H37Rv) and Mycobacterium smegmatis (mc2155) strains with minimal inhibitory concentrations (MICs) of 100 μg/mL and 200 μg/mL, respectively. Currently, the basis for the observed antimycobaterial activity is unclear.
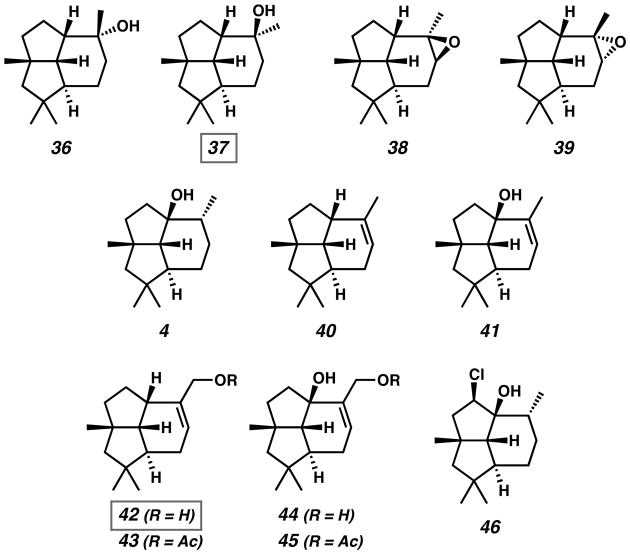
Non-natural presilphiperfolane analogs have also been investigated for their biological properties. Presilphiperfolane derivatives 4, 36–46 were investigated as novel antifungal agents by Collado (Figure 5).[25] Of these compounds, alcohols 37 and 42 showed the most promising inhibition in fungal growth assays with Botryis cinerea. Tertiary hydroxyl 37 showed complete suppression of fungal growth for four days with continued growth reduction after seven days. Primary alcohol 42 effectively reduced the size of fungal colonies and triggered changes in fungal morphology. For both of these active tricyclic terpenoid compounds, the hydroxyl groups are believed to be essential for inhibition, as the evaluation of acetylated derivates such as 43 led to no observable activity.
Figure 5.
Natural and Non-natural Presilphiperfolanol Analogs Investigated for Antifungal Activity.
2. Synthetic Studies Toward the Presilphiperfolanol Natural Products
Although the presilphiperfolanols are vitally important to the biosynthesis of numerous polycyclic sesquiterpenes, reports of synthetic efforts directed toward these natural products have been scarce. A number of biomimetic synthetic approaches have aimed to convert advanced biosynthetic precursors to the tricyclic alcohols 1–3, but these approaches have not been successful. More recently, research directed toward the chemical synthesis of the presilphiperfolanols has led to compounds that possess the tricyclic core of the targeted natural products and two completed total syntheses.
2.1 Biomimetic Cyclizations of Caryophyllene and Isocaryophyllene
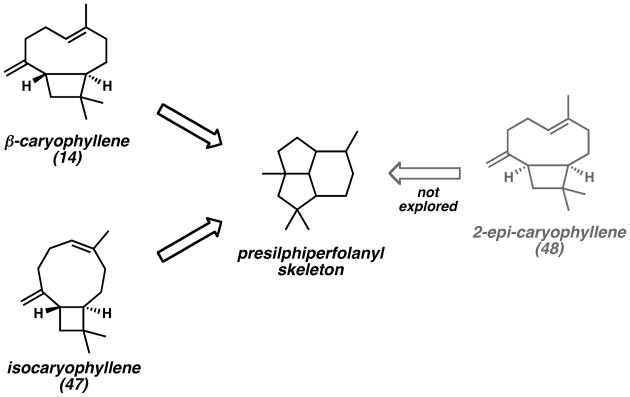
Based on the substantial evidence for the biosynthetic conversion of FPP (21) to caryophyllenyl cations en route to presilphiperfolanyl cations through cation-polyene cyclizations, many researchers have sought to achieve biomimetic syntheses of the presilphiperfolanols by rearrangement of β-caryophyllene (14) or isocaryophyllene (47) (Scheme 7).[26] To date, however, these efforts have not resulted in the formation of any of the naturally occurring tricyclic alcohols 1–3.
Scheme 7.
Strategy for the Rearrangement of Caryophyllenyl and Isocaryophyllenyl Skeletons.
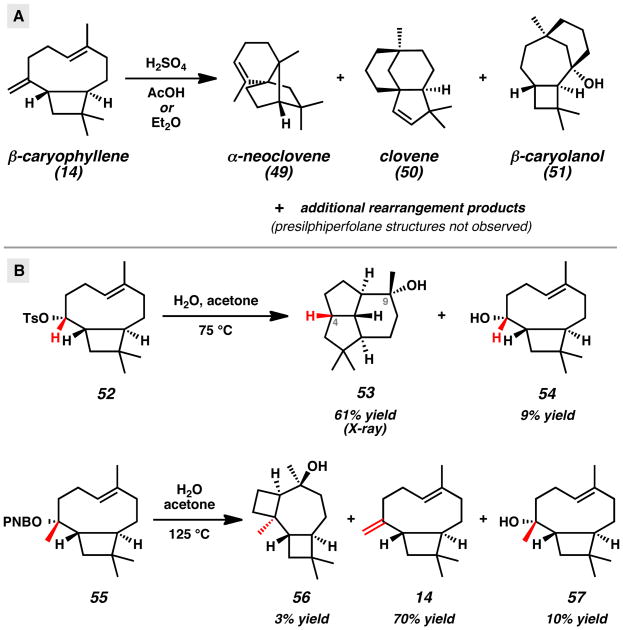
Research by numerous groups has explored the rearrangement of β-caryophyllene under acidic conditions (Scheme 8A).[27,28] These reactions typically have led to complex mixtures with product distributions that change over time. In this context, numerous rearrangement products such as α-neoclovene (49), clovene (50), and β-caryolanol (51) have been isolated and characterized. A supporting computational study was also performed to help understand the complex nature of the diverse rearrangement pathways.[28a] To date, however, presilphiperfolane structures have not been observed in any of these detailed studies.
Scheme 8.
Reported Rearrangements of Caryophyllene Skeletons.
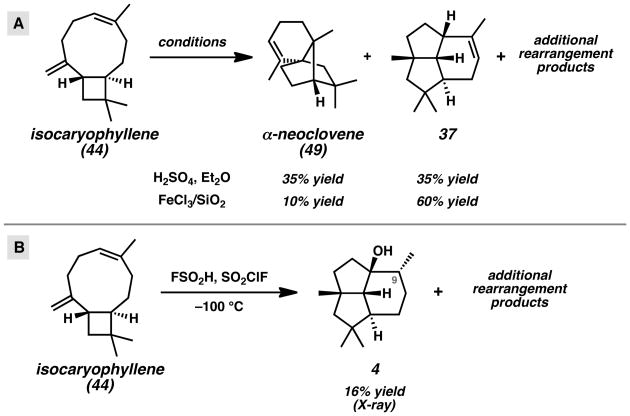
More recently, Coates and co-workers studied the solvolytic rearrangement of β-caryophyllene-derived structures with intriguing results (Scheme 8B).[28b] The ionization and rearrangement β-caryophyllene-derived tosylate 52 in water and acetone at 75 °C provided 12-nor-8α-presilphiperfolan-9β-ol (53) and alcohol 54. Compound 53 resembles presilphiperfolan-9α-ol (2), but notably lacks the methyl group attached to C(4) in the natural product. Rearrangement reactions employing β-caryophyllenyl precursors with the requisite methyl group were also investigated. Subjection of the p-nitrobenzoate ester 55 to similar solvolytic rearrangement conditions at a higher temperature did not furnish presilphiperfolan-9α-ol (2), but instead led to 5,8-cyclocaryophyllen-4α-ol (56), β-caryophyllene (14), and alcohol 57. The different product distributions under nearly identical reaction conditions suggests that the non-enzymatic cyclization is highly sensitive to the substitution of the caryophyllenyl framework and nature of the leaving group.
The rearrangement of isocaryophyllene (44) to presilphiperfolane-type structures has also been investigated.[25,29,30] Robertson and co-workers treated isocaryophyllene (44) with sulfuric acid in diethyl ether to obtain α-neoclovene (49) and tricyclic olefin 37, which resembles the tricyclic core of the presilphiperfolanols (Scheme 9A). Since these early studies, Collado was able to favor the formation of olefin 37 by employing silica-supported FeCl3.[25b] Further work by Khomenko and co-workers has produced alcohol-containing tricyclic structures that more closely resemble the presilphiperfolanols.[30] Treatment of isocaryophyllene (44) with fluorosulfonic acid and sulfuryl fluorochloride at −100 °C followed by a careful quenching of the acidic solution led to the formation of tricyclic alchohol 4 in 16% yield (Scheme 9B). The structure was assigned based on 1H and 13C NMR studies and confirmed by single crystal X-ray diffraction. Notably, this compound is the C(9)-epimer of presilphiperfolan-1β-ol (3) and identical to the structure originally assigned by König as “presilphiperfolan-1-ol” (4).
Scheme 9.
Reported Rearrangements of Isocaryophyllene (44).
While the variation of the endocyclic double bond geometry of caryophyllene has been explored in numerous contexts, biomimetic cyclizations with 2-epi-caryophyllene (48)[18] have not been explored. Since the compound was proposed as a key intermediate in Cane's biosynthetic proposal (Scheme 3),[17] successful chemical conversion to presilphiperfolane structures would provide further evidence for this hypothesis.
2.3 Weyerstahl Total Synthesis of (±)-Presilphiperfolan-9α-ol (2)
Driven by a keen interest in the biosynthetic importance, intriguing polycyclic structure, and olfactory properties of presilphiperfolan-9α-ol (2), Weyerstahl and co-workers aimed to prepare the natural product by total synthesis.[8] Central to their synthetic approach was the design of an intramolecular olefination strategy for the construction of the tricyclic core.
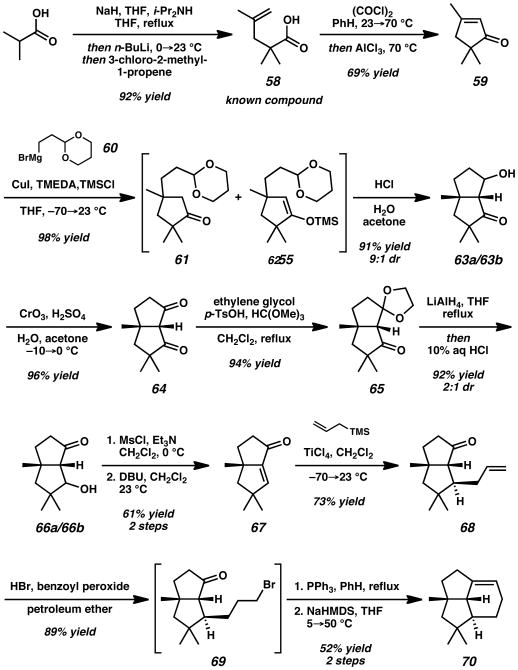
Beginning from isobutyric acid, enolization and alkylation with methallyl chloride provided functionalized pentenoic acid 58 (Scheme 10). Subsequent carboxylate activation with oxalyl chloride and cyclization with AlCl3 provided cyclopentenone 59 in 69% yield. The conjugate addition of organocuprate 60 with TMSCl as an activator followed by acidic deprotection and aldolization provided a mixture of β-hydroxyketones 63a and 63b in 89% yield and 9:1 dr over two steps. A subsequent Jones oxidation afforded diketone 64 in 96% yield. Selective protection of the less hindered carbonyl proceeded smoothly with p-TsOH, ethylene glycol, and trimethyl orthoformate in CH2Cl2 at reflux. Reduction of the remaining ketone in 65 with LiAlH4 followed by acidic workup provided β-hydroxyketones 66a and 66b in 92% yield and 2:1 dr. Dehydration was achieved by initial mesylation and elimination with DBU to give bicyclic enone 67 in 61% yield over two steps. Alternatively, the elimination was achieved with Burgess' reagent[31] in 64% yield. A subsequent diastereoselective Sakurai allylation[32] afforded ketone 68 in 73% yield. Regioselective radical hydrobromination of the terminal C=C double bond followed by an intramolecular Wittig reaction completed the tricyclic core of the target tricyclic molecules (70) in 52% yield over two steps.
Scheme 10.
Synthesis of Key Tricyclic Olefin Intermediate 70. TMEDA = N,N,N′,N′-tetramethylethylenediamine, TMSCl = chlorotrimethylsilane, MsCl = methanesulfonyl chloride, p-TsOH = p-toluenesulfonic acid, DBU = 1,8-Diazabicyclo[5.4.0]undec-7-ene.
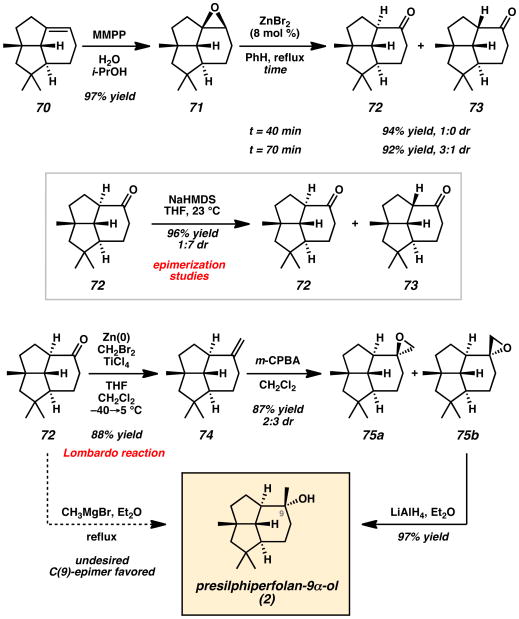
With key tricyclic olefin 70 in hand, a highly diastereoselective epoxidation with magnesium bis(monoperoxyphthalate) (MMPP)[33] afforded epoxide 71 in 97% yield (Scheme 11). The epoxidation could also be achieved with m-CPBA, but yields were typically lower. A subsequent stereospecific epoxy-keto rearrangement catalyzed by ZnBr2 was effective, giving the expected ketone 72 with α-H stereochemistry at C(1) in 94% yield after 40 min. While these reaction conditions proved successful, longer reaction times led to significant C(1)-epimerization to the undesired ketone epimer 73. The gradual conversion of tricyclic ketone 72 to its epimer 73 over time suggests that the desired ketone is thermodynamically unstable. This hypothesis was also supported by epimerization studies on ketone 72 with NaHMDS, which provided a mixture of 72 and 73 in a 1:7 ratio of diastereomers.
Scheme 11.
Weyerstahl's Completion of (±)-Presilphiperfolan-9α-ol (2). MMPP = magnesium monoperoxyphthalate, NaHMDS = sodium bis(trimethylsilyl)amide, m-CPBA = 3-chloroperbenzoic acid.
With 14 of the 15 carbons of the target compound installed, it was anticipated that the addition of MeMgBr to 72 could give presilphiperfolan-9α-ol (2) in a direct and straightforward manner. Unfortunately, this transformation predominantly led to the undesired C(9)-epimer with only trace amounts of the desired natural product 2. The steric environment of the tricycle as well as the favorable Bürgi–Dunitz trajectory from the α-face of the molecule dictated the facial bias of nucleophilic additions to the ketone of 72. In order to arrive at the natural product through alternative means, a Lombardo reaction was employed to give olefin 74 in 88% yield. A subsequent epoxidation with m-CPBA gave a 2:3 ratio of diastereomers 75a and 75b in 87% yield. After chromatographic separation, LiAlH4 reduction of epoxide 75b provided (±)-presilphiperfolan-9α-ol (2) in 97% yield. The total synthesis was completed in 17 steps and 4.0% overall yield from commercial starting materials.
2.3 Piers Approach to the Synthesis of (±)-Presilphiperfolan-9α-ol (2)
Subsequent synthetic efforts toward the presilphiperfolanol natural products aimed to assemble the tricyclic framework in a more efficient manner by forging multiple rings in a single key step. In developing a novel approach to presilphiperfolan-9α-ol (2), Piers employed a radical polycyclization strategy to enable rapid construction of central bonds in the core structure.[34,35]
The synthesis proceeded from 3-methyl-2-cyclopentenone (76) (Scheme 12). An initial Luche reduction[36] provided alcohol 77 in excellent yield. The method of Wilson[37] was used to convert the allylic alcohol was to dianionic intermediate 78, which undergoes a thermal Carroll rearrangement[38] and decarboxylation to form functionalized cyclopentene 80 in 77% yield over two steps. With the C(4) quaternary carbon installed, a Wittig homologation with ylide 81[39] followed by methyl enol ether hydrolysis and α-methylation provided aldehyde 82 in 65% yield. Addition of alkyllithium reagent 83 to aldehyde 82 and subsequent xanthate ester formation led to radical cyclization precursor 84.
Scheme 12.
Synthesis of Radical Cyclization Precursor 84. DMAP = 4-(dimethylamino)pyridine, TFA = trifluoroacetic acid.
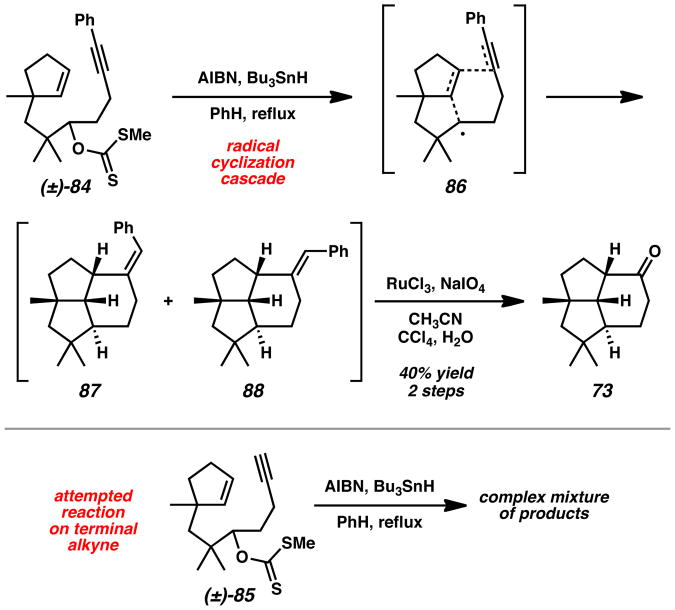
The slow addition of Bu3SnH and AIBN to xanthate 84 in benzene at reflux provided a mixture of tricyclic olefins 87 and 88 (Scheme 13). Oxidative styrene C=C double bond cleavage with RuCl3 and NaIO4[40] afforded (±)-epi-9-nor-presilphiperfolan-9-one (73)[8] in 40% yield over two steps. The disubstituted alkyne was essential for efficient cyclization since precursor 85 only led to a complex mixture of volatile hydrocarbon products.
Scheme 13.
Radical Cyclization Cascades with Precursors 84 and 85. AIBN = 2,2′-azobis(2-methylpropionitrile).
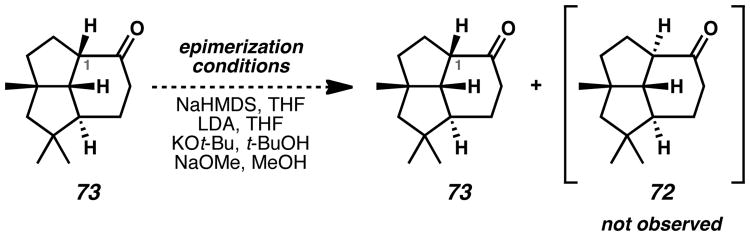
Epimerization of the C(1) methine hydrogen of ketone 73 was necessary in order to proceed toward presilphiperfolan-9α-ol (2) (Scheme 14). Thermodynamic equilibration according to Weyerstahl's procedure[8] (Scheme 11) failed, returning only starting material. Other strong bases such as LDA, KOt-Bu, and NaOMe provided no trace of the desired ketone 72. Due to the synthetic difficulties arising from the thermodynamic preferences of the tricyclic scaffold, the synthesis was not advanced further.
Scheme 14.
Attempted Epimerization of the C(1)-Methine Hydrogen of Ketone 73. NaHMDS = sodium bis(trimethylsilyl)amide, LDA = lithium diisopropylamide.
2.4 Ito Approach to the Synthesis of (−)-Presilphiperfolan-8α-ol (1)
While previous synthetic routes offered different strategies for the construction of the presilphiperfolanols, they did not provide access to the target natural products in enantioenriched form. To address this problem, Ito and coworkers devised a concise, enantiospecific approach to the synthesis of presilphiperfolan-8α-ol (1) from chiral pool starting material.[41] The route aimed to forge the tricyclic core using two complementary transannular cyclization reactions.
A Sakurai conjugate allylation[42] of (+)-pulegone (89) followed by base-mediated epimerization provided ketone 90 in 65% yield and 4:1 dr over two steps (Scheme 15). Selective formation of the less substituted, kinetic enolate and subsequent allylation provided α,α-dialkylated ketone 91 in 75% yield and 5:1 dr. A key ring-closing metathesis event was achieved by treatment of diene 91 with Grubbs–Hoveyda 2nd generation catalyst (92),[43] efficiently forging the necessary 8-membered ring in 83% yield. Hydroboration/oxidation of bicyclic alkene 93 led to a mixture of diketones 94 (28% yield) and 95 (41% yield).
Scheme 15.
Construction of Diketones 94 and 95 from Chiral Pool. LDA = lithium diisopropylamide, PCC = pyridinium chlorochromate.
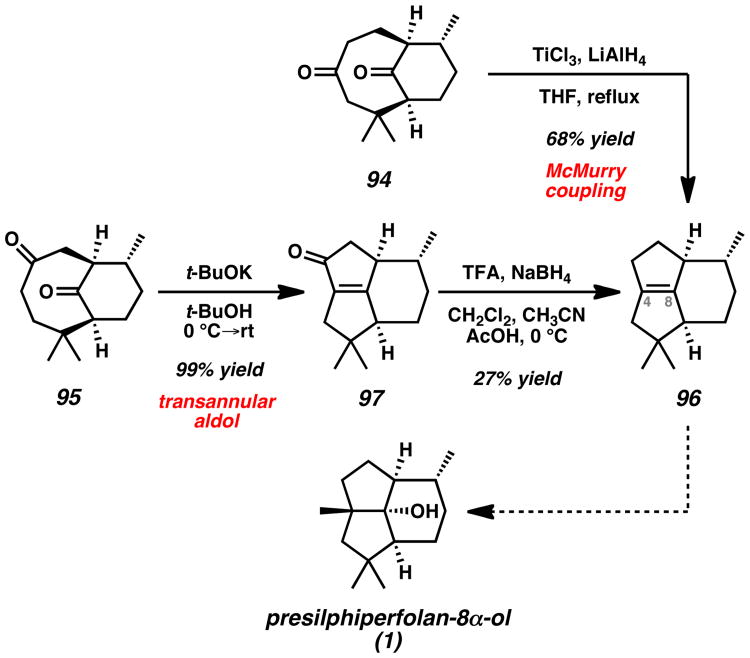
With isomeric diketones in hand, two transannular cyclization strategies provided rapid access to the presilphiperfolanol core by construction of the key C(4)–C(8) bond (Scheme 16). The first strategy toward the tricyclic architecture employed diketone 94 in a reductive coupling strategy. The application of McMurry conditions[44] provided the desired tetrasubstituted alkene 96 in 68% yield. The second strategy, which alternatively employed isomeric diketone 95, relied on an intramolecular aldol reaction to forge the same fully substituted C=C double bond. Addition of the bicyclic compound to a solution of KOt-Bu in t-BuOH provided enone 97 in excellent yield. Subsequent reductive deoxygenation using the Gribble protocol[45] provided tricyclic alkene 96 in 27% yield. Notably, the two routes provided efficient access to the tricyclic olefin core in 7 or 8 steps without the use of protecting groups. The all-carbon quaternary center at C(4) and tertiary hydroxyl group at C(8) still must be installed in a stereoselective manner in order to advance core structure 96 to (−)-presilphiperfolan-8α-ol (1).
Scheme 16.
Conversion of Diketones 94 and 95 to Tricyclic Core 96. TFA = trifluoroacetic acid.
2.5 Stoltz Total Synthesis of (−)-Presilphiperfolan-1β-ol (3)
Motivated by the presilphiperfolanols' important role in sesquiterpene biosynthesis and the unique challenges posed by their strained, stereochemically dense architectures, the Stoltz group initiated studies toward the total synthesis[46] of presilphiperfolan-1 β-ol (3)[3,4] and isomer 4 with the goal of developing a catalytic, asymmetric route. The application of an intramolecular Diels–Alder (IMDA) strategy was a key component of the overall strategy. At the outset of their investigations, the discrepancy of the structural assignments of “presilphiperfolan-1-ol” (4) and “9-epi-presilphiperfolan-1-ol” (3) was unknown (Figure 2), so synthetic efforts were directed toward both reported presilphiperfolanol compounds.[3,4]
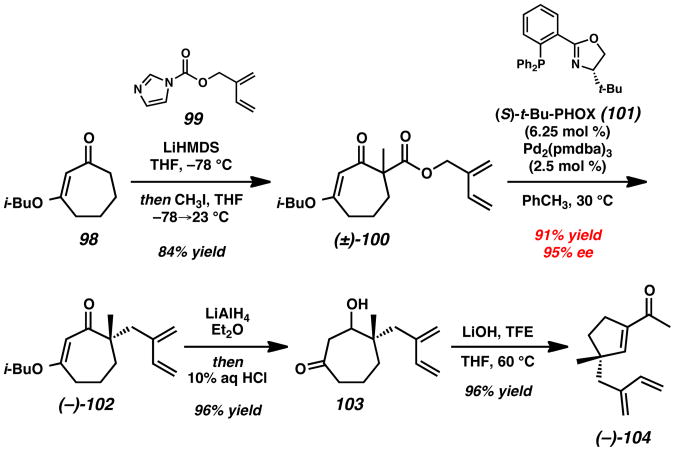
The commercial vinylogous ester 98 was treated with carbamate 99, followed by the addition of CH3I, which gave rise to racemic α-quaternary β-ketoester 100 in 84% yield (Scheme 17). With the requisite isoprenyl fragment in place, the application of the group's previously developed Pd-catalyzed asymmetric allylic alkylation methodology[47, 48] with Pd2(pmdba)3 and (S)-t-Bu-PHOX (101) smoothly provided enantioenriched vinylogous ester 102 in 91% yield and 95% ee. Conversion of the compound to acylcyclopentene 104 was achieved by employing a recently developed two-carbon ring contraction sequence.[49] Treatment of asymmetric alkylation product 102 with LiAlH4 in Et2O followed by acid workup provided intermediate β-hydroxyketone 103, which undergoes retro-aldol fragmentation and aldol cyclization in the presence of LiOH and TFE in THF at 60 °C. In this manner, γ-quaternary enone 104 was obtained in 92% yield over 2 steps.
Scheme 17.
Construction of Enantioenriched Acylcyclopentene 104. LiHMDS = lithium bis(trimethylsilyl)amide, pmdba = 4,4′-methoxybenzylideneacetone, TFE = 2,2,2-trifluoroethanol.
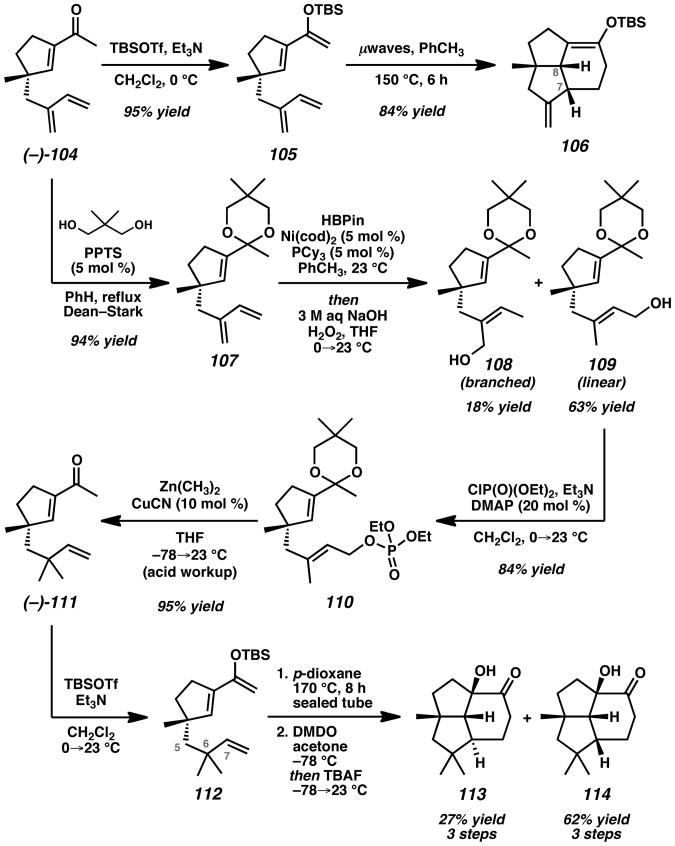
With the key all-carbon quaternary stereocenter of the target installed, the planned IMDA bicyclization was evaluated (Scheme 18). Silylation of acylcyclopentene 104 and heating in the presence of microwave irradiation led to the exclusive formation of undesired tricyclic silyl enol ether 106 without any trace of the desired product containing the α-oriented C–H methine hydrogen at C(7). Based on these results, modification of the IMDA strategy was necessary to complete the synthesis of presilphiperfolan-1β-ol (3).
Scheme 18.
Investigation of IMDA Bicyclizations with Acylcyclopentenes 104 and 111. TBSOTf = tert-butyldimethylsilyl trifluoromethanesulfonate, PPTS = pyridinium p-toluenesulfonate, HBPin = pinacolborane (4,4,5,5-tetramethyl-1,3,2-dioxaborolane), cod = 1,5-cyclooctadiene, PCy3 = tricyclohexylphosphine, DMAP = 4-(dimethylamino)pyridine, DMDO = dimethyldioxirane, TBAF = tetrabutylammonium fluoride.
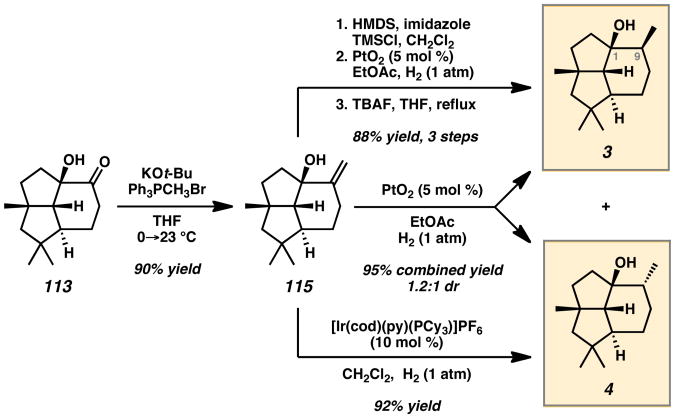
Subsequent efforts focused on the construction of acylcyclopentene 111, a compound having the gem-dimethyl substituents at C(6), as an alternative IMDA precursor (Scheme 18). Following ketal formation, Ni-catalyzed regioselective 1,4-hydroboration/oxidation[50] of diene 107 provided allylic alcohols 108 and 109 in 81% combined yield and 1:3.5 ratio favoring the desired isomer. Phosphorylation and Cu-catalyzed allylic substitution followed by acid workup led to key enone 111. Silylation and heating produced a mixture of intermediate tricyclic silyl enol ethers. Treatment of these compounds with DMDO led to diastereoselective epoxidation, providing α-hydroxyketone 113 in 27% yield and α-hydroxyketone 114 in 62% yield.
Methylenation of isomer 113 using Wittig conditions led to the formation of tricyclic alkene 115 in 90% yield (Scheme 19). Hydrogenation using Adam's catalyst provided a separable mixture of tricyclic alcohols 3 and 4 in 95% combined yield and 1.2:1 d.r. Diastereoselective formation of alcohol 3 could be achieved by employing a bulky trimethylsilyl group on the C(1) hydroxyl, while preferential formation of alcohol 4 could be achieved by using the sterically sensitive Crabtree catalyst. The total synthesis of target 3 was completed in 15 steps and 7.9% overall yield while target 4 was completed in 13 steps and 8.3% overall yield.[46]
Scheme 19.
Completion of Presilphiperfolan-1β-ol (3) and Synthesis of Isomer 4. HMDS = hexamethyldisilazane, TMSCl = chlorotrimethylsilane, TBAF = tetrabutylammonium fluoride.
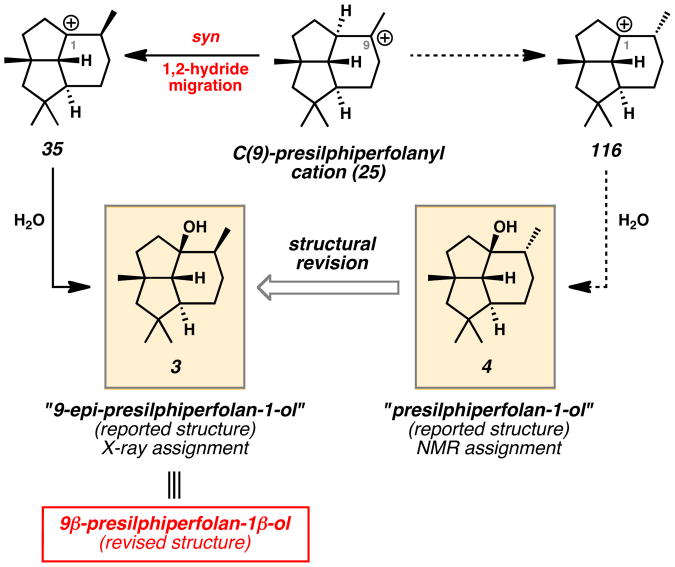
Upon completion of the synthesis of tricyclic alcohols 3 and 4, subsequent comparison of spectral data for the synthetic presilphiperfolanols and the reported natural products led to unanticipated findings, which prompted structural reevaluation and a new biosynthetic proposal. While the synthetic sample of compound 3 matched literature reports,[4] synthetic isomer 4 clearly showed significant discrepancies with reported 1H and 13C NMR spectra.[3] To explain these results, we examined possible biosynthetic routes toward alcohols 3 and 4 (Scheme 20). In accordance with previous biosynthetic proposals,[1,2,5,7,13,14,15,17,19,20] FPP (21) can undergo polycyclization and rearrangement to C(9)-presilphiperfolanyl cation 25 (Schemes 1–4). A syn 1,2-hydride migration provided a reasonable path to alcohol 3 (Scheme 20), but the formation of isomer 4 through similar hydride shifts was difficult to rationalize. Thus, inspection of the likely biosynthetic pathway in conjunction with spectroscopic data for the synthetic compounds suggested that the true structure of presilphiperfolan-1β-ol is alcohol 3 while isomeric alcohol 4 currently does not correspond to a known natural product.[46]
Scheme 20.
Proposed Biosynthesis of Presilphiperfolan-1β-ol (3) and Structural Revision of Reported “Presilphiperfolan-1-ol” (4)
3. Conclusion
The presilphiperfolanol terpenoids have been studied intensely in natural products, biosynthesis, computational, and fragrance chemistry research, but reports documenting synthetic efforts toward these molecules have been relatively scarce. Early studies of the biomimetic rearrangement of caryophyllene, isocaryophyllene, and their derivatives have provided structures resembling the presilphiperfolanol natural products. More recent work by several research groups has provided unique strategies for accessing the strained tricyclic presilphiperfolanol core through total synthesis. To date, presilphiperfolan-9α-ol (2),[2] has been prepared in racemic form and presilphiperfolan-1β-ol (3)[3,4] has been prepared in enantioenriched form, but presilphiperfolan-8α-ol (1)[1] has remained elusive to total synthesis.
Understanding of the biosynthetic relationships between presilphiperfolanes and related sesquiterpenes continues to grow and synthetic chemistry has made contributions in this area by not only providing access to members of the natural product family, but by also suggesting new biosynthetic rearrangement pathways. Much remains to be learned about the biosynthetic rearrangement pathways connecting the strained, high-energy structures of the presilphiperfolanols to diverse sesquiterpene natural products, and chemical synthesis can greatly aid these research efforts.
Acknowledgments
The authors thank Dr. Scott Virgil, Prof. Sarah Reisman, Dr. Douglas Behenna, Dr. Mike Krout, Dr. Thomas Jensen, Dr. Phil Kun-Liang Wu, Dr. Alex Marziale, Dr. Jimin Kim, Douglas Duquette, Nick O'Connor, Jeffrey Holder, Nathan Bennett and our reviewers for helpful discussions and suggestions.
Biographies

Brian M. Stoltz was born in Philadelphia, Pennsylvania, USA in 1970. After spending a year at the Ludwig Maximilians Universität in München, Germany, he obtained his BS in Chemistry and BA in German from Indiana University of Pennsylvania in 1993. He then earned his Ph. D. in 1997 under the direction of Professor John L. Wood at Yale University specializing in synthetic organic chemistry. Following an NIH postdoctoral fellowship in the laboratories of Professor E. J. Corey at Harvard University (1998–2000), he joined the faculty at Caltech in 2000 where he is currently Professor of Chemistry. His research focuses on the design and implementation of new synthetic strategies for the synthesis of complex molecules possessing important biological properties, in addition to the development of new synthetic methods including asymmetric catalysis and cascade processes.

Allen Y. Hong was born in San Francisco, California in 1984. He began studying chemistry in 2004 at UC Berkeley. In 2005 he joined Prof. Richmond Sarpong's laboratory and to pursue undergraduate research. Allen graduated from UC Berkeley in 2006 with a B.S. in chemical biology. He moved south to Pasadena, California in 2007 to begin doctoral studies in synthetic organic chemistry at the California Institute of Technology. In January 2008 he joined the research group of Prof. Brian M. Stoltz to study asymmetric catalysis and total synthesis. Allen earned his Ph.D. in chemistry in 2012. In January 2013, he continued the southward trend and began NIH-sponsored postdoctoral research under the guidance of Prof. Chris D. Vanderwal at UC Irvine.
Footnotes
The authors thank NIH-NIGMS (R01GM080269-01), Roche, Abbott Laboratories, Amgen, Boehringer Ingelheim, the Gordon and Betty Moore Foundation, and Caltech for awards and financial support.
References
- 1.For the isolation of presilphiperfolan-8α-ol (1), see: Bohlmann F, Zdero C, Jakupovic J, Robinson H, King RM. Phytochemistry. 1981;20:2239–2244.
- 2.For the isolation of presilphiperfolan-9α-ol (2), see: Weyerstahl P. In: Newer Trends in Essential Oils and Flavours. Dhar KL, Thappa RK, Agarwal SG, editors. Tata McGraw-Hill Publishing Company Ltd.; New Delhi: 1993. pp. 24–41.Marco JA, Sanz-Cervera JF, Morante MD, García-Lliso V, Vallès-Xirau J, Jakupovic J. Phytochemistry. 1996;41:837–844.
- 3.Presilphiperfolan-1β-ol (3) was originally assigned as structure 4. For the first records of its isolation, see: Melching S, König WA. Phytochemistry. 1999;51:517–523.“Isolierung, Strukturaufklärung und stereochemische Untersuchungen neuer sesquiterpenoider Verbindungen aus vier Chemotypen des Lebermooses Conocephalum conicum”: Melching S. Ph D Thesis. Universität Hamburg; Apr, 1999.
- 4.Presilphiperfolan-1β-ol (3) was subsequently isolated by another research group, but reported as a unique natural product with structure 5. This structure was later revised to structure 3. For these reports, see: Pinto SC, Leitão GG, Bizzo HR, Martinez N, Dellacassa E, dos Santos FM, Jr, Costa FLP, de Amorim MB, Leitão SG. Tetrahedron Lett. 2009;50:4785–4787.Joseph-Nathan P, Leitão SG, Pinto SC, Leitão GG, Bizzo HR, Costa FLP, de Amorim MB, Martinez N, Dellacassa E, Hernández-Barragán A, Pérez-Hernández N. Tetrahedron Lett. 2010;51:1963–1965.
- 5.Davis CE, Duffy BC, Coates RM. J Org Chem. 2003;68:6935–6943. doi: 10.1021/jo0343580. [DOI] [PubMed] [Google Scholar]
- 6.Osawa E, Aigami K, Takaishi N, Inamoto Y, Fujikura Y, Majerski Z, Schleyer P von R, Engler EM, Farcasiu M. J Am Chem Soc. 1977;99:5361–5373. [Google Scholar]
- 7.Coates RM, Ho Z, Klobus M, Wilson SR. J Am Chem Soc. 1996;118:9249–9254. [Google Scholar]
- 8.Weyerstahl P, Marschall H, Schulze M, Schwope I. Liebigs Ann. 1996:799–807. [Google Scholar]
- 9.Weyerstahl P, Marschall H, Seelmann I, Jakupovic J. Eur J Org Chem. 1998:1205–1212. [Google Scholar]
- 10.Yang JL, Liu LL, Shi YP. Tetrahedron Lett. 2009;50:6315–6317. [Google Scholar]
- 11.a) Bohlmann F, Ziesche J, Gupta RK. Phytochemistry. 1982;21:1331–1334. [Google Scholar]; b) Bohlmann F, Zdero C. Phytochemistry. 1982;21:2537–2541. [Google Scholar]; c) Mericli AH, Mericli F, Jakupovic J, Bohlmann F, Dominguez XA, Vega HS. Phytochemistry. 1989;28:1149–1153. [Google Scholar]
- 12.Fehlhaber HW, Geipel R, Mercker HJ, Tschesche R, Welmar K, Schönbeck F. Chem Ber. 1974;107:1720–1730. [Google Scholar]
- 13.Bohlmann F, Jakupovic J. Phytochemistry. 1980;19:259–265. [Google Scholar]
- 14.a) Bohlmann F, Zdero C. Phytochemistry. 1981;20:2529–2534. [Google Scholar]; b) Bohlmann F, Zdero C, King RM, Robinson H. Phytochemistry. 1981;20:2425–2427. [Google Scholar]
- 15.a) Hanson JR. Pure Appl Chem. 1981;53:1155–1162. [Google Scholar]; b) Bradshaw APW, Hanson JR, Nyfeler R. J Chem Soc, Perkin Trans. 1981;1:1469–1472. [Google Scholar]; c) Bradshaw APW, Hanson JR, Nyfeler R, Sadler IH. J Chem Soc, Chem Commun. 1981:649–650. [Google Scholar]; d) Bradshaw APW, Hanson JR, Nyfeler R, Sadler IH. J Chem Soc, Perkin Trans. 1982;1:2187–2192. [Google Scholar]
- 16.Pinedo C, Wang CM, Pradier JM, Dalmais B, Choquer M, Le Pêcheur P, Morgant G, Collado IG, Cane DE, Viaud M. ACS Chem Biol. 2008;3:791–801. doi: 10.1021/cb800225v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CM, Hopson R, Lin X, Cane DE. J Am Chem Soc. 2009;131:8360–8361. doi: 10.1021/ja9021649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.While 2-epi-caryophyllene (45) was not observed in Bohlmann's presilphiperfolan-8α-ol isolation report, it was found in a different plant, Carydium cupressinium: Hinkley SFR, Perry NB, Weavers RT. Phytochemistry. 1994;35:1489–1494.
- 19.Wang SC, Tantillo DJ. Org Lett. 2008;10:4827–4830. doi: 10.1021/ol801898v. [DOI] [PubMed] [Google Scholar]
- 20.Barquera-Lozada JE, Cuevas G. J Org Chem. 2011;76:1572–1577. doi: 10.1021/jo101869z. [DOI] [PubMed] [Google Scholar]
- 21.While presilphiperfol-1(8)-ene (34) is a structural isomer of presilphiperfol-1(8)-ene (6), it has not yet been observed as a natural product.
- 22.a) Weyerstahl P, Marschall H, Schröder M, Wahlburg HC, Kaul VK. Flavour Fragr J. 1997;12:315–325. [Google Scholar]; b) Menut C, Lamaty G, Weyerstahl P, Marschall H, Seelmann I, Amvam Zollo PH. Flavour Fragr J. 1997;12:415–421. [Google Scholar]
- 23.González-Coloma A, Valencia F, Martín N, Hoffmann JF, Hutter L, Marco JA, Reina M. J Chem Ecol. 2002;28:117–129. doi: 10.1023/a:1013566919874. [DOI] [PubMed] [Google Scholar]
- 24.Pinto SC, Leitão GG, de Oliveira DR, Bizzo HR, Ramos DF, Coelho TS, Silva PEA, Lourenço MCS, Leitão SG. Nat Prod Comm. 2009;4:1675–1678. [PubMed] [Google Scholar]
- 25.a) Collado IG, Aleu J, Macías-Sánchez AJ, Hernández-Galán R. J Chem Ecol. 1994;20:2631–2644. doi: 10.1007/BF02036197. [DOI] [PubMed] [Google Scholar]; b) Collado IG, Aleu J, Macías-Sánchez AJ, Hernández-Galán R. J Nat Prod. 1994;57:738–746. [Google Scholar]
- 26.For a review discussing the rearrangements of β-caryophyllene and isocaryophyllene, see: Collado IG, Hanson JR, Macías-Sánchez A. J Nat Prod Rep. 1998;15:187–204. doi: 10.1021/np9801054.
- 27.To our knowledge, the rearrangement of β-caryophyllene or β-caryophyllene derivatives in biomimetic reactions has not produced any of the presilphiperfolanol natural products. For representative studies, see the following and references therein: Wallach O, Walker W. Liebigs Ann. 1892;271:285–299.Asahina Y, Tsukamoto T. J Pharm Soc Jpn. 1922:463–473.Henderson GG, McCrone ROO, Robertson JM. J Chem Soc. 1929:1368–1372.Aebi A, Barton DHR, Burgstahler AW, Lindsey AS. J Chem Soc. 1954:4659–4665.Barton DHR, Nickon AJ. J Chem Soc. 1954:4665–4669.Parker W, Raphael RA, Roberts JS. Tetrahedron Lett. 1965;6:2313–2316.Parker W, Raphael RA, Roberts JS. J Chem Soc C. 1969:2634–2643.
- 28.a) Fitjer L, Malich A, Paschke C, Kluge S, Gerke R, Rissom B, Weiser J, Noltemeyer M. J Am Chem Soc. 1995;117:9180–9189. [Google Scholar]; b) Shankar S, Coates RM. J Org Chem. 1998;63:9177–9182. [Google Scholar]
- 29.a) Gollnick K, Schade G, Cameron AF, Hannaway C, Roberts JS, Robertson JM. J Chem Soc, Chem Commun. 1970:248–249. [Google Scholar]; b) Gollnick K, Schade G, Cameron AF, Hannaway C, Robertson JM. J Chem Soc D: Chem Commun. 1971:46. [Google Scholar]; c) Cameron AF, Hannaway C, Robertson JM. J Chem Soc, Perkin Trans. 1973;2:1938–1942. [Google Scholar]
- 30.a) Khomenko TM, Bagryanskaya IY, Gatilov YV, Korchagina DV, Gatilova VP, Dubovenko ZV, Barkhash VA. Zh Org Khim. 1985;21:677–678. [Google Scholar]; Russ J Org Chem (Engl Transl) 1985;21:614–615. [Google Scholar]; b) Khomenko TM, Korchagina DV, Gatilov YV, Bagryanskaya YI, Tkachev AV, Vyalkov AI, Kun OB, Salenko VL, Dubovenko ZV, Barkash VA. Zh Org Khim. 1990;26:2129–2145. [Google Scholar]; Russ J Org Chem (Engl Transl) 1990;26:1839–1852. [Google Scholar]
- 31.Burgess EM, Penton HR, Jr, Taylor EA. J Org Chem. 1973;38:26–31. [Google Scholar]
- 32.a) Hosomi A, Endo M, Sakurai H. Chem Lett. 1976;5:941–942. [Google Scholar]; b) Hosomi A, Sakurai H. Tetrahedron Lett. 1976;17:1295–1298. [Google Scholar]; c) Sakurai H, Hosomi A, Hayashi J. Org Synth. 1984;62:86–93. [Google Scholar]
- 33.For comparisons of the reactivity of MMPP and m-CPBA, see: Brougham P, Cooper MS, Cummerson DA, Heaney H, Thompson N. Synthesis. 1987:1015–1017.
- 34.For a review on radical cascade reactions, see: McCarroll AJ, Walton JC. Angew Chem. 2001;113:2282–2307. doi: 10.1002/1521-3773(20010618)40:12<2224::aid-anie2224>3.0.co;2-f.Angew Chem Int Ed. 2001;40:2224–2248. doi: 10.1002/1521-3773(20010618)40:12<2224::aid-anie2224>3.0.co;2-f.
- 35.“Carbocycle Construction in Terpenoid Synthesis. The Total Synthesis of (±)-Sarcodonin G and (±)-1-Epi-9-Norpresilphiperfolan-9-one”: Gilbert MW. Ph D Thesis. University of British Columbia; Jun, 2002.
- 36.Luche JL. J Am Chem Soc. 1978;100:2226–2227. [Google Scholar]
- 37.Wilson SR, Price MF. J Org Chem. 1984;49:722–725. [Google Scholar]
- 38.a) Carroll MF. J Chem Soc. 1940:704–706. [Google Scholar]; b) Carroll MF. J Chem Soc. 1940:1266–1268. [Google Scholar]
- 39.Levine SG. J Am Chem Soc. 1958;80:6150–6151. [Google Scholar]
- 40.Carlsen PHJ, Katsuki T, Martin VS, Sharpless KB. J Org Chem. 1981;46:3936–3938. [Google Scholar]
- 41.Kobayashi T, Shiroi H, Abe H, Ito H. Chem Lett. 2013;42:975–976. [Google Scholar]
- 42.a) Hosomi A, Sakurai H. J Am Chem Soc. 1977;99:1673–1675. [Google Scholar]; b) Miles RB, Davis CE, Coates RM. J Org Chem. 2006;71:1493–1501. doi: 10.1021/jo052142n. [DOI] [PubMed] [Google Scholar]
- 43.Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 44.McMurry JE, Fleming MP, Kees KL, Krepski LR. J Org Chem. 1978;43:3255–3266. [Google Scholar]
- 45.Gribble GW, Leese RM, Evans BE. Synthesis. 1977:172–176. [Google Scholar]
- 46.Hong AY, Stoltz BM. Angew Chem. 2012;124:9812–9816.Angew Chem Int Ed. 2012;51:9674–9678. doi: 10.1002/anie.201205276.This communication is corrected by: Hong AY, Stoltz BM. Angew Chem. 2013;125:2201.Angew Chem Int Ed. 2013;52:2147.
- 47.a) Behenna DC, Stoltz BM. J Am Chem Soc. 2004;126:15044–15045. doi: 10.1021/ja044812x. [DOI] [PubMed] [Google Scholar]; b) Mohr JT, Behenna DC, Harned AM, Stoltz BM. Angew Chem. 2005;117:7084–7087. doi: 10.1002/anie.200502018. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:6924–6927. doi: 10.1002/anie.200502018. [DOI] [PubMed] [Google Scholar]; c) Behenna DC, Mohr JT, Sherden NH, Marinescu SC, Harned AM, Tani K, Seto M, Ma S, Novák Z, Krout MR, McFadden RM, Roizen JL, Enquist JA, Jr, White DE, Levine SR, Petrova KV, Iwashita A, Virgil SC, Stoltz BM. Chem Eur J. 2011;17:14199–14223. doi: 10.1002/chem.201003383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.For selected reviews of Pd-catalyzed asymmetric allylic alkylation reactions in total synthesis, see: Hong AY, Stoltz BM. Eur J Org Chem. 2013:2745–2759. doi: 10.1002/ejoc.201201761.Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w.
- 49.a) Hong AY, Krout MR, Jensen T, Bennett NB, Harned AM, Stoltz BM. Angew Chem. 2011;123:2808–2812. doi: 10.1002/anie.201007814. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:2756–2760. doi: 10.1002/anie.201007814. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hong AY, Bennett NB, Krout MR, Jensen T, Harned AM, Stoltz BM. Tetrahedron. 2011;67:10234–10248. doi: 10.1016/j.tet.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bennett NB, Hong AY, Harned AM, Stoltz BM. Org Biomol Chem. 2012;10:56–59. doi: 10.1039/c1ob06189e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.a) Ely RJ, Morken JP. J Am Chem Soc. 2010;132:2534–2535. doi: 10.1021/ja910750b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ely RJ, Morken JP. Org Synth. 2011;88:342–352. [Google Scholar]