Abstract
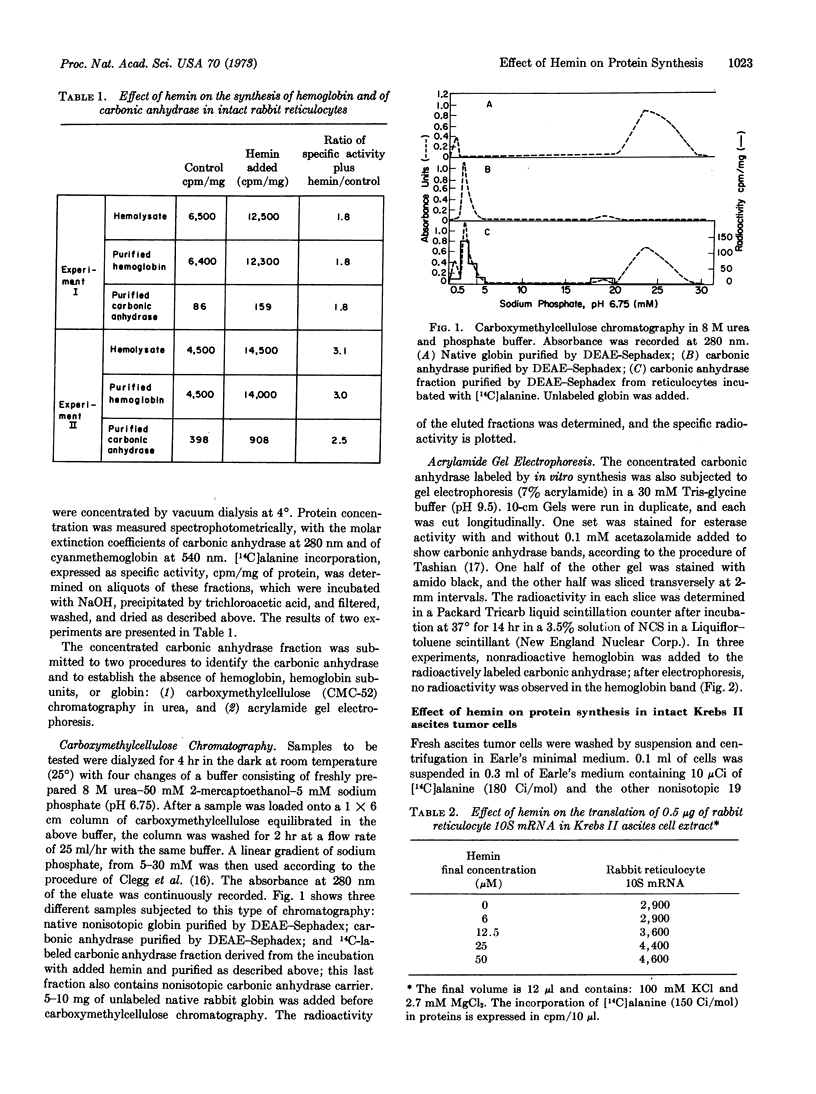
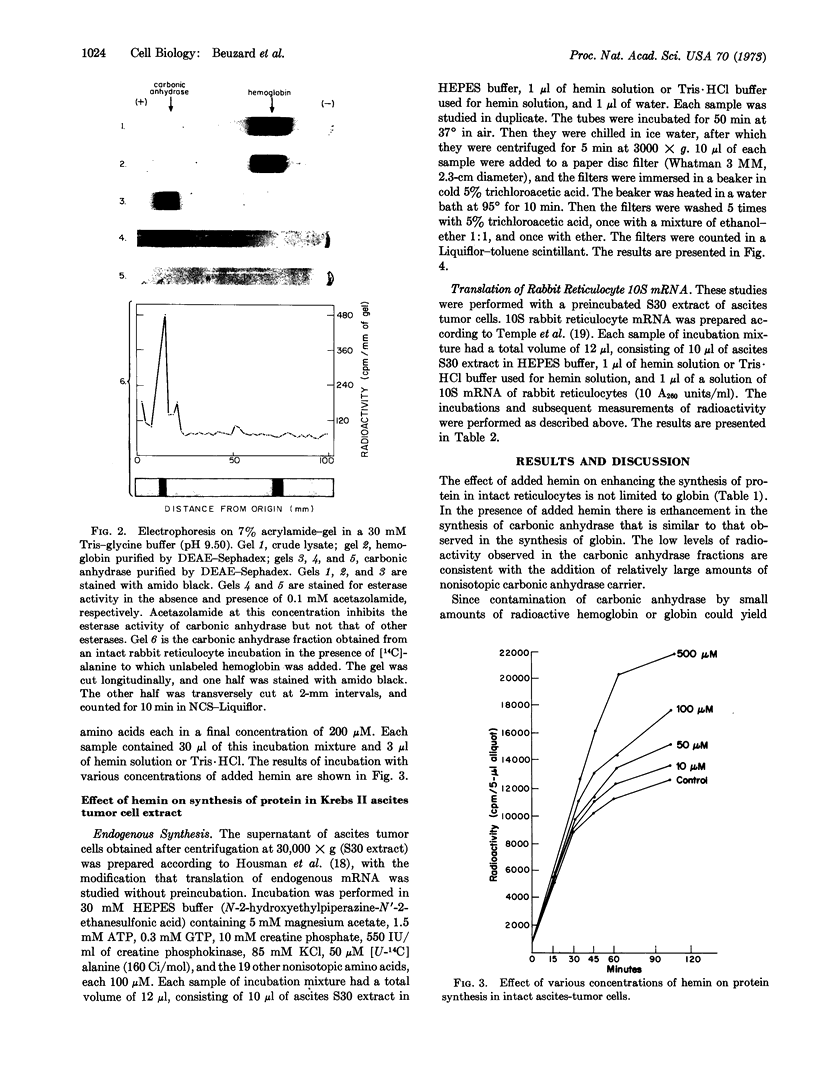
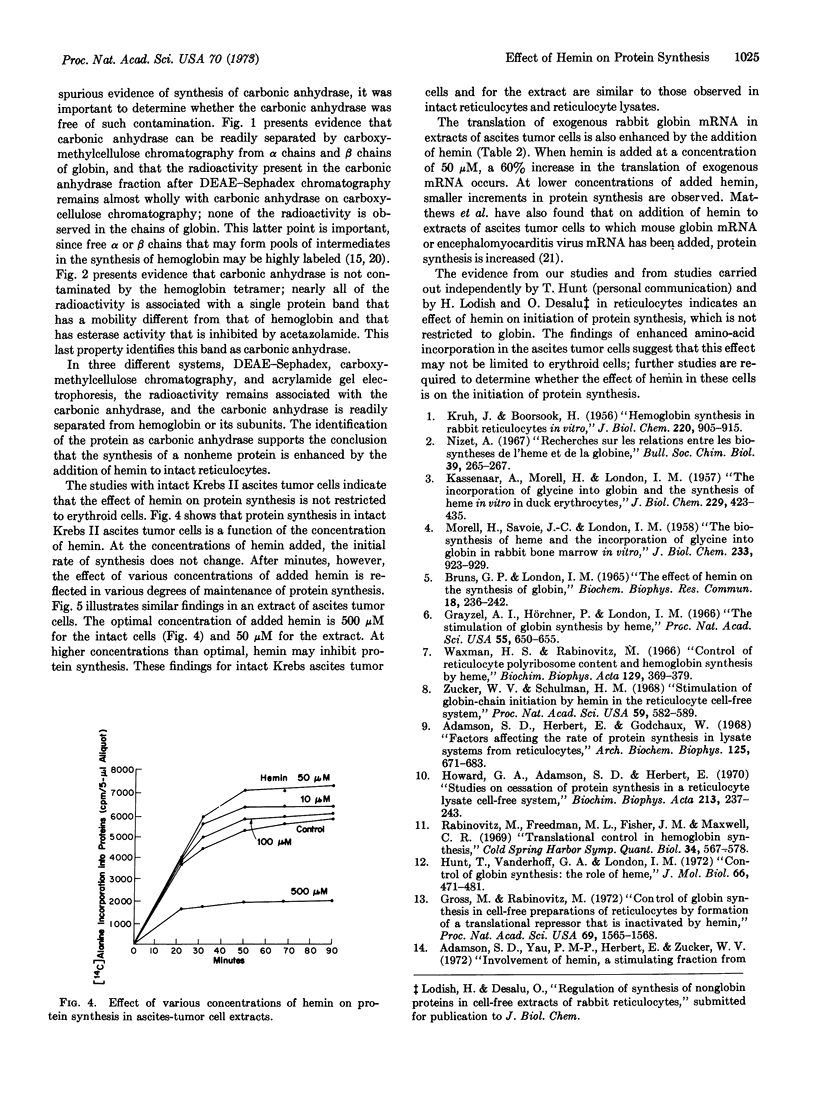
The initiation of globin synthesis in intact reticulocytes and in reticulocyte lysates is maintained by the addition of hemin. The specificity of this effect has been studied to determine whether it is restricted to hemoglobin and erythroid cells. In intact reticulocytes, hemin (500 μM) enhances the synthesis of carbonic anhydrase as well as of hemoglobin. Similar enhancement of protein synthesis is observed on addition of hemin (500 μM) to intact Krebs II ascites tumor cells, in cell-free extracts of these cells, added hemin (50 μM) increases endogenous protein synthesis and the translation of exogenous rabbit globin messenger RNA. These results provide evidence that the effect of hemin is not restricted to globin, and they suggest that hemin may enhance protein synthesis in at least some nonerythroid cells.
Keywords: carbonic anhydrase, Krebs II ascites tumor cells, reticulocytes
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Grayzel A. I., Hörchner P., London I. M. The stimulation of globin synthesis by heme. Proc Natl Acad Sci U S A. 1966 Mar;55(3):650–655. doi: 10.1073/pnas.55.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Pemberton R., Taber R. Synthesis of and chains of rabbit hemoglobin in a cell-free extract from Krebs II ascites cells. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2716–2719. doi: 10.1073/pnas.68.11.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Studies on cessation of protein synthesis in a reticulocyte lysate cell-free system. Biochim Biophys Acta. 1970 Jul 16;213(1):237–240. doi: 10.1016/0005-2787(70)90028-6. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- KASSENAAR A., MORELL H., LONDON I. M. The incorporation of glycine into globin and the synthesis of heme in vitro in duck erythrocytes. J Biol Chem. 1957 Nov;229(1):423–435. [PubMed] [Google Scholar]
- KRUH J., BORSOOK H. Hemoglobin synthesis in rabbit reticulocytes in vitro. J Biol Chem. 1956 Jun;220(2):905–915. [PubMed] [Google Scholar]
- MORELL H., SAVOIE J. C., LONDON I. M. The biosynthesis of heme and the incorporation of glycine into globin in rabbit bone marrow in vitro. J Biol Chem. 1958 Oct;233(4):923–929. [PubMed] [Google Scholar]
- Mathews M. B. Further studies on the translation of globin mRNA and encephalomyocarditis virus RNA in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1972 Jun 22;272(1):108–118. doi: 10.1016/0005-2787(72)90038-x. [DOI] [PubMed] [Google Scholar]
- NIZET A. Recherches sur les relations entre les biosynthéses de l'hème et de la globine. Bull Soc Chim Biol (Paris) 1957 Mar 15;39(2-3):265–277. [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- TASHIAN R. E., SHAW M. W. Inheritance of an erythrocyte acetylesterase variant in man. Am J Hum Genet. 1962 Sep;14:295–300. [PMC free article] [PubMed] [Google Scholar]
- Tabill A. S., Vanderhoff G. A., London I. M. The control of hemoglobin synthesis. A comparison of the role of heme in rabbit bone marrow and reticulocytes. J Biol Chem. 1972 Jan 25;247(2):326–333. [PubMed] [Google Scholar]
- Tavill A. S., Grayzel A. I., London I. M., Williams M. K., Vanderhoff G. A. The role of heme in the synthesis and assembly of hemoglobin. J Biol Chem. 1968 Oct 10;243(19):4987–4999. [PubMed] [Google Scholar]
- Temple G. F., Housman D. E. Separation and translation of the mRNAs coding for and chains of rabbit globin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1574–1577. doi: 10.1073/pnas.69.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]





