Abstract
Pseudomonas aeruginosa virulence components are subject to complex regulatory control primarily through two-component regulatory systems that allow for sensing and responding to environmental stimuli. In this study, the expression and regulation of the P. aeruginosa AlgZR two-component regulatory system were examined. Primer extension and S1 nuclease protection assays were used to identify two transcriptional initiation sites for algR within the algZ coding region, and two additional start sites were identified upstream of the algZ coding region. The two algR transcriptional start sites, RT1 and RT2, are directly regulated by AlgU, consistent with previous reports of increased algR expression in mucoid backgrounds, and RpoS additionally plays a role in algR transcription. The expression of the first algZ promoter, ZT1, is entirely dependent upon Vfr for expression, whereas Vfr, RpoS, or AlgU does not regulate the second algZ promoter, ZT2. Western blot, real-time quantitative PCR (RT-qPCR), and transcriptional fusion analyses show that algZR expression is Vfr dependent. The algZ and algR genes also are cotranscribed in both nonmucoid and mucoid backgrounds. Furthermore, algZR was found to be cotranscribed with hemCD by RT-PCR. RT-qPCR confirmed that hemC transcription in the PAO1 ΔalgZ mutant was 40% of the level of the wild-type strain. Taken together, these results indicate that algZR transcription involves multiple factors at multiple start sites that control individual gene expression as well as coexpression of this two-component system with heme biosynthetic genes.
INTRODUCTION
Pseudomonas aeruginosa is a human opportunistic pathogen capable of causing fatal infections in individuals with compromised innate immunity, such as those undergoing cancer treatment or those with severe burn wounds or cystic fibrosis (CF) (1–3). P. aeruginosa is one of the most clinically relevant organisms in CF patients due to its ability to establish chronic infections, characterized by the overproduction of an exopolysaccharide called alginate. Alginate overproduction (a phenotype called mucoidy) increases P. aeruginosa resistance against antimicrobials and phagocytosis and is a hallmark of clinical decline and worsening prognosis in patients with CF.
Alginate production is regulated in a complex manner by several transcriptional regulators, including the extracytoplasmic sigma factor AlgU (AlgT) (4–6). The activity of AlgU is increased in mucoid P. aeruginosa due to mutations in the mucA anti-sigma factor gene that releases AlgU from the inner membrane (6–8). The enzymes encoded by the algC gene and the algD operon are responsible for alginate biosynthesis, modification, and export. The AlgR transcriptional regulator is an essential activator for the algD operon and the algC gene; deletion of algR in mucoid P. aeruginosa backgrounds results in a nonmucoid phenotype (9–11). As part of its regulon, AlgU also activates the transcription of the algR gene to increase alginate production. An AlgU-dependent algR transcriptional start site was determined in mucA22 strains to be located 73 bp upstream of the translational start site (4, 12). A constitutively active promoter had previously been proposed to be located in the algR promoter region, although it has not been mapped (4, 10, 12–15).
Although transcriptional regulation of algR has been examined briefly in previous work, the regulation of algZ has remained virtually unexplored. There are several pieces of data demonstrating that Vfr (for virulence factor regulator), a member of the 3′,5′-cyclic AMP (cAMP) receptor protein (CRP) family of transcriptional regulators (16), is tied into the regulation of the algZR system. A Vfr binding site has been identified upstream of the algZ coding region and was shown by gel shifts to be bound by Vfr (17). Additionally, it has been shown that the cAMP/Vfr-dependent (CVS) signaling pathway is suppressed in a mucA22 background, and that this suppression can be relieved by deletion of either algZR or algU (48).
Besides alginate production, AlgR also regulates a number of virulence factors in P. aeruginosa, including type IV pili, hydrogen cyanide and rhamnolipid production, the type III secretion system, the Rhl quorum-sensing system, and biofilm formation (19–26, 48, 67, 68). The response regulator encoded by algR (PA5261) and the putative cognate histidine kinase encoded by algZ-fimS (PA5262) are proposed to form a two-component regulatory system (TCS) (25, 27). TCSs transduce an environmental signal to the intracellular environment through a phosphotransfer reaction between the sensor kinase and response regulator. AlgR phosphorylation functions to modulate AlgR activity either as an activator or repressor of its target genes (14, 19, 20, 23, 24, 28, 29).
Because AlgZ and AlgR regulate a number of virulence factors, this TCS plays a key role in overall P. aeruginosa virulence. Therefore, understanding how these genes are transcriptionally controlled is important in dissecting their physiological role in the organism. Currently, one transcriptional start site has been identified upstream of algR that is AlgU dependent (4, 12). However, an additional start site also was detected but not characterized (12). In this study, transcriptional start site mapping was performed to provide a better understanding of the regulation of this two-component system. We present evidence that (i) two promoters control algR transcription, (ii) two promoters control algZ transcription, (iii) algZ and algR are cotranscribed, (iv) the algZR genes are cotranscribed with hemCD, (v) both promoters regulating algR transcription are directly AlgU dependent, (vi) RpoS plays a role in algR expression but not algZ expression, and (vii) Vfr regulates algZR expression.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. P. aeruginosa was grown at 37°C in lysogeny broth-Miller (LB-Miller) or Pseudomonas isolation agar (PIA; Difco). The LB-Miller medium used for P. aeruginosa was supplemented with tetracycline (300 μg/ml), gentamicin (150 μg/ml), or carbenicillin (300 μg/ml) as needed. Escherichia coli was cultivated at 37°C in LB-Miller and supplemented when necessary with ampicillin (100 μg/ml), gentamicin (15 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (34 μg/ml).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or other characteristics | Reference or source |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Wild type | 30 |
| PSL317 | PAO1 ΔalgR | 20 |
| PAZ | PAO1 ΔalgZ | 22 |
| PAO1 Δvfr | PAO1 Δvfr | Matthew Wolfgang |
| PAO1 mucB | PAO1 mucB::Tcr | 7 |
| SS24 | PAO1 rpoS101::aacCI | 31 |
| PDO300 | PAO1 mucA22 | 32 |
| PAO1 ΔalgU | This study | |
| PAO1 rpoS::Gm ΔalgU | SS24 (rpoS101::aacC1) and deletion of algU | This study |
| PAO1 ΔmucA | PAO1 with mucA deleted | This study |
| PAO1-Z | PAO1 containing an attB site single copy of algZ–S-Tag gene with 1,212 bp of upstream promoter | This study |
| PAO1-Z ΔalgU | PAO1 ΔalgU containing an attB site single copy of algZ–S-Tag gene with 1,212 bp of upstream promoter | This study |
| PAO1-Z rpoS::Gm | PAO1 rpoS::Gm containing an attB site single copy of algZ–S-Tag gene with 1,212 bp of upstream promoter | This study |
| PAO1-Z rpoS::Gm ΔalgU | PAO1ΔalgU rpoS::Gm containing an attB site single copy of algZ–S-Tag gene with 1,212 bp of upstream promoter | This study |
| PAO1-Z Δvfr | PAO1 Δvfr containing an attB site single copy of algZ–S-Tag gene with 1,212 bp of upstream promoter | This study |
| PAO1-RTF | PAO1 containing 1,077-bp algR::lacZ promoter fusion inserted at chromosomal attB site | This study |
| PAO1 vfr RTF | PAO1 Δvfr containing 1,077-bp algR::lacZ promoter fusion inserted at chromosomal attB site | This study |
| PAO1 ZTF | PAO1 containing 796-bp algZ::lacZ promoter fusion inserted at chromosomal attB site | This study |
| PAO1 vfr ZTF | PAO1 Δvfr containing 796-bp algZ::lacZ promoter fusion inserted at chromosomal attB site | This study |
| PAO1-RT2 | PAO1 containing 422 bp of the algR RT2::lacZ promoter fusion inserted at chromosomal attB site | This study |
| mucA-RT2 | PAO1 ΔmucA containing 422 bp of the algR RT2::lacZ promoter fusion inserted at chromosomal attB site | This study |
| algU-RT2 | PAO1 ΔalgU containing 422 bp of the algR RT2::lacZ promoter fusion inserted at chromosomal attB site | This study |
| PAO1-SDM | PAO1 with 422 bp of the algR RT2::lacZ promoter fusion inserted at chromosomal attB site; RT2 −35 AlgU binding region mutated from 5′-GAACTG-3′ to 5′-TGGAGT-3′ | This study |
| mucA-SDM | PAO1 ΔmucA with 422 bp of algR RT2::lacZ promoter fusion inserted at chromosomal attB site; RT2 −35 AlgU binding region mutated from 5′-GAACTG-3′ to 5′-TGGAGT-3′ | This study |
| E. coli | ||
| DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Invitrogen |
| Plasmids | ||
| pRK2013 | tra+ mob+ functions, Kmr | 33 |
| Mini-CTXlacZ | Self-proficient integration vector with tet, Ω-FRT-attP-MCS, ori, int, and oriT | 34 |
| pFLP2 | Flp recombinase | 35 |
| pVDZ′2R | algR complementation plasmid, tetracycline resistant | |
| Mini-CTX algZ–S-Tag | Mini-CTX containing 2.2-kb BamHI/EcoRV fragment of algZ and its promoter fused to carboxy-terminal S-Tag | |
| pCTX-lacZRTF | Mini-CTXlacZ, containing 1,077 bp of the algR promoter, including RT1 and RT2 | This study |
| pCTX-lacZZTF | Mini-CTXlacZ, containing 796 bp of the algZ promoter, including ZT1 | This study |
| pCTX-lacZRT1 | Mini-CTXlacZ, containing 395 bp upstream of the algR promoter RT1 | This study |
| pCTX-lacZRT2 | Mini-CTXlacZ, containing 422 bp upstream of the algR RT2 promoter | This study |
| −136 | pVDX18 algR::xylE, 110-bp fragment, −27 to −136 (RT1) | This study |
| −423 | pVDX18 algR::xylE, 397-bp fragment, −27 to −423 (RT1-RT2) | This study |
| −802 | pVDX18 algR::xylE, 776-bp fragment, −27 to −802 (RT1-RT2) | This study |
| −1428 | pVDX18 algR::xylE, 1,402-bp fragment, −27 to −1428 (RT1-ZT1) | This study |
| −1877 | pVDX18 algR::xylE, 1,851-bp fragment, −27 to −1877 (RT1-ZT1) | This study |
| pVDX18 | IncQ, AmpR (CbR), mob+ xylE+ | 36 |
| pRK2013 | tra+ mob+ functions, Kmr | 33 |
The PAO1 ΔalgU, PAO1 ΔmucA, and PAO1 rpoS::Gm ΔalgU strains were constructed by cloning an in-frame deletion of algU or mucA into pEXG18Gm or pEX18Tc (35). The in-frame algU deletion was created by overlap extension (OE) PCR (37) using the oligonucleotides algUKO1, algUSOEF, algUSOER, and algUKO4 (see Table S1 in the supplemental material). The in-frame mucA deletion was created by OE PCR (37) using the oligonucleotides mucA-SOE-YO-1, mucA-SOE-YO-2, mucA-SOE-YO-3, and mucA-SOE-YO-4 (see Table S1 in the supplemental material). The pEX18Gm ΔalgU plasmid was conjugated into PAO1, and pEX18Tc ΔalgU plasmid was conjugated into the PAO1 rpoS::Gm strain (SS24 [31]). The pEX18Gm ΔmucA plasmid was conjugated into PAO1. Single recombinants were selected on Vogel-Bonner minimal medium (VBMM) supplemented with the appropriate antibiotic to create merodiploid strains. The merodiploids were resolved by an overnight outgrowth in LB followed by plating on VBMM (38), supplemented with 7.5% sucrose for counterselection. The algU or mucA mutation was confirmed by PCR using oligonucleotides algUKO1 and algU KO4 or mucA-SOE-YO-1 and mucA-SOE-YO-4, respectively, and the nucleotide sequences of the amplicons were determined.
A single algZ–S-Tag gene was integrated at the attB site on the P. aeruginosa chromosomes of the PAO1, PAO1 rpoS::Gm, PAO1 ΔalgU, PAO1 rpoS::Gm ΔalgU, and PAO1 Δvfr strains to create PAO1-Z, PAO1 rpoS::Gm-Z, PAO1 ΔalgU-Z, PAO1 rpoS::Gm ΔalgU-Z, and PAO1 Δvfr-Z strains, respectively. We could not insert this algZ–S-Tag allele into its correct location on the chromosome, because the S-Tag gene would disrupt algR transcription. The algZ gene was cloned by PCR using oligonucleotides algZ-1212FXbaI and S-Tag–HindIIIR and cloned into PCR2.1. The algZ–S-Tag gene was excised by BamHI and EcoRV and subcloned into the mini-CTX vector. The mini-CTX algZ–S-Tag construct includes 1,212 bp of algZ's upstream promoter region and encodes a carboxy-terminal S-Tag (Lys-Glu-Thr-Ala-Ala-Ala-Lys-Phe-Glu-Arg-Gln-His-Met-Asp-Ser). The mini-CTX algZ–S-Tag vector was moved into each strain using the helper conjugative E. coli strain harboring plasmid pRK2013, and single recombinants were selected as tetracycline resistant on PIA plates supplemented with tetracycline (200 μg/ml). The inserted mini-CTX vector was removed from each construct's chromosome by conjugation with pFLP2 and plated on PIA supplemented with carbenicillin. After an overnight outgrowth without antibiotics, individual colonies were counterselected on 10% sucrose. The sucrose-resistant colonies were replica plated to PIA supplemented with tetracycline to confirm tetracycline sensitivity.
S1 nuclease protection assay analysis.
The P. aeruginosa strain PAO1 total cellular RNA for the S1 nuclease protection assay was isolated from cells of cultures grown to stationary phase (optical density at 600 nm [OD600] of 1.0 to 2.0) through a cushion of 5.7 M CsCl as previously described (12) or using the Qiagen RNeasy kit (Qiagen). The S1 nuclease protection assays were performed using 50 to 100 μg of RNA with the following modifications (12). A 1,910-bp PCR amplicon containing upstream sequence of algR using the primers algRS1R and algZko1 (see Table S1 in the supplemental material) was cloned in plasmid pCR2.1 (Invitrogen) and subsequently transferred into M13mp18. This fragment ranges from bp +34 to −1877 upstream of the algR translational start site. Single-stranded phage DNA was isolated and used as the template for the uniformly labeled [α-32P]dCTP DNA probes generated using Klenow. The probes were digested using the appropriate restriction enzymes (New England BioLabs, Ipswich, MA) and purified on a 5% denaturing polyacrylamide-urea gel. The probes were hybridized to the RNA at 67°C for 12 to 24 h in 40 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 6.4), 0.1 mM EDTA (pH 8.0), and 0.4 M NaCl. Hybridizations were treated with 3,000 U of S1 nuclease (Promega) for 60 min at 37°C. A control containing the single-stranded probe alone and S1 nuclease also was performed. Reactions were precipitated and separated on a 6% polyacrylamide-8 M urea gel along with a sequencing ladder generated using the same oligonucleotides as the probes.
Primer extension.
P. aeruginosa total RNA (20 to 30 μg) was isolated as described for the S1 nuclease protection assays. Radiolabeled oligonucleotides algRB5R′ and ZT2-875R (see Table S1 in the supplemental material) were used in reverse transcription reactions using Thermoscript (Invitrogen). A temperature of 55°C was used for the extension. The same oligonucleotides used for primer extension also were used in sequencing reactions with the algZ upstream sequence in pCR2.1 (Invitrogen) using the Sequenase 7-deaza-dGTP kit (USB). Both primer extensions and sequencing reactions were run on a 6% acrylamide-8 M urea gel. The gel was dried and extension products detected using a phosphorimager (Bio-Rad).
Reverse transcriptase PCR (RT-PCR).
Total RNA was isolated from cells grown in 100-ml LB cultures at 37°C and 250 rpm to an OD600 of 2.0 and 2.5 (early and late stationary phases). RNA (250 ng) was reverse transcribed at 50°C using random primers and Superscript II as outlined by the manufacturer (Invitrogen). For PCR amplification of the cDNA, five different primer sets were used to generate 4 amplicons. To test for genomic contamination, oligonucleotides algZ-1212FXbaI and algRGSR6 were used and generated a 2,165-bp fragment. The algR-specific oligonucleotides algRTOPOF and algR-Y99F2-YO-R generated a 304-bp fragment. The algZ-specific oligonucleotides used were algRSF2 and algRSR6 and generated a 611-bp fragment. The algZR-specific oligonucleotides algRSF2 and algR-Y99F2-YO-R were used to detect cDNA that spanned algR and algZ and generated a 1,045-bp fragment. The PCR cycle used was 30 s of denaturation at 94°C, 30 s of annealing at 60°C, and 2 min of elongation at 68°C and was repeated for 30 cycles using the AccuPrime Taq DNA polymerase (Invitrogen) and an optimized buffer of 2.5 mM MgCl2 at pH 8.5.
Transcriptional fusion analysis.
Promoter fragments were generated by PCR using both algRTFRHindIII and algZNotIF2 for algR fusions or algZTFRHindIII and algZ3forward for algZ (see Table S1 in the supplemental material). The algR RT2 promoter fusion was created by PCR using algZNotIF2 and algRP2HindIII to generate a 395-bp product (PAO1 genomic positions 5923976 to 5924370). The −35 region of algR RT2 was changed from 5′-GAACTG-3′ to 5′-TGGAGT-3′ using the mutagenic oligonucleotides AlgUSDM2F and AlgUSDM2R (see Table S1). PCR products were cloned into pCR2.1 and then subcloned into miniCTXlacZ using the restriction enzymes NotI/HindIII (NEB). Fusion constructs were introduced into P. aeruginosa strains by triparental filter conjugation (39). Strains were selected for tetracycline resistance and then conjugated with pFLP2 (35). Strains were selected for carbenicillin resistance, grown overnight without selection, and plated on PIA supplemented with 10% sucrose. To confirm the presence of the fusion constructs, PCR was performed using the forward oligonucleotide used to construct the fusion and the reverse oligonucleotide, lacZR-for-TF (see Table S1). Sonic cell extracts were prepared from strains grown on PIA or LB plates. Total protein was determined by the method of Bradford (Bio-Rad). The kinetic readings at OD420 were measured using a spectrophotometer in cuvettes with a 1-cm light path. The values were calculated based on the following formula: units/mg = (Abs/min)/(ε · [scr1] · P), where Abs/min is the change in OD420 units per minute, ε is the extinction coefficient taken as 4,500 M−1cm−1 at OD420, [scr1] is the light path, and P is the protein amount (0.01 mg) (40). The activity was expressed as mU/mg, where 1 U was defined as the enzymatic activity of β-galactosidase to convert 1 μmol o-nitrophenyl-â-d-galactopyranoside (ONPG) to o-nitrophenol per minute. Each strain was tested with at least three biological replicates.
Five algR::xylE promoter deletion fragments were generated by PCR using algRecoR as the 3′ primer. The following are the 5′ oligonucleotides used to construct the five fusions (see Table S1 in the supplemental material for sequences): algRXbaF1, algRXbaF2, algRXbaF4, algZHindF5, and algZko1. PCR products were cloned into pCR2.1 and then subcloned into pVDX18. Fusion constructs were introduced into P. aeruginosa by triparental filter conjugation (39). Sonic cell extracts were prepared from strains grown on PIA. Total protein was determined by the method of Bradford (Bio-Rad). Catechol 2,3-dioxygenase (CDO) activity was determined as previously described (15). The appropriate strain (PAO1 or PDO300) harboring the empty vector, pVDX18, was assayed and subtracted from the algR::xylE constructs.
Western blot analysis of AlgR and S-tagged AlgZ.
P. aeruginosa strains were grown in liquid LB at 37°C for the times indicated in the figure legends. The bacteria were collected by centrifugation, resuspended in sterile phosphate-buffered saline (PBS), and lysed by sonication. Total protein concentrations were quantified by the Bradford protein assay (Bio-Rad). Ten micrograms of protein from cell extracts was separated by SDS-PAGE on 12% polyacrylamide gels and transferred to a polyvinylidene difluoride membrane (Bio-Rad), and the membranes were blocked overnight in TBST containing 5% skim milk and 1% bovine serum albumin (BSA).
For AlgR Western blotting, the membranes were probed using a 1:2,500 dilution of preabsorbed anti-AlgR rabbit polyclonal antibody followed by a 1:30,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit antibody (41) and detected using the ECL plus kit (GE Healthcare) and a Chemi-doc XRS system (Bio-Rad).
For the AlgZ-S-tagged Western blotting, the membranes were probed using a 1:5,000 dilution of anti-S-Tag mouse monoclonal antibody (Novagen, EMD Chemicals) followed by a 1:20,000 dilution of horseradish peroxidase-conjugated goat anti-mouse antibody (41) and detected using the Clarity Western ECL substrate kit (Bio-Rad) and a Chemi-doc XRS system (Bio-Rad).
RT-qPCR.
For relative real-time quantitative PCR (RT-qPCR) analysis of algR and algZ expression, total RNA was extracted from cultures using RNeasy spin columns (Qiagen) and treated with DNase I (New England BioLabs). cDNA was generated using Superscript II reverse transcriptase and random primers (Invitrogen Life Science Technologies) according to the manufacturer's instructions under the following conditions: 25°C for 10 min, 37°C for 60 min, 42°C for 60 min, and 70°C for 10 min. Real-time PCRs were conducted in a LightCycler 480 instrument with LightCycler 480 probe master mix (Roche Applied Science). Fragments were amplified using primer/probe sets for algR (algR RTq-F, algR RTq-R, and algR probe), algZ (algZ RTq-F, algZ RTq-R, and algZ probe), algZ upstream (algZ Up RTq-F, algZ Up RTq-R, and AlgZ Up probe) and compared to the amplification of rpoD (rpoD RTq-F, rpoD RTq-R, and rpoD probe). Data were analyzed using LightCycler 480 software, release 1.5.0 (Roche Applied Science). Relative expression was normalized to rpoD cDNA levels as the reference gene in each sample.
Relative real-time quantitative PCR analysis also was performed using SYBR green incorporation for quantification of fimU, hemC, and hemY expression in PAO1 and the PAO1 ΔalgZ mutant. RNA isolation was performed from stationary-phase cells grown to an OD600 of 2.5 by using RNeasy plus (Qiagen), and then cDNA was synthesized with Superscript III (Invitrogen) using the recommended protocols. Real-time PCR was performed using a Bio-Rad CFXConnect real-time system with primer pairs shown in Table S1 in the supplemental material and Power SYBR green PCR master mix (Life Technologies). Data were analyzed using CFX Manager 3.1 (Bio-Rad). Results are from three independent experiments performed in triplicate. All samples were normalized to the expression of the reference gene rpoD via the Pfaffl method (40).
RESULTS
Two transcriptional start sites control algR gene expression.
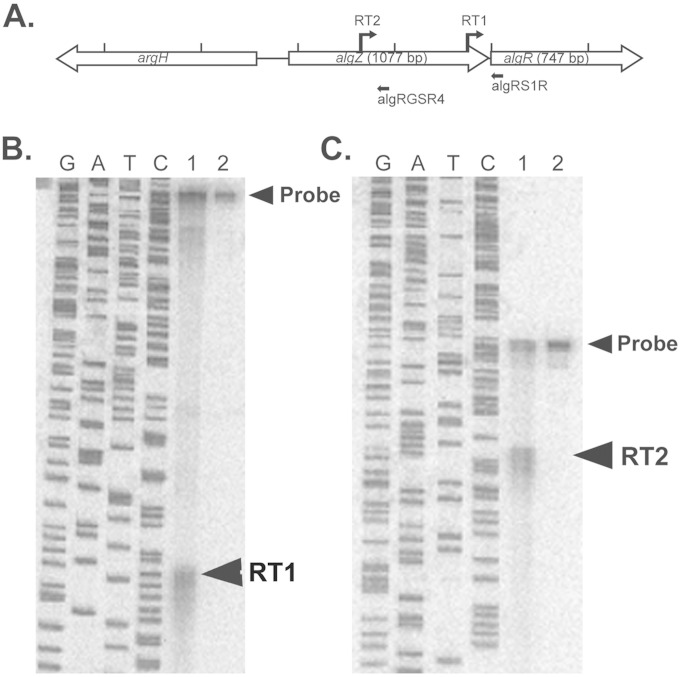
Previous studies demonstrated that algR transcription was activated by the AlgU sigma factor in phenotypically mucoid P. aeruginosa. An AlgU-dependent transcriptional start site for the algR gene was identified 73 bp upstream of the algR open reading frame in mucoid strain PAO568 and a mucA22 cystic fibrosis clinical isolate, FRD1 (4, 10, 12–15). However, algR transcription has not been examined in a wild-type nonmucoid strain, such as PAO1. Therefore, S1 nuclease protection assays were performed using two different oligonucleotides (Fig. 1A), which confirmed the previously identified proximal start site, RT1 (4, 12) (Fig. 1B). In addition, there was evidence that algR is transcribed from at least one other transcriptional start (10, 12, 14). S1 nuclease protection assays were performed to identify these other transcriptional start sites. A band of protection was identified at 698 bp upstream of the algR open reading frame (RT2), located within the algZ gene from cells grown to stationary phase (OD600 of 1.5) in LB-Miller medium (Fig. 1C, lane 1). S1 nuclease protection assays indicated that the algR gene has two transcriptional start sites located 73 and 698 bp upstream from its translational start site (see Fig. S1 in the supplemental material).
FIG 1.
Two transcriptional start sites are located directly upstream of algR. (A) Diagram showing the relative location and orientation of argH (PA5263), algZ (PA5262), and algR (PA5261). The transcriptional start sites for algR identified previously (RT1) and in this paper (RT2) are indicated. Black arrows indicate the location of probes utilized for S1 nuclease protection assays and sequencing reactions. Each tick represents 500 bp. (B) S1 nuclease protection assay of algR transcriptional start site RT1 from nonmucoid PAO1. The four lanes on the left show sequencing reactions with corresponding nucleotides. Lane 1, hybridized probe from PAO1 cDNA; lane 2, S1 probe alone. The upper arrowhead indicates undigested probe, and the lower arrowhead indicates the transcriptional start site. (C) Identification of algR transcriptional start site RT2. Lanes: 1, protected probe; 2, probe alone.
The algZ gene has two transcriptional start sites.
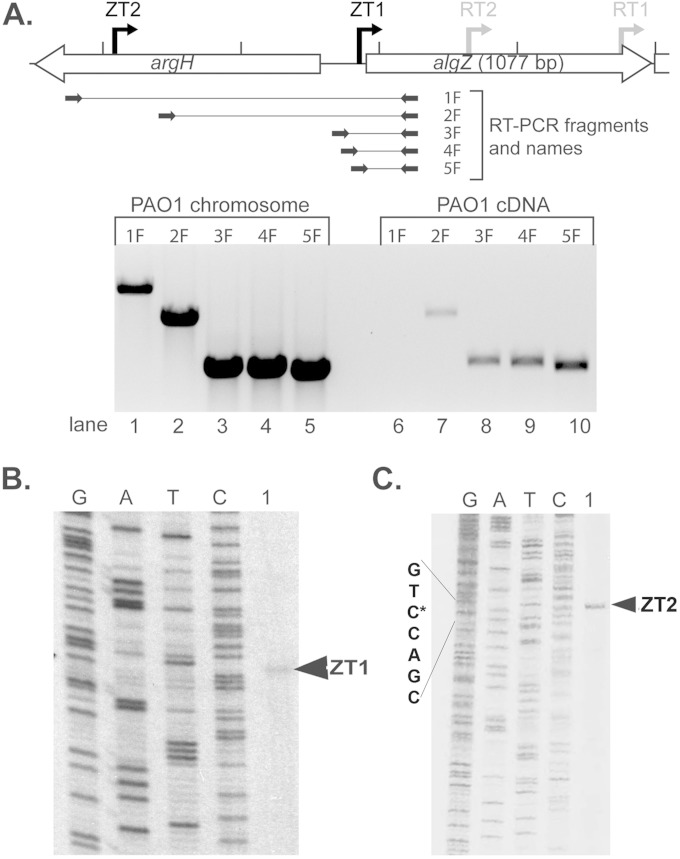
The location of the algZ transcriptional start sites were identified by RT-PCR analysis. An anchoring 3′ oligonucleotide (algRGSR6) within the algZ coding region was used with various 5′ oligonucleotides upstream of algZ (Fig. 2A). PAO1 chromosomal DNA (Fig. 2A, lanes 1 to 5) and no-RT (data not shown) control templates were utilized as positive and negative controls, respectively. RT-PCR results showed that an algZ transcript is present at least 766 bp (Fig. 2A, lane 7) but less than 1.2 kb (Fig. 2A, lane 6) upstream of the algZ open reading frame.
FIG 2.
Two transcriptional start sites are located upstream of algZ. (A) Reverse transcription-PCR to elucidate the transcriptional start site(s) for algZ. (Top) Diagram showing the relative locations of algZ transcriptional start sites identified in this paper (ZT1 and ZT2), as well as the primers (black arrow) and amplicons (solid line) utilized for reverse transcription reactions. (Bottom) Representative agarose gel with amplification of promoter regions from PAO1 genomic template control (lanes 1 to 5) or cDNA generated from PAO1 (lanes 6 to 10). Amplified products 1F (lanes 1 and 6), 2F (lanes 2 and 7), 3F (lanes 3 and 8), 4F (lanes 4 and 9), and 5F (lanes 5 and 10) correspond to those in the top diagram, utilizing the algRGSR6 oligonucleotide in every reaction and the following oligonucleotides for specific reactions: 1F, algZ-1212FXbaI; 2F, algZpromoter-766-F; 3F, algZpromoter-184-F; 4F, algZpromoter-167-F; and 5F, algZpromter-126-F. (B) Primer extension using primer algZ Up RTq-R to identify the first algZ transcriptional start site, ZT1. The four lanes on the left show sequencing reactions with corresponding nucleotides. Lane 1, hybridized probe from PAO1 cDNA. (C) Primer extension using primer ZT2-875R to identify the second algZ transcriptional start site, ZT2.
Primer extension analyses were performed to define the location of the algZ start site using cDNA from 10-h PAO1 broth cultures. A start site was identified 23 bp upstream of the algZ open reading frame (Fig. 2B) and named ZT1. Because the RT-PCR results suggested a larger transcript existed that contained at least 766 bp upstream of algZ (Fig. 2A; also see Fig. S2 in the supplemental material), primer extension also was performed using a primer at bp −875. The second transcriptional start site was identified 1,044 bp upstream of the algZ open reading frame (Fig. 2C; also see Fig. S2) and named ZT2.
The algR gene is cotranscribed with algZ in the nonmucoid and mucoid backgrounds.
The band of full protection that runs with the probe alone in the S1 nuclease protection assay shown in Fig. 1C, and the close proximity of the two genes (separated by 4 bp), led to the hypothesis that algZ and algR are cotranscribed. Deletion of algZ in a mucA22 background increased alginate production (27); therefore, RT-PCR analysis was performed to determine if algZR expression is similar in a different genotype (mucB) that is also phenotypically mucoid and in wild-type strains. Oligonucleotides were designed for RT-PCR to amplify algZ, algR, algZR, and a region beyond the algZ ZT2 promoter (Fig. 3A). RNA isolated from both PAO1 mucB (Fig. 3B, lanes 1 to 4) and wild-type PAO1 (Fig. 3B, lanes 5 to 8) strains were converted to cDNA and compared to a PAO1 chromosomal control (Fig. 3B, lanes 9 to 12). Primer pairs were designed to amplify either the algZ (amplicon termed algZ) (Fig. 3B, lanes 2, 6, and 10) or algR (amplicon termed algR) (Fig. 3B, lanes 3, 7, and 11) gene alone, the 5′ end of algZ into the coding region of algR (amplicon termed algZR) (Fig. 3B, lanes 4, 8, and 12), and a position well upstream of algZ ZT2 into the coding region of algR as a control for genomic DNA contamination (amplicon termed algZR-C) (Fig. 3B, lanes 1, 5, and 9). Amplification indicated that both algZ and algR are expressed and that they also are expressed as a cotranscript in both the mucB and wild-type backgrounds.
FIG 3.
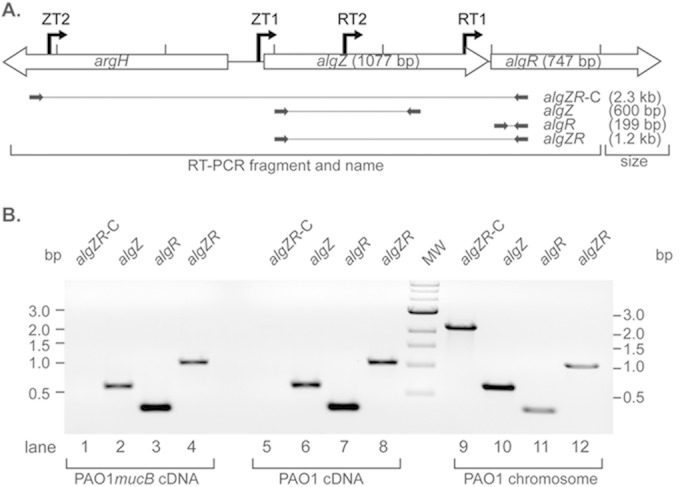
algR and algZ genes are cotranscribed in both the nonmucoid and mucoid backgrounds. (A) Diagram showing the relative location and orientation of argH (PA5263), algZ (PA5262), and algR (PA5261) with the locations of the transcriptional start sites (bent arrows) ZT2, ZT1, RT2, and RT1. Primers (black arrows) and amplicons (solid lines) utilized for reverse transcriptase reactions are indicated. (B) Representative agarose gel with amplification of cDNA generated from the PAO1 mucB strain (lanes 1 to 4), PAO1 (lanes 5 to 8), or PAO1 genomic template control (lanes 9 to 12). The algZ and algR products correspond to the expression of the respective genes; algZR amplifies both in a single transcript, and algZR-C controls for chromosomal contamination.
The algZR genes are cotranscribed with hemCD.
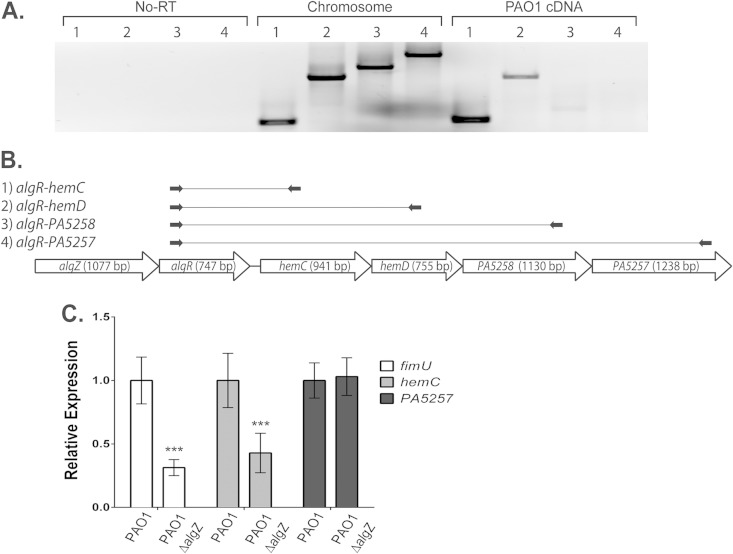
Previous characterization of an algR insertion mutant (CDM5/2) identified the location of the hemC and hemD genes directly downstream of algR (42). Additionally, this study showed that algR transcription was linked to these downstream genes in mucoid strains (the PAO568 mucA22 mutant and a CF clinical isolate) but not a nonmucoid clinical derivative (42). These results indicated that algR is cotranscribed with heme biosynthetic genes in a mucoid but not a nonmucoid background. This discrepancy between mucoid and nonmucoid strains led to further investigation of whether the hemCD genes are cotranscribed with algZR. To test this possibility in PAO1, RNA from stationary-phase cells was converted to cDNA and examined by RT-PCR (Fig. 4). Initially, the transcription from algR to each downstream gene was examined (Fig. 4A and B, amplicon schematic 1 to 4). Figure 4A, lanes 1 and 2, show PCR products generated from PAO1 cDNA, indicating that an mRNA transcript from algR through hemD is expressed at this stage of growth (OD600 of 2.0; early stationary phase). However, Fig. 4A, lanes 3 and 4, do not show clear products generated from PAO1 cDNA, suggesting that this cotranscription does not extend into the downstream genes PA5258 (hemX) and PA5257 (hemY). The separation of expression between hemCD and PA5258 (hemX)-PA5257 (hemY) has been shown previously in a PA14 RNA sequencing (RNA-seq) experiment (43).
FIG 4.
algZR and hemCD genes are cotranscribed in PAO1. (A) Amplification of algR and progressively further downstream genes hemC, hemD, PA5258, and PA5257 from the no-RT control, PAO1 chromosomal DNA, and cDNA resolved in an agarose gel. (B) Diagram showing the locations of oligonucleotide sets used in panels A (oligonucleotide sets 1 to 4) and C (oligonucleotide sets 5 to 9), which use the following oligonucleotides: set 1, algRGSF7 and hemCNR; set 2, algRGSF7 and hemD-R2; set 3, algRGSF7 and PA5258-R2; set 4, algRGSF7 and PA5257-R2. Arrows indicate primers, and solid lines indicate amplicons. (C) RT-qPCR of fimU, hemC, and PA5257 in the PAO1 and PAO1 ΔalgZ mutant backgrounds normalized to rpoD expression.
From our algR transcriptional mapping results (Fig. 1), we now know that the algR RT2 promoter was deleted when the PAO1 ΔalgZ strain (PAZ) was created. In a comparison of PAO1 to the PAO1 ΔalgZ mutant using RT-qPCR, a clear reduction in fimU expression was observed, which was expected, because AlgZR is known to regulate the expression of the fimU operon (Fig. 4C) (20, 44). These experiments also demonstrated that the PAO1 ΔalgZ strain retained 40% of the hemC transcript found in PAO1, whereas the PA5257 (hemY) transcript was unaltered (Fig. 4C). Taken together, algR and hemC transcription are linked according to both qualitative and quantitative assessment measures.
Expression of algR increases throughout growth.
A previous report comparing the transcriptomes of PAO1 and a PAO1 rpoS::aacC1 mutant (via microarray) showed a 2.5-fold reduction in algR expression in the mutant strain, suggesting that algR gene expression is regulated by RpoS in a nonmucoid background (45). This is consistent with a previous study that showed rpoS deletion abrogated mucoidy in FRD1 (31). To investigate the possible involvement of RpoS in AlgR levels, the expression of algR was monitored through the growth curve (Fig. 5). Transcriptional fusion data, utilizing a single-copy chromosomal algR::lacZ fusion, indicated a large increase in algR transcriptional activity from 6 to 16 h of growth and then a subsequently slight increase in expression from 16 to 24 h (Fig. 5A). A more rigorous examination of AlgR protein levels over 16 h showed a corresponding increase in AlgR levels and the CFU/ml of the culture (in Fig. 5B, bars indicate AlgR and the line depicts CFU/ml). When this was evaluated by Western blotting using anti-AlgR antibody and compared to OmlA levels as a loading control, there is a clear increase in the amount of AlgR over time into stationary phase (Fig. 5C).
FIG 5.
AlgR expression increased throughout the growth phase. (A) β-Galactosidase activity of an algR::lacZ fusion chromosomal transcription in PAO1 was measured over time. The fusion contains algR promoters RT1 and RT2 and was assayed in triplicate. (B) AlgR concentrations in PAO1 were quantified by comparison to purified AlgR over time by Western blotting. The left axis depicts CFU per milliliter (line) of culture. The right axis depicts concentration of AlgR (bars; values are ng AlgR). (C) Western blot of LB-grown PAO1 extracts to detect AlgR and OmlA (loading control) at 4, 6, 8, 10, 12, and 14 h. Anti-AlgR, rabbit polyclonal antibody used to detect AlgR; anti-OmlA, rabbit polyclonal antibody used to detect OmlA.
The RT1 and RT2 algR promoters are AlgU dependent.
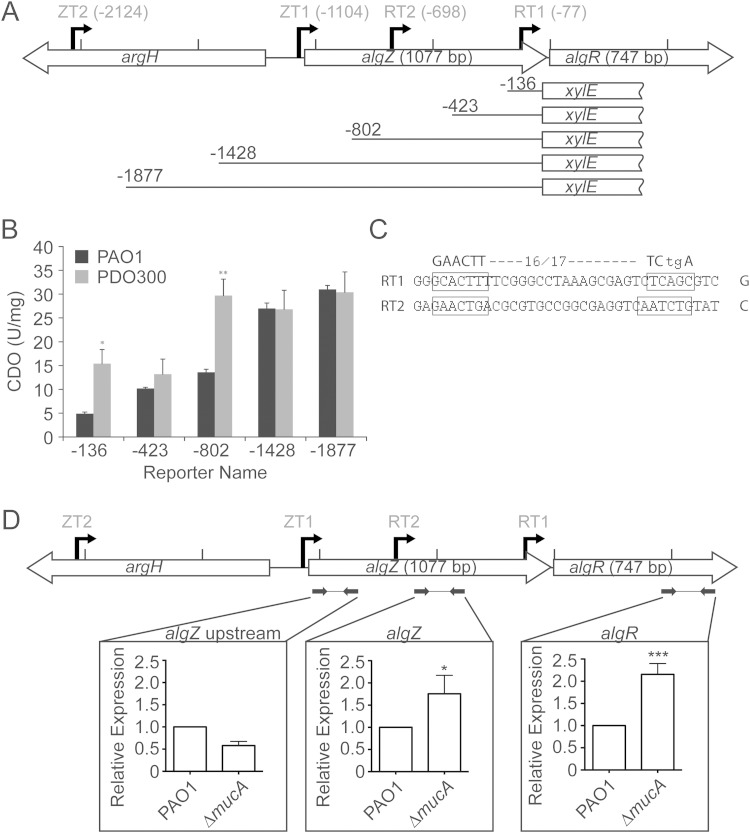
Previous studies identified RT1 as the main AlgU-dependent algR promoter in a mucoid background (12). In light of the newly identified transcriptional start site, RT2, and the detection of an algZ-algR cotranscript, algR transcription was examined by transcriptional reporters with various fragments of the algZR promoter analyzed in PAO1 or mucoid PAO1 mucA22 (strain PDO300) backgrounds. As shown in Fig. 6A, various 5′ primers and a common 3′ anchor were utilized to construct a set of xylE reporter fusions containing the promoter regions for RT1 (−136 and −423), RT1 and RT2 (−802), or RT1, RT2, and ZT1 (−1428 and −1877) (Fig. 6A). In a nonmucoid background, a fusion containing both algR promoters (RT2 and RT1) resulted in a higher level of xylE reporter activity than that of a fusion containing only RT1 (Fig. 6B, −802, −423, and −136). Additionally, a 2-fold increase in transcriptional activity was observed when an algZ promoter (ZT1) was included (Fig. 6B, −1428 versus −802). These results demonstrate that ZT1 may contribute to an algZ-algR cotranscript and enhance algR expression in the nonmucoid background.
FIG 6.
algR promoters RT1 and RT2 are AlgU dependent. (A) Diagram showing the relative location and orientation of argH (PA5263), algZ (PA5262), and algR (PA5261) with the locations of promoter fragments fused to the xylE gene. (B) Expression of XylE from the promoter fragments measured in CDO (U/mg) in both PAO1 and the mucoid strain PDO300. (C) Proposed AlgU binding sites upstream of RT1 and RT2. (D) RT-qPCR performed with cDNA generated from both PAO1 and the PAO1 ΔmucA mutant. Three regions were tested in each background, which included different promoters, including algZ upstream (ZT2 and ZT1), algZ (ZT2, ZT1, and RT2), and algR (ZT2, ZT1, RT2, and RT1). Data were analyzed by analysis of variance (ANOVA). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In the mucoid PDO300 strain (PAO1 containing a mucA22 allele; thus, increased AlgU activity), algR expression from the RT1 promoter was higher than that of wild-type PAO1 (Fig. 6B, −136 and −423). There was also a 2-fold increase in transcriptional activity when RT2 was included in the reporter (Fig. 6B, −423 versus −802), indicating that the RT1 and RT2 promoters are required for maximal transcriptional activity. In addition, the alignment of RT1 and RT2 promoters with the AlgU-binding consensus sequence (13) shows an identifiable AlgU-binding site in both RT1 and RT2 (Fig. 6C; also see Fig. S1 in the supplemental material), an appropriate distance from the transcriptional start site. These results suggest that AlgU activates both RT1 and RT2 in mucA strains and are in agreement with the presently accepted model that AlgU activates algR expression (4, 46). Unlike in PAO1, the addition of ZT1 in the reporter (Fig. 6B, −802 versus −1428) in a mucA22 background failed to significantly increase transcriptional activity. Although a cotranscription product was detected in the mucB background (Fig. 3B, lanes 1 to 4), these transcriptional fusion data suggest that cotranscription is less relevant in a mucA22 background.
To confirm that AlgU activates RT1 and RT2 in a mucA background, RT-qPCR experiments were performed using three primer sets. The following primer set labels were used: (i) algZ upstream measured the contribution of ZT2 and ZT1, (ii) algZ measured the contribution of ZT2, ZT1, and RT2, and (iii) algR measured the contribution of all four promoters (Fig. 6D). The relative abundances of transcripts of the PAO1 ΔmucA strain was compared to those of PAO1. The primer sets termed algZ and algR showed an approximately 2-fold increase in transcript levels in the ΔmucA strain compared to those of the wild type, which would be expected if the algR promoters are regulated by AlgU. The relative amount of algZ upstream transcript was lower in the ΔmucA strain than in the wild type, but this decrease was not statistically significant. Because the algZ upstream primer set only measured the contributions of ZT2 and ZT1 and both the algZ and algR primer sets include algR promoters, the increased algR transcript in the ΔmucA background was due primarily to the contributions of both RT2 and RT1.
Transcription of algR in the nonmucoid background is directly AlgU dependent and additionally regulated by RpoS.
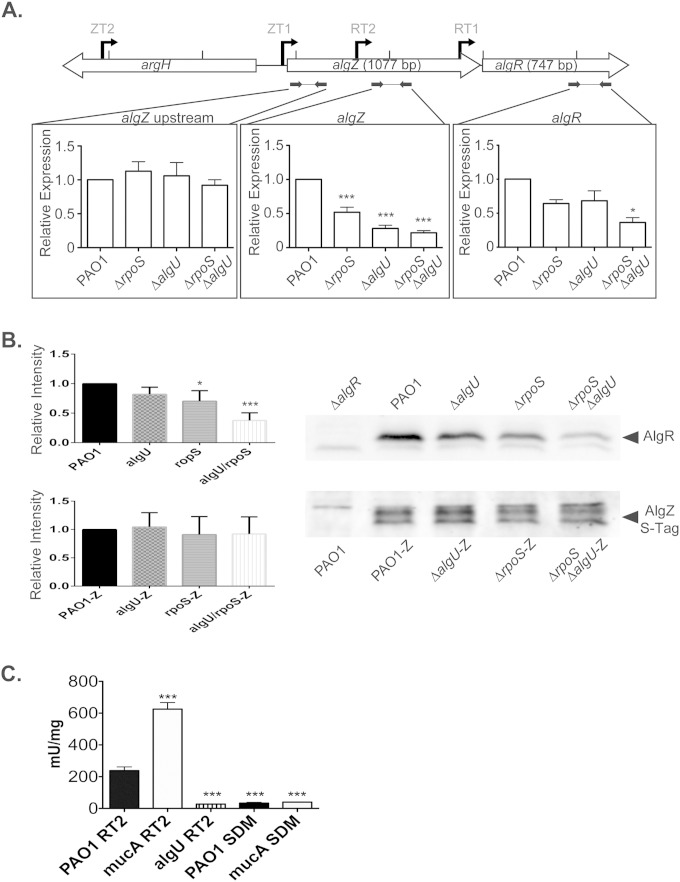
To further investigate the possibility of RpoS involvement and confirm a role for AlgU in algR expression, RT-qPCR was performed using three primer sets (as described for Fig. 6D) to evaluate algZR expression in (i) the PAO1 wild type and the (ii) PAO1ΔalgR, (iii) PAO1 rpoS::Gm, (iv) PAO1ΔalgU, and (v) PAO1 rpoS::Gm ΔalgU mutants (Fig. 7). Comparison of the transcript levels in the PAO1 rpoS::Gm strain to those in wild-type PAO1 revealed that transcript levels downstream of RT2 (primer set algZ) were reduced to 52%, while transcript levels downstream of RT1 (primer set algR) were reduced to 64% relative to the wild type (Fig. 7A). On the other hand, there were no differences in transcript levels downstream of ZT1 and ZT2 (primer set algZ upstream) for all strains tested. Together, these results suggest that RpoS affects RT1 and RT2 transcription either through direct regulation or indirectly through AlgU but does not affect the transcription from the ZT1 and ZT2 promoters.
FIG 7.
Transcription dependence of algR on both rpoS and algU in the nonmucoid background. (A) RT-qPCR performed with cDNA generated from PAO1 and the mutant strains rpoS::Gm, ΔalgU, and rpoS::Gm ΔalgU in the PAO1 background. Three regions were tested in each background, which included different start sites for different genes, algZ upstream (ZT2 and ZT1), algZ (ZT2, ZT1, and RT2), and algR (ZT2, ZT1, RT2, and RT1). ns, not statistically significant compared to PAO1. (B) Densitometry and representative Western blotting for expression of AlgR in the tested backgrounds, as well as the PAO1ΔalgR mutant, and for the expression of AlgZ in the tested backgrounds containing a single copy of algZ with a fused S-Tag and a PAO1 negative control. Densitometry was measured as fluorescent intensity relative to PAO1 and was averaged from four blots. (C) β-Galactosidase activity of an algR::lacZ fusion chromosomal transcription in PAO1 was measured over time. The RT2 fusion contains the algR RT2 promoter on the chromosomes of the PAO1 (PAO1 RT2), PAO1 ΔmucA (mucA RT2), and PAO1 ΔalgU (algU RT2) strains. The −35 region of RT2 was mutated from 5′-GAACTG-3′ to 5′-ACTCCA-3′ in the RT2 fusion, termed SDM, and conjugated into PAO1 (PAO1 SDM, and the PAO1 ΔmucA mutant (mucA SDM). All of the constructs were assayed in triplicate. Data were analyzed by ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In the ΔalgU background, transcript levels from RT2 also were reduced to 28% of the wild type (primer set algZ), supporting the findings shown in Fig. 6D. Interestingly, algR transcript levels downstream of both RT2 and RT1 (primer set algZ and algR) were further reduced in the rpoS::Gm ΔalgU double mutant compared to the wild type (Fig. 7A). AlgR protein levels then were examined in (i) PAO1 and the mutant (ii) PAO1ΔalgR, (iii) PAO1 rpoS::Gm, (iv) PAO1ΔalgU, and (v) PAO1 rpoS::Gm ΔalgU strains (Fig. 7B) to support the RT-qPCR data. In agreement with such data, AlgR protein levels were noticeably reduced in the rpoS::Gm ΔalgU background compared to those in wild-type PAO1 at an OD600 of 2.1 (Fig. 7B) and OD600 of 2.5 (data not shown). These results indicate that both AlgU and RpoS are required for maximal expression of AlgR in the nonmucoid background. As transcript levels from the ZT1 and ZT2 promoters (primer set algZ upstream) were not reduced in the backgrounds examined by RT-qPCR, Western blotting additionally was performed using a chromosomal single-copy AlgZ expressing a C-terminal S-Tag and a monoclonal anti-S-Tag antibody to confirm expression levels. The AlgZ protein levels were unaffected in these backgrounds, confirming the RT-qPCR results that transcription of algZ is neither RpoS nor AlgU dependent (Fig. 7B). To determine if AlgU controlled transcription from the RT2 promoter, a 413-bp transcriptional fusion containing only RT2 was assayed in PAO1 and the PAO1 mucA22 strain. As indicated in Fig. 7C, there was a 2.6-fold increase in β-galactosidase activity in the mucA22 background compared to PAO1 for this construct. When this fusion was tested in the PAO1 ΔalgU strain, there was an 8-fold decrease in β-galactosidase activity compared to that of wild-type PAO1. Site-directed mutagenesis was performed on the −35 region of the RT2 transcriptional fusion to mutate the AlgU −35 binding site (from 5′-GAACTG-3′ to 5′-ACTCCA-3′; see Fig. S1 in the supplemental material) and assayed in various strains. This site-directed AlgU −35 binding site mutant exhibited significantly decrease β-galactosidase activity compared to both the PAO1 (8-fold) and the mucA22 mutant (15-fold) backgrounds. Together with the data from Fig. 6, these data indicate that AlgU controls the expression from the RT2 algR promoter located within the algZ coding region.
Expression of algZ is Vfr dependent.
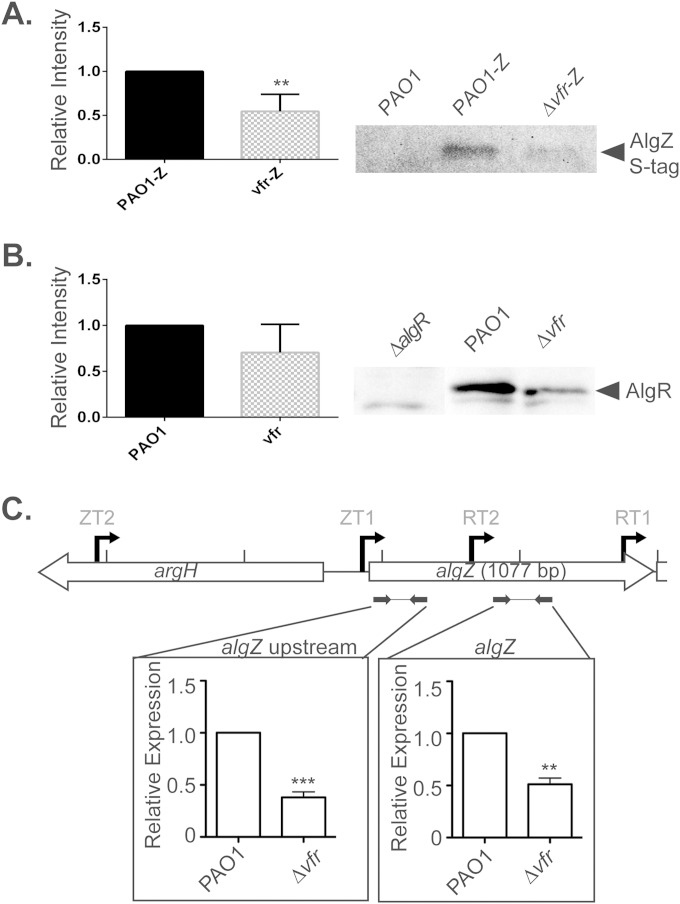
While the expression of algZ was unaffected in the tested sigma factor backgrounds shown in Fig. 7, previous reports suggested that there is a transcriptional link between Vfr and algZR, where Kanack et al. identified a putative Vfr binding site upstream of algZ ZT1 (see Fig. S2, boxed sequence, in the supplemental material). It also has been demonstrated that elevated levels of AlgR repress vfr transcription, resulting in the downregulation of the Vfr-dependent virulence factor regulon (17, 47, 48). To determine whether Vfr regulates algZ transcription, transcriptional reporters utilizing either the first algZ promoter (ZT1) or both algR promoters (RT1 and RT2) were constructed and integrated in single copy into wild-type PAO1 or Δvfr mutant backgrounds (Table 2). Reporter activity from the algR promoter was unaffected in the Δvfr mutant, indicating that Vfr is not required for its expression alone. On the other hand, reporter activity from the algZ ZT1 promoter was significantly reduced in the Δvfr background to undetectable levels compared to the activity of wild-type PAO1. This indicates that while Vfr has no direct regulatory role on RT1 and RT2, the algZ ZT1 promoter is substantially, if not completely, dependent upon Vfr for expression, which is consistent with the previously identified Vfr binding site upstream of algZ ZT1 (17).
TABLE 2.
Vfr controls algZ expression
| Strain | Chromosomal fusiona | β-Galactosidase activity (mU/mg) |
|---|---|---|
| PAO1 RTF | algR::lacZb | 499 ± 51 |
| PAO1 vfr RTF | algR::lacZ | 494 ± 32 |
| PAO1 ZTF | algZ::lacZc | 164 ± 24 |
| PAO1 vfr ZTF | algZ::lacZ | NDd |
Transcriptional fusions. β-Galactosidase activity was recorded as mU/mg of protein and was averaged for three separate experiments. Data are represented as mU/mg ± standard error.
algR::lacZ promoter fusion of 1,077 bp inserted at the chromosomal attB site.
algZ::lacZ promoter fusion of 796 bp inserted at the chromosomal attB site.
ND, not detected.
Western blotting and RT-qPCR were performed to examine AlgR, AlgZ, and AlgZR expression levels in PAO1 and PAO1 Δvfr strains. Western blot analysis using anti-S-Tag antibody directed to AlgZ expressing a C-terminal S-Tag and anti-AlgR polyclonal sera against cell extracts from PAO1 and PAO1 Δvfr strains showed decreased AlgZ and AlgR levels in the Δvfr mutant background (Fig. 8A and B). Confirmatory RT-qPCR performed on both PAO1 and PAO1 Δvfr strains indicated that transcript levels from ZT1 and ZT2 (algZ upstream) were reduced 62%, and transcript levels from ZT1, ZT2, and RT2 (algZ) were reduced by 49% in the PAO1 Δvfr mutant (Fig. 8C). Collectively, these data indicate that Vfr heavily influences the expression of algZ. Nevertheless, algZ expression is not totally abolished by the absence of Vfr, suggesting that AlgZ expression from the ZT2 promoter is controlled by a different transcription factor and accounts for approximately 40% of algZ transcription at late stationary phase (OD600 of 2.5). Diminished algR expression likely is due to reduced cotranscription, because the PAO1 Δvfr mutant showed no detectable decrease in algR promoter activity (Table 2). Moreover, expression levels measured by RT-qPCR increased when the fragment tested moved from only including ZT1 and ZT2 (algZ upstream) to including RT2 (algZ) (Fig. 8C).
FIG 8.
Vfr dependence of algZR transcription. (A) Densitometry and representative Western blotting for expression of AlgZ and AlgR in the PAO1 and mutant PAO1 ΔalgR and PAO1 Δvfr backgrounds and of AlgZ in the PAO1-Z and mutant PAO1 Δvfr-Z backgrounds containing a single copy of algZ with a fused S-Tag and a PAO1 negative control. Densitometry was measured as fluorescent intensity relative to that of PAO1 averaged from four blots. (B) RT-qPCR performed with cDNA generated from PAO1 and PAO1 Δvfr strains. Two regions were tested in each background and included different promoters, algZ upstream (ZT2 and ZT1) and algZ (ZT2, ZT1, and RT2). Data were analyzed by ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
As a pathogen, P. aeruginosa is extremely versatile and survives in a wide variety of niches. P. aeruginosa is capable of rapidly responding to changes in its environment by the expression of the most appropriate genes and virulence factors for a particular environment. To maintain this responsiveness, layers of regulation are employed, including two-component regulatory systems (18), alternative sigma factors (49), quorum-sensing systems (50, 51), regulatory RNAs (52), and a variety of more complex and specialized regulatory elements (53). The expression of virulence determinants is especially tightly controlled to prevent unnecessary expression of metabolically burdening systems, such as the exopolysaccharide alginate (54–57). One of the transcriptional regulators that controls alginate overproduction as well as other virulence factors, such as type IV pili, the type III secretion system, and rhamnolipid and cyanide production, is the response regulator AlgR (58). The proposed histidine kinase for AlgR, AlgZ (FimS), is required for type IV pili (25) function but also modulates alginate and rhamnolipid production by two- to threefold (24, 27). Consequently, determining the transcriptional regulators that control the AlgZR two-component regulatory system provides insights into the functions of many different P. aeruginosa virulence determinants.
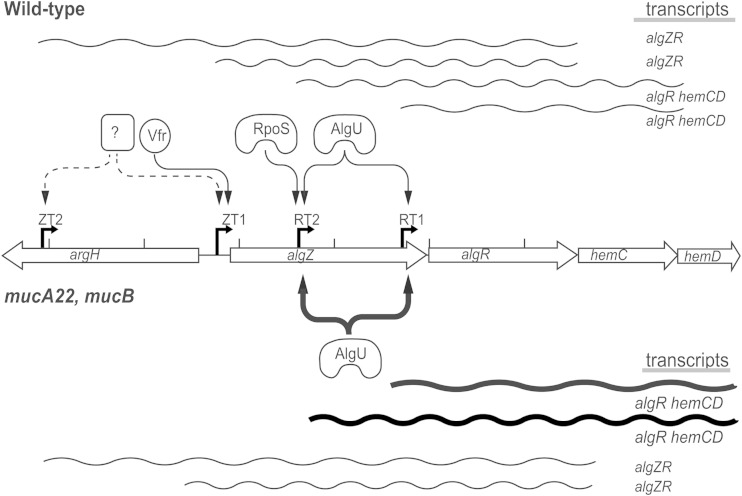
The algZR transcriptional analysis revealed that multiple regulatory layers control this two-component regulatory system (Fig. 9). This study identified four transcriptional start sites, three of which were previously unknown. While an algR transcriptional start site dependent upon AlgU was identified previously in the mucoid strain PAO568 and the clinical isolate FRD1 (4, 12), it had not been examined previously in a wild-type background. There also have been prior indications that algR is transcribed from at least one additional transcriptional start site (12, 14). Using primer extensions and S1 nuclease protection assays with the wild-type strain PAO1, the previously identified transcriptional start site (RT1), located at 73 bp upstream from the translational start site, was confirmed, and a newly identified start site was found 698 bp upstream of the algR open reading frame within the algZ gene (RT2). Previous data suggested a role for RpoS in algR expression based on microarray analysis (31). Our data are consistent with this study and showed increased AlgR expression in the stationary phase of growth (Fig. 5). However, when RT-qPCR was performed to examine expression from ZT1 and ZT2 (algZ upstream); ZT1, ZT2, and RT2 (algZ); and all four promoters (algR) in various sigma factor deletion strains, it appears that RpoS affects algR expression, either directly by activating RT1 and/or RT2 or indirectly, potentially through AlgU (Fig. 7). Interestingly, it appears that RpoS has no effect on algZ expression (Fig. 7A and B). Evidence that expression from RT2 is directly AlgU dependent includes (i) decreased algR expression in the PAO1 ΔalgU mutant (Fig. 7A); (ii) decreased AlgR levels in the PAO1 ΔalgU mutant (Fig. 7B); (iii) significantly decreased RT2 expression when the promoter was isolated and examined in the PAO1 ΔalgU mutant (Fig. 7C); (iv) increased RT2 expression in the PAO1 mucA22 mutant (Fig. 7C); and (v) abrogation of algR expression when the AlgU −35 region was mutated in strains tested (Fig. 7C). While control of RT1 by AlgU in a mucA background was known previously, the control of both RT1 and RT2 by AlgU in the wild-type PAO1 background is a bit more surprising and shows that this sigma factor may play a larger role in gene regulation in wild-type P. aeruginosa than previously appreciated. Interestingly, the algR promoter is the second promoter region identified where AlgU controls two different start sites within the same gene, the other promoter being algU itself (59). The reason for two independent start sites within the same gene promoters that are controlled by the same sigma factor is unknown. Perhaps there are differences in promoter affinity that respond to increased levels of the sigma factor. However, both of the AlgU-dependent start sites in the algU and algR promoters were discovered in PAO1, indicating that increased AlgU levels were not required to initiate transcription from both promoters. While the role of AlgU in wild-type P. aeruginosa has not been examined as thoroughly as in the mucoid backgrounds, it is known to play a regulatory role, since about 33% of AlgU present in nonmucoid cells is free in the cytoplasm (60, 61). Interestingly, the PAO1 ΔalgU strain does not display the twitching motility phenotype, which has been explained by the reduced expression of both lectin-encoding genes lecA and lecB, since LecB is required for type IV pilus biogenesis (62). Our data suggest that the deletion of algU would decrease AlgR expression at least 2-fold, which likely would abrogate twitching motility through decreased fimU expression. The algR deletion strain also is impaired in twitching motility (20, 25).
FIG 9.
Model of the regulation and expression of the algZR system. The diagram shows the relative location and orientation of argH (PA5263), algZ (PA5262), algR (PA5261), hemC (PA5260), and hemD (PA5259), with the transcriptional start site locations of ZT2, ZT1, RT2, and RT1 indicated by bent arrows. Transcripts and regulatory elements are split horizontally between regulation in wild-type cells and mucoid (mucA22, mucB) cells. In wild-type cells, the regulation of RT1 and RT2 by AlgU (Fig. 7A and C), RT2 by RpoS (Fig. 7A), and ZT1 by Vfr (Fig. 8) are indicated by solid lines, with dashed lines indicating additional unknown elements regulating ZT2 and possibly ZT1. Above the regulatory elements are several transcripts produced by cotranscription from several promoters extending into hemCD (Fig. 4). In mucoid cells, the regulation of RT1 and RT2 is heavily dependent upon AlgU, as indicated by line thickness, and the regulation of ZT1 and ZT2 is unknown in this background, although algZ transcripts are detectable (Fig. 3B). The transcripts of algR are shown with thicker lines, as the mucA background was shown to have higher levels of algR transcripts by RT-qPCR (Fig. 6D).
Little has been reported on algZ transcriptional regulation. After identifying the furthest upstream transcriptional end, primer extensions were performed and identified two transcriptional start sites at bp −23 (ZT1) and −1044 (ZT2; numbering relative to the translational start site). Unlike the algR expression that was found to be AlgU and RpoS dependent, levels of expression from ZT1, ZT2, and AlgZ were unaffected when these sigma factors were not present. Although algZ expression was unaffected in the tested sigma factor deletion backgrounds, further experimentation identified stationary-growth-phase dependence on the transcriptional factor Vfr for AlgZ through ZT1 (Fig. 8). Evidence that ZT1 is Vfr dependent includes (i) decreased algZ transcription levels from ZT1 and ZT2 by 62% in the Δvfr mutant background relative to the wild-type PAO1 (Fig. 8C); (ii) reduced AlgZ and AlgR levels according to Western blot analysis (Fig. 8A and B); and (iii) abrogation of transcriptional activity from an isolated ZT1 algZ promoter fusion in the PAO1 Δvfr mutant (Table 2). Another indication that Vfr controls AlgZ is the presence of a Vfr binding site (see Fig. S2 in the supplemental material) upstream of ZT1 identified by Kanack et al. (17).
Vfr is a member of the 3′,5′-cAMP receptor protein (CRP) family of transcriptional regulators involved in regulating a variety of virulence phenotypes in P. aeruginosa (16). These virulence determinants include exotoxin A production, type IV pili, type III secretion, the las quorum-sensing system, and flagellar expression (47, 63–66). Interestingly, AlgR controls two of these same phenotypes, specifically type IV pili (20, 25, 44) and the type III secretion system (48, 67), but it does so through different genes than those controlled by Vfr. Interplay between AlgR and Vfr was shown previously when AlgR indirectly suppressed cAMP/Vfr-dependent (CVS) signaling in a mucA22 background (48). Deletion of algZR or algU in a mucA22 mutant restored CVS activity to wild-type levels, as measured through a synthetic E. coli lac P1 strain fused to lacZ. In support of these data, Vfr levels returned to normal in the same mutants, and plasmid-borne overexpression of algZR alone suppressed Vfr levels. Here, we show that AlgR-Vfr regulation is reciprocal in that Vfr directly controls expression from the algZ ZT1 promoter and algZR expression in a wild-type background. While ZT1 contains an adjacent Vfr binding site, the ZT2 start site further upstream contains no such consensus sequence. Our RT-qPCR and Western analyses showed reduction but not total loss of algZ and AlgZ expression in the Δvfr mutant background. These results indicate that Vfr does not influence expression from ZT2, but it is likely controlled by another transcriptional regulator. Further experiments are necessary to identify such a regulator(s). We have observed algZ expression in multiple growth phases and have not observed any conditions that result in a total loss of expression (data not shown). These data suggest that the expression from ZT2 is constitutive, like that through RpoD.
The analysis of the algZR transcript indicated that these genes also are expressed as a cotranscript even in the mucoid background, where the AlgU-dependent algR promoters seem to dominate. The cotranscription of algZR has been reported in a PA14 RNA-seq experiment (43). This same study showed that hemCD and PA5258 (hemX)-PA5257 (hemY) are two separate transcriptional units. The differences between our data and that reported previously may be strain dependent, as our studies were conducted in PAO1 and the RNA-seq data are from PA14. However, the RNA-seq data may be a reflection of the most prevalent transcript identified. The cotranscription of algR-hemC previously has been demonstrated in a mucA22 background, and another transcriptional start site has been mapped between algR and hemC by others and us, but nothing else was known (21, 42, 43). Our data suggest that algZR is at least transcriptionally connected to heme biosynthesis and also plays a role in controlling heme levels in P. aeruginosa. Data that support this suggestion include alterations in the regulation of catalase and cytochrome oxidase genes observed in our microarray studies where the PAO1 algR::Tcr mutant was compared to PAO1 (20, 23).
The AlgZR system is known to be involved in regulating a considerable number of virulence factors, so fully elucidating the regulation of this system is vital for fully understanding their physiological role in both virulence and the biology of the organism as a whole. Transcriptional control by at least three distinct regulatory elements, Vfr, RpoS, and AlgU, allows for highly regulated expression based on several environmental conditions. However, several questions remain. (i) How is ZT2 regulated? (ii) What is the function of the large 1-kb noncoding intergenic region between ZT2 and the start of algZ translation? (iii) What is the biological significance of tying algZ expression to cAMP levels through Vfr? (iv) What is the biological significance of algZR and hemCD cotranscription?
ACKNOWLEDGMENTS
This work was supported by funding from NIH grant 1R01 AI050812-01 and 1R21 AI 1094487 to M.J.S. and NIH training grant 2T32 AI 052066 to Y.O. Funding also was provided in part by RDC grant 12-025M to C.L.P.
We thank Matthew Wolfgang for providing the PAO1 Δvfr strain. We thank Michael Vasil for providing the anti-OmlA antibody and critical reading of the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02290-14.
REFERENCES
- 1.Bodey GP, Bolivar R, Fainstein V, Jadeja L. 1983. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis 5:279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- 2.Berghmans T, Sculier JP, Klastersky J. 2003. A prospective study of infections in lung cancer patients admitted to the hospital. Chest 124:114–120. doi: 10.1378/chest.124.1.114. [DOI] [PubMed] [Google Scholar]
- 3.Santucci SG, Gobara S, Santos CR, Fontana C, Levin AS. 2003. Infections in a burn intensive care unit: experience of seven years. J Hosp Infect 53:6–13. doi: 10.1053/jhin.2002.1340. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak DJ, Ohman DE. 1994. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J Bacteriol 176:6007–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVries CA, Ohman DE. 1994. Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternate sigma factor, and shows evidence for autoregulation. J Bacteriol 176:6677–6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin DW, Holloway BW, Deretic V. 1993. Characterization of a locus determining the mucoid status of Pseudomonas aeruginosa: AlgU shows sequence similarities with a Bacillus sigma factor. J Bacteriol 175:1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schurr MJ, Yu H, Martinez-Salazar JM, Boucher JC, Deretic V. 1996. Control of AlgU, a member of the sigma E-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J Bacteriol 178:4997–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie ZD, Hershberger CD, Shankar S, Ye RW, Chakrabarty AM. 1996. Sigma factor-anti-sigma factor interaction in alginate synthesis: inhibition of AlgT by MucA. J Bacteriol 178:4990–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deretic V, Dikshit R, Konyecsni WM, Chakrabarty AM, Misra TK. 1989. The algR gene, which regulates mucoidy in Pseudomonas aeruginosa, belongs to a class of environmentally responsive genes. J Bacteriol 171:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohr CD, Deretic V. 1990. Gene-scrambling mutagenesis: generation and analysis of insertional mutations in the alginate regulatory region of Pseudomonas aeruginosa. J Bacteriol 172:6252–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielinski NA, Maharaj R, Roychoudhury S, Danganan CE, Hendrickson W, Chakrabarty AM. 1992. Alginate synthesis in Pseudomonas aeruginosa: environmental regulation of the algC promoter. J Bacteriol 174:7680–7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin DW, Schurr MJ, Yu H, Deretic V. 1994. Analysis of promoters controlled by the putative sigma factor AlgU regulating conversion to mucoidy in Pseudomonas aeruginosa: relationship to sigma E and stress response. J Bacteriol 176:6688–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firoved AM, Boucher JC, Deretic V. 2002. Global genomic analysis of AlgU (sigma(E))-dependent promoters (Sigmulon) in Pseudomonas aeruginosa and implications for inflammatory processes in cystic fibrosis. J Bacteriol 184:1057–1064. doi: 10.1128/jb.184.4.1057-1064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr CD, Martin DW, Konyecsni WM, Govan JR, Lory S, Deretic V. 1990. Role of the far-upstream sites of the algD promoter and the algR and rpoN genes in environmental modulation of mucoidy in Pseudomonas aeruginosa. J Bacteriol 172:6576–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deretic V, Konyecsni WM. 1989. Control of mucoidy in Pseudomonas aeruginosa: transcriptional regulation of algR and identification of the second regulatory gene, algQ. J Bacteriol 171:3680–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West SE, Sample AK, Runyen-Janecky LJ. 1994. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J Bacteriol 176:7532–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanack KJ, Runyen-Janecky LJ, Ferrell EP, Suh SJ, West SE. 2006. Characterization of DNA-binding specificity and analysis of binding sites of the Pseudomonas aeruginosa global regulator, Vfr, a homologue of the Escherichia coli cAMP receptor protein. Microbiology 152:3485–3496. doi: 10.1099/mic.0.29008-0. [DOI] [PubMed] [Google Scholar]
- 18.Sivaneson M, Mikkelsen H, Ventre I, Bordi C, Filloux A. 2011. Two-component regulatory systems in Pseudomonas aeruginosa: an intricate network mediating fimbrial and efflux pump gene expression. Mol Microbiol 79:1353–1366. doi: 10.1111/j.1365-2958.2010.07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carterson AJ, Morici LA, Jackson DW, Frisk A, Lizewski SE, Jupiter R, Simpson K, Kunz DA, Davis SH, Schurr JR, Hassett DJ, Schurr MJ. 2004. The transcriptional regulator AlgR controls cyanide production in Pseudomonas aeruginosa. J Bacteriol 186:6837–6844. doi: 10.1128/JB.186.20.6837-6844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lizewski SE, Schurr JR, Jackson DW, Frisk A, Carterson AJ, Schurr MJ. 2004. Identification of AlgR-regulated genes in Pseudomonas aeruginosa by use of microarray analysis. J Bacteriol 186:5672–5684. doi: 10.1128/JB.186.17.5672-5684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lizewski SE, Lundberg DS, Schurr MJ. 2002. The transcriptional regulator AlgR is essential for Pseudomonas aeruginosa pathogenesis. Infect Immun 70:6083–6093. doi: 10.1128/IAI.70.11.6083-6093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cody WL, Pritchett CL, Jones AK, Carterson AJ, Jackson D, Frisk A, Wolfgang MC, Schurr MJ. 2009. Pseudomonas aeruginosa AlgR controls cyanide production in an AlgZ dependent manner. J Bacteriol 191:2993–3002. doi: 10.1128/JB.01156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morici LA, Carterson AJ, Wagner VE, Frisk A, Schurr JR, Zu Bentrup KH, Hassett DJ, Iglewski BH, Sauer K, Schurr MJ. 2007. Pseudomonas aeruginosa AlgR represses the Rhl quorum-sensing system in a biofilm-specific manner. J Bacteriol 189:7752–7764. doi: 10.1128/JB.01797-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okkotsu Y, Tieku P, Fitzsimmons LF, Churchill ME, Schurr MJ. 2013. Pseudomonas aeruginosa AlgR phosphorylation modulates rhamnolipid production and motility. J Bacteriol 195:5499–5515. doi: 10.1128/JB.00726-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitchurch CB, Alm RA, Mattick JS. 1996. The alginate regulator AlgR and an associated sensor FimS are required for twitching motility in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 93:9839–9843. doi: 10.1073/pnas.93.18.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitchurch CB, Erova TE, Emery JA, Sargent JL, Harris JM, Semmler AB, Young MD, Mattick JS, Wozniak DJ. 2002. Phosphorylation of the Pseudomonas aeruginosa response regulator AlgR is essential for type IV fimbria-mediated twitching motility. J Bacteriol 184:4544–4554. doi: 10.1128/JB.184.16.4544-4554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Mudd M, Boucher JC, Schurr MJ, Deretic V. 1997. Identification of the algZ gene upstream of the response regulator algR and its participation in control of alginate production in Pseudomonas aeruginosa. J Bacteriol 179:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohr CD, Hibler NS, Deretic V. 1991. AlgR, a response regulator controlling mucoidy in Pseudomonas aeruginosa, binds to the FUS sites of the algD promoter located unusually far upstream from the mRNA start site. J Bacteriol 173:5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohr CD, Leveau JH, Krieg DP, Hibler NS, Deretic V. 1992. AlgR-binding sites within the algD promoter make up a set of inverted repeats separated by a large intervening segment of DNA. J Bacteriol 174:6624–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holloway BW. 1955. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol 13:572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- 31.Suh SJ, Silo-Suh L, Woods DE, Hassett DJ, West SE, Ohman DE. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol 181:3890–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathee K, Ciofu O, Sternberg C, Lindum PW, Campbell JI, Jensen P, Johnsen AH, Givskov M, Ohman DE, Molin S, Hoiby N, Kharazmi A. 1999. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 145(Part 6):1349–1357. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 33.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoang TT, Kutchma AJ, Becher A, Schweizer HP. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- 35.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 36.Konyecsni WM, Deretic V. 1988. Broad-host-range plasmid and M13 bacteriophage-derived vectors for promoter analysis in Escherichia coli and Pseudomonas aeruginosa. Gene 74:375–386. doi: 10.1016/0378-1119(88)90171-0. [DOI] [PubMed] [Google Scholar]
- 37.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 38.Vogel HJ, Bonner DM. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106. [PubMed] [Google Scholar]
- 39.Deretic V, Govan JR, Konyecsni WM, Martin DW. 1990. Mucoid Pseudomonas aeruginosa in cystic fibrosis: mutations in the muc loci affect transcription of the algR and algD genes in response to environmental stimuli. Mol Microbiol 4:189–196. doi: 10.1111/j.1365-2958.1990.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 40.Skoneczny M, Rytka J. 2000. Oxygen and haem regulate the synthesis of peroxisomal proteins: catalase A, acyl-CoA oxidase and Pex1p in the yeast Saccharomyces cerevisiae; the regulation of these proteins by oxygen is not mediated by haem. Biochem J 350(Part 1):313–319. doi: 10.1042/0264-6021:3500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deretic V, Leveau JH, Mohr CD, Hibler NS. 1992. In vitro phosphorylation of AlgR, a regulator of mucoidy in Pseudomonas aeruginosa, by a histidine protein kinase and effects of small phospho-donor molecules. Mol Microbiol 6:2761–2767. doi: 10.1111/j.1365-2958.1992.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 42.Mohr CD, Sonsteby SK, Deretic V. 1994. The Pseudomonas aeruginosa homologs of hemC and hemD are linked to the gene encoding the regulator of mucoidy AlgR. Mol Gen Genet 242:177–184. doi: 10.1007/BF00391011. [DOI] [PubMed] [Google Scholar]
- 43.Wurtzel O, Yoder-Himes DR, Han K, Dandekar AA, Edelheit S, Greenberg EP, Sorek R, Lory S. 2012. The single-nucleotide resolution transcriptome of Pseudomonas aeruginosa grown in body temperature. PLoS Pathog 8:e1002945. doi: 10.1371/journal.ppat.1002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belete B, Lu H, Wozniak DJ. 2008. Pseudomonas aeruginosa AlgR regulates type IV pilus biosynthesis by activating transcription of the fimU-pilVWXY1Y2E operon. J Bacteriol 190:2023–2030. doi: 10.1128/JB.01623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster M, Hawkins AC, Harwood CS, Greenberg EP. 2004. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol Microbiol 51:973–985. doi: 10.1046/j.1365-2958.2003.03886.x. [DOI] [PubMed] [Google Scholar]
- 46.Firoved AM, Deretic V. 2003. Microarray analysis of global gene expression in mucoid Pseudomonas aeruginosa. J Bacteriol 185:1071–1081. doi: 10.1128/JB.185.3.1071-1081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beatson SA, Whitchurch CB, Sargent JL, Levesque RC, Mattick JS. 2002. Differential regulation of twitching motility and elastase production by Vfr in Pseudomonas aeruginosa. J Bacteriol 184:3605–3613. doi: 10.1128/JB.184.13.3605-3613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones AK, Fulcher NB, Balzer GJ, Urbanowski ML, Pritchett CL, Schurr MJ, Yahr TL, Wolfgang MC. 2010. Activation of the Pseudomonas aeruginosa AlgU regulon through mucA mutation inhibits cAMP/Vfr signaling. J Bacteriol 192:5709–5717. doi: 10.1128/JB.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potvin E, Sanschagrin F, Levesque RC. 2008. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol Rev 32:38–55. doi: 10.1111/j.1574-6976.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 50.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. 2013. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu Rev Microbiol 67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 51.Schuster M, Greenberg EP. 2006. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int J Med Microbiol 296:73–81. doi: 10.1016/j.ijmm.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 52.Sonnleitner E, Romeo A, Blasi U. 2012. Small regulatory RNAs in Pseudomonas aeruginosa. RNA Biol 9:364–371. doi: 10.4161/rna.19231. [DOI] [PubMed] [Google Scholar]
- 53.Balasubramanian D, Schneper L, Kumari H, Mathee K. 2013. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res 41:1–20. doi: 10.1093/nar/gks1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okkotsu Y, Pritchett C, Schurr MJ. 2013. Regulation of exopolysaccharide biosynthesis in Pseudomonas aeruginosa, p 171–189. In Vasil ML, Darwin AJ (ed), Regulation of bacterial virulence ASM Press, Washington, DC. [Google Scholar]
- 55.Govan JR, Deretic V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Damron FH, Goldberg JB. 2012. Proteolytic regulation of alginate overproduction in Pseudomonas aeruginosa. Mol Microbiol 84:595–607. doi: 10.1111/j.1365-2958.2012.08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franklin MJ, Nivens DE, Weadge JT, Howell LP. 2011. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides alginate, Pel, and Psl. Front Cell Infect Microbiol 2:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okkotsu Y, Little AS, Schurr MJ. 2014. The Pseudomonas aeruginosa AlgZR two-component system coordinates multiple phenotypes. Front Cell Infect Microbiol 4:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schurr MJ, Yu H, Boucher JC, Hibler NS, Deretic V. 1995. Multiple promoters and induction by heat shock of the gene encoding the alternative sigma factor AlgU (sigma E) which controls mucoidy in cystic fibrosis isolates of Pseudomonas aeruginosa. J Bacteriol 177:5670–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowen DW, Deretic V. 2000. Membrane-to-cytosol redistribution of ECF sigma factor AlgU and conversion to mucoidy in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Mol Microbiol 36:314–327. doi: 10.1046/j.1365-2958.2000.01830.x. [DOI] [PubMed] [Google Scholar]
- 61.Wood LF, Ohman DE. 2009. Use of cell wall stress to characterize sigma 22 (AlgT/U) activation by regulated proteolysis and its regulon in Pseudomonas aeruginosa. Mol Microbiol 72:183–201. doi: 10.1111/j.1365-2958.2009.06635.x. [DOI] [PubMed] [Google Scholar]
- 62.Bazire A, Shioya K, Soum-Soutera E, Bouffartigues E, Ryder C, Guentas-Dombrowsky L, Hemery G, Linossier I, Chevalier S, Wozniak DJ, Lesouhaitier O, Dufour A. 2010. The sigma factor AlgU plays a key role in formation of robust biofilms by nonmucoid Pseudomonas aeruginosa. J Bacteriol 192:3001–3010. doi: 10.1128/JB.01633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albus AM, Pesci EC, Runyen-Janecky LJ, West SE, Iglewski BH. 1997. Vfr controls quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179:3928–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dasgupta N, Ferrell EP, Kanack KJ, West SE, Ramphal R. 2002. fleQ, the gene encoding the major flagellar regulator of Pseudomonas aeruginosa, is sigma70 dependent and is downregulated by Vfr, a homolog of Escherichia coli cyclic AMP receptor protein. J Bacteriol 184:5240–5250. doi: 10.1128/JB.184.19.5240-5250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185:2080–2095. doi: 10.1128/JB.185.7.2080-2095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Intile PJ, Diaz MR, Urbanowski ML, Wolfgang MC, Yahr TL. 2014. The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. J Bacteriol 196:357–366. doi: 10.1128/JB.01199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu W, Badrane H, Arora S, Baker HV, Jin S. 2004. MucA-mediated coordination of type III secretion and alginate synthesis in Pseudomonas aeruginosa. 186:7575–7585. doi: 10.1128/JB.186.22.7575-7585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]