Abstract
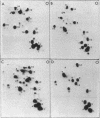
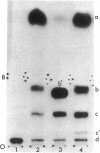
The nucleotide sequence G-T-Ψ-C-G(A)- has previously been found in every tRNA of known sequence that is active in protein biosynthesis. An exception to this generalization is the recently sequenced initiator tRNA from yeast cytoplasm. It is now reported that cytoplasmic initiator tRNAs from wheat germ, rabbit liver, and sheep mammary gland also lack the G-T-Ψ-C-G(A)- sequence. Thus: (i) nucleoside composition analyses show the absence of T in all these tRNAs; (ii) analyses of oligonucleotide fragments produced by T1 ribonuclease show the absence not only of the T-Ψ-C-G(A)- sequence, but also of U-Ψ-C-G(A)- or U-U-C-G(A)- sequences in such digests. The absence of G-T-Ψ-C-G(A)- in the eukaryotic cytoplasmic initator tRNAs is, therefore, not simply due to lack of enzymatic modification of U to T.
Keywords: tritium fluorography, polynucleotide kinase labeling, fingerprinting
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatterjee N. K., Bose K. K., Woodley C. L., Gupta N. K. Protein synthesis in rabbit reticulocytes: factors controlling terminal and internal methionine codon (AUG) recognition by methionyl tRNA species. Biochem Biophys Res Commun. 1971 May 21;43(4):771–779. doi: 10.1016/0006-291x(71)90683-8. [DOI] [PubMed] [Google Scholar]
- Dirheimer G., Ebel J. P., Bonnet J., Gangloff J., Keith G., Krebs B., Kuntzel B., Roy A., Weissenbach J., Werner C. Structure primaire des tRN. Biochimie. 1972;54(2):127–144. doi: 10.1016/s0300-9084(72)80097-x. [DOI] [PubMed] [Google Scholar]
- Drews J., Grasmuk H., Weil R. Utilization of methionine-accepting tRNA species form Escherichia coli, ascites-tumor cells, and yeast in homologous and heterologous cell-free systems. Eur J Biochem. 1972 Aug 18;29(1):119–127. doi: 10.1111/j.1432-1033.1972.tb01965.x. [DOI] [PubMed] [Google Scholar]
- Drews J., Högenauer G., Unger F., Weil R. Incorporation of methionine from met-tRNA-Met-F into internal positions of polypeptides by mouse liver polysomes. Biochem Biophys Res Commun. 1971 May 21;43(4):905–912. doi: 10.1016/0006-291x(71)90703-0. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Ghosh K. Specificity of the initiator methionine tRNA for terminal and internal recognition. Biochem Biophys Res Commun. 1972 Oct 17;49(2):550–557. doi: 10.1016/0006-291x(72)90446-9. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Grishko A., Ghosh H. P. Initiation of protein synthesis in eukaryotes. Biochem Biophys Res Commun. 1971 Feb 5;42(3):462–468. doi: 10.1016/0006-291x(71)90393-7. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J. N., Landy A., Zadrazil S., Smith J. D. The nucleotide sequences of tyrosine transfer RNAs of Escherichia coli. Eur J Biochem. 1970 Apr;13(3):461–483. doi: 10.1111/j.1432-1033.1970.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Johnson L., Hayashi H., Söll D. Isolation and properties of a transfer ribonucleic acid deficient in ribothymidine. Biochemistry. 1970 Jul 7;9(14):2823–2831. doi: 10.1021/bi00816a011. [DOI] [PubMed] [Google Scholar]
- Kimura-Harada F., Saneyoshi M., Nishimura S. 5-methyl-2-thiouridine: A new sulfur-containing minor constituent from rat liver glutamic acid and lysine tRNAs. FEBS Lett. 1971 Apr 2;13(6):335–338. doi: 10.1016/0014-5793(71)80254-5. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA. Biochem Biophys Res Commun. 1970 Jul 27;40(2):416–421. doi: 10.1016/0006-291x(70)91025-9. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Petrissant G., Boisnard M., Puissant C. Purification d'un tRNA accepteur de la méthionine dans le foie de lapin. Biochim Biophys Acta. 1970 Jul 16;213(1):223–225. [PubMed] [Google Scholar]
- Petrissant G. Etude du méthionine-tARN dans la glande mammaire de brebis. Hétérogénéité et purification. Biochimie. 1971;53(4):523–531. doi: 10.1016/s0300-9084(71)80170-0. [DOI] [PubMed] [Google Scholar]
- Petrissant G. Evidence for the absence of the G-T-psi-C sequence from two mammalian initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1046–1049. doi: 10.1073/pnas.70.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Studies on polynucleotides. IV. Enzymic degradation; the stepwise action of venom phosphodiesterase on deoxyribo-oligonucleotides. J Biol Chem. 1959 Aug;234(8):2114–2117. [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- RajBhandary U. L., Kumar A. A formylatable methionine transfer ribonucleic acid from yeast: comparison of coding properties and sequences around the anticodon with Escherichia coli formylatable methionine transfer RNA. J Mol Biol. 1970 Jun 28;50(3):707–711. doi: 10.1016/0022-2836(70)90095-1. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L. Studies on polynucleotides. LXXVII. The labeling of end groups in polynucleotide chains: the selective modification of diol end groups in ribonucleic acids. J Biol Chem. 1968 Feb 10;243(3):556–564. [PubMed] [Google Scholar]
- Randerath E., Yu C. T., Randerath K. Base analysis of ribopolynucleotides by chemical tritium labeling: a methodological study with model nucleosides and purified tRNA species. Anal Biochem. 1972 Jul;48(1):172–198. doi: 10.1016/0003-2697(72)90181-9. [DOI] [PubMed] [Google Scholar]
- Randerath K., Rosenthal L. J., Zamecnik P. C. Base composition differences between avian myeloblastosis virus transfer RNA and transfer RNA isolated from host cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3233–3237. doi: 10.1073/pnas.68.12.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Structures of two glycyl-tRNAs from Staphylococcus epidermidis. Nat New Biol. 1972 May 10;237(71):44–45. doi: 10.1038/newbio237044a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972 Oct 17;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]
- Székely M., Sanger F. Use of polynucleotide kinase in fingerprinting non-radioactive nucleic acids. J Mol Biol. 1969 Aug 14;43(3):607–617. doi: 10.1016/0022-2836(69)90362-3. [DOI] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Studies on polynucleotides. CI. Escherichia coli tyrosine and formylmethionine transfer ribonucleic acids: effect of chemical modification of 4-thiouridine to uridine on their biological properties. J Biol Chem. 1972 Aug 10;247(15):4879–4892. [PubMed] [Google Scholar]
- Wu R. Nucleotide sequence analysis of DNA. I. Partial sequence of the cohesive ends of bacteriophage lambda and 186 DNA. J Mol Biol. 1970 Aug;51(3):501–521. doi: 10.1016/0022-2836(70)90004-5. [DOI] [PubMed] [Google Scholar]