Abstract
Neurotransmitter vesicles are known to concentrate hydrogen ions (or protons), the simplest ion, and to release them during neurotransmission. Furthermore, receptors highly sensitive to protons, acid-sensing ion channels (ASICs), were previously localized on the opposite side of the synaptic cleft on dendritic spines. Now, recent experiments provide some of the strongest support to date that protons function as a neurotransmitter in mice, crossing synapses onto medium spiny neurons of the nucleus accumbens (NAc), activating ASICs, and ultimately suppressing drug abuse-related behaviors.
Keywords: ASIC1A, synaptic transmission, protons, nucleus accumbens, cocaine, addiction
Hydrogen ions, or protons, are loaded into presynaptic neurotransmitter-containing vesicles by H+-ATPase, creating an electrochemical gradient that powers vesicle uptake of neurotransmitters including glutamate, GABA, and acetylcholine. Thus, most, if not all, neurotransmitter-containing synaptic vesicles are acidic, estimated to range between pH 5.2 and 5.7 by 31P NMR spectroscopy and by imaging pH-sensitive fluorescent proteins. During neurotransmission, protons are released along with the other vesicle contents. In addition, the vesicle membrane fuses with the presynaptic membrane, presenting an opportunity for vesicular H+-ATPase to continue to pump protons into the synaptic cleft. Extracellular pH measurements and pH-mediated inhibition of presynaptic Ca2+ channels indicate that protons are indeed released by presynaptic terminals during neurotransmission. These findings suggested the possibility that protons communicate with receptors on the opposite side of the synapse. Two new papers focusing on acid-sensing ion channels (ASICs) support the likelihood that protons function as a neurotransmitter at glutamatergic synapses in the mammalian brain.1,2 Moreover, these studies suggest that this form of neurotransmission may play a critical role in psychiatric illnesses such as drug addiction.
Background
ASIC receptors are members of the DEG/ENaC family of Na+ channels; they are defined by their sensitivity to pH and are activated when extracellular pH falls into the acidic range.3 ASICs are formed by homomeric and heteromeric combinations of subunits assembled into a trimeric channel structure. ASIC1A, ASIC2A, and ASIC2B are the predominant subunits in the brain, where they are located on neurons on the cell body and along dendrites. Previous studies suggest ASICs are concentrated on postsynaptic dendritic spines, which make contact with presynaptic axon terminals, suggesting they are well positioned to respond to changes in synaptic pH. Indeed, prior work indicates ASICs contribute to synaptic plasticity and play a role in learning and memory. However, direct evidence implicating ASICs in synaptic transmission has been lacking.
ASICs, Synaptic Transmission, and Cocaine
Knowing that presynaptic terminals release protons, and that ASICs are positioned to potentially respond to these protons (Figure 1), investigators from the University of Iowa explored the possibility that ASICs are activated during neurotransmission in two recent papers.1,2 One of these examined synaptic transmission in the lateral amygdala, a brain region involved in fear learning and memory, and observed that a relatively small percentage of the current evoked during glutamatergic synaptic transmission was insensitive to ionotropic glutamate and GABA receptor antagonists.2 However, this previously unidentified synaptic current was eliminated by ASIC inhibitors and by genetically disrupting ASIC1A, and it was sensitive to changes in pH buffering capacity. The second paper found a similar synaptic current evoked in medium-spiny neurons in the nucleus accumbens (NAc), where about 5% of the total evoked synaptic current depended on ASICs (Figure 2).1 Supporting the likelihood that the synaptic ASIC currents were proton-activated, disrupting or inhibiting carbonic anhydrase 4 (CA4), an enzyme critical for extracellular pH buffering in brain, resulted in larger ASIC-mediated synaptic currents (Figures 1, 2).1
Figure 1.
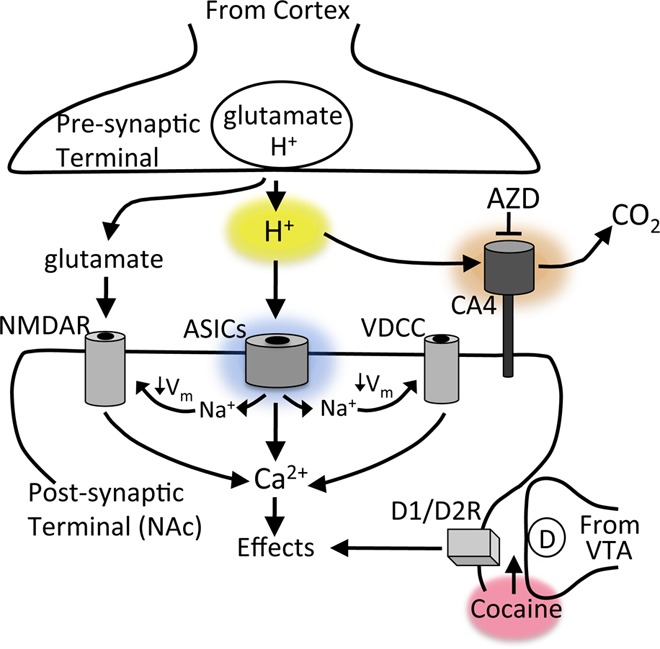
Model for ASICs and cocaine action at cortico-accumbens synapses. Neurotransmitter (NT)-containing vesicles are acidic (H+) and lower pH in the synaptic cleft to activate ASICs in postsynaptic dendritic spines, which coincides with glutamate receptor activation. This activity is regulated by the membrane bound pH-buffering enzyme, carbonic anhydrase IV (CA4). Over time, ASIC activity alters synapse structure and function likely through membrane depolarization (↓Vm) and by raising Ca2+. Loss of ASIC1A produces effects very similar to effects of cocaine withdrawal such as increased AMPA-to-NMDA ratio and increased cocaine-evoked plasticity.
Figure 2.
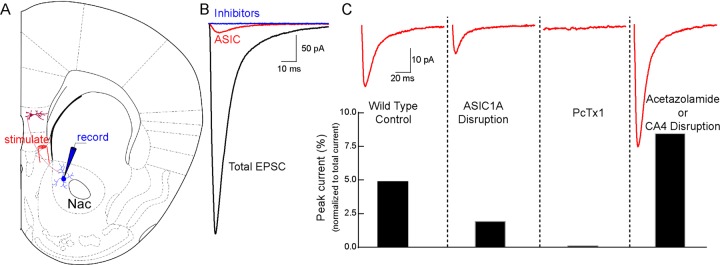
Synaptic ASIC currents in nucleus accumbens. (A) Stimulating inputs from medial prefrontal cortex (mPFC) evoked excitatory postsynaptic currents (EPSCs) in medium spiny neurons of the nucleus accumbens (NAc) core, recorded by whole cell voltage clamp in acute mouse brain slices. (B) Total EPSC (black trace) was largely eliminated by blocking AMPAR, NMDAR, and GABAAR, respectively, with their inhibitors CNQX, APV, and picrotoxin, leaving a residual current (red trace) that depended on ASICs and was completely abolished by the ASIC antagonist amiloride (blue trace, all above-mentioned inhibitors). (C) ASIC-dependent EPSC (red trace) was reduced by ASIC1A gene disruption and by the ASIC1A inhibitor PcTx1 (in ASIC2–/– mice). And the ASIC-dependent EPSC was potentiated by the carbonic anhydrase inhibitor acetazolamide, by genetically disrupting carbonic anhydrase 4 (CA4) and by reducing pH buffering capacity (not shown) (adapted from ref (1)).
Because the NAc is thought to play a critical role in drug abuse, Kreple et al. examined whether the synaptic ASIC currents were involved in responses to drugs of abuse.1 Accumulating evidence suggests that cocaine administration and withdrawal from cocaine alter the structure and function of synapses in the NAc and that these rearrangements may underlie craving for drugs. For example, synapse number and shape are altered by cocaine withdrawal.4 Glutamate receptor function and composition are also altered, including an increase in the ratio of the synaptic current mediated by AMPA receptors relative to that mediated by NMDA receptors (AMPA-to-NMDA ratio).4 A very similar profile was found in mice lacking ASIC1A, even though they had never been exposed to cocaine.1 ASIC1A knockout mice had higher AMPA-to-NMDA ratios and a higher density of dendritic spines, due largely to an increase in immature stubby and thin spines. Moreover, like cocaine-withdrawn animals, in which the synaptic AMPA-to-NMDA ratio is rapidly changed by a single cocaine dose, the synaptic AMPA-to-NMDA ratio in drug-naïve mice lacking ASIC1A was also rapidly changed by cocaine.1 This exaggerated molecular response to cocaine was absent in drug-naïve control mice, suggesting that ASIC1A disruption increased the synaptic sensitivity to cocaine. These observations suggest an interaction between ASICs and cocaine (Figure 1), though the molecular details of this interaction remain to be identified.
The exaggerated synaptic response to cocaine in the ASIC1A knockout mice suggested that ASIC1A disruption might also alter cocaine-evoked behavior. Consistent with this possibility, conditioned place preference to cocaine, which requires the memory of a learned association between the rewarding properties of cocaine injections and the environmental context, was exaggerated in mice completely lacking ASIC1A and also in mice with ASIC1A disrupted specifically in the NAc.1 Restoring ASIC1A to the NAc via a viral vector produced the opposite effect and reduced cocaine conditioned place preference. Conditioned place preference to morphine was also increased by ASIC1A disruption, suggesting that these findings may extend to multiple drugs of abuse. Finally, to explore whether ASICs might suppress the desire to consume cocaine, the authors investigated cocaine-self-administration in rats and found that overexpressing ASIC1A in the NAc, again via viral vector, greatly increased the synaptic ASIC current and reduced the amount of cocaine that the rats self-administered.1
Discussion
These new data suggest protons and ASICs comprise a neurotransmitter/receptor pair in the mammalian central nervous system.1,2 They suggest this pair contributes to synapse morphology and plasticity. Furthermore, they suggest important roles in synaptic and behavioral responses to cocaine and potentially other drugs of abuse.
With all of the known neurotransmitters, these new data raise the question: why protons? One potential answer may be found in evolution. For single-celled organisms, extracellular pH is a critical environmental factor, and thus detecting changes in pH is likely essential for survival. With the capacity to detect extracellular pH established, an ability to concentrate and release protons in vesicles may have provided a simple and convenient way for one cell to communicate with others. Furthermore, this simple signal may have provided a way for organisms to develop a more diverse repertoire of signaling molecules as organisms became more complex with evolving nervous systems. With the proton electrochemical gradient, vesicles need only one additional protein, a transporter, to uptake neurotransmitters such as glutamate, monoamines, or GABA. Interestingly, protons have been suggested to function as a transmitter in the worm, and ASICs are present in the evolutionarily ancient comb jelly Pleurobrachia bachei of the phylum ctenophora, suggesting ASICs and protons may have played an important role in early, simple nervous systems.5
Previously it was thought that each synapse uses only one neurotransmitter, but this view is changing. Accumulating evidence suggests synaptic vesicles can load multiple neurotransmitters. Indeed, if protons function as neurotransmitters, then most mammalian synaptic vesicles contain at least two. The findings that glutamatergic synapses use protons and ASICs1,2 raise the possibility that other types of synapses, such as GABAergic and cholinergic synapses, might also use protons as cotransmitters. ASICs have not been localized at GABAergic synapses, although they have been suggested to play a role at cholinergic synapses at the neuromuscular junction. Furthermore, it is possible that other pH receptors contribute to neurotransmission since other pH-sensitive receptors exist, including pH-sensitive G-protein coupled receptors (GPCRs).
Previous studies have implicated ASICs in chemosensation and in learning and memory.3 These new data suggest additional or alternative functions for ASICs. The increased density of immature synaptic structures in mice lacking ASIC1A suggests ASICs might normally contribute to synapse maintenance or turnover.1 Interestingly, drugs of abuse have also been suggested to increase the proportion of immature synapses, which are considered highly plastic.4 Thus, ASICs may somehow oppose the synaptic changes induced by drugs of abuse. Because ASICs allow Na+ and Ca2+ to enter the cell,3 the observed effects likely stem from membrane depolarization and Ca2+-dependent signaling (Figure 1). However, further studies will be necessary to understand the downstream molecular consequences of ASIC activation.
Like other neurotransmitter systems, synaptic protons appear to be regulated. pH-buffering catalyzed by carbonic anhydrases provides a way to rapidly terminate the proton-mediated signal. Reducing pH-buffering capacity and eliminating carbonic anhydrase 4 significantly increased the synaptic ASIC current (Figure 2).1,2 This finding, coupled with the observation that increasing ASIC1A expression above normal levels suppressed cocaine self-administration, suggests that augmenting ASIC expression or function might help suppress drug craving.1 Medications capable of blocking carbonic anhydrases, such as acetazolamide, have already been developed and are considered safe, offering one way that these new results might be realistically extended to treat drug addiction in humans.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Kreple C. J.; Lu Y.; Taugher R. J.; Schwager-Gutman A. L.; Du J.; Stump M.; Wang Y.; Ghobbeh A.; Fan R.; Cosme C. V.; Sowers L. P.; Welsh M. J.; Radley J. J.; LaLumiere R. T.; Wemmie J. A. (2014) Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat. Neurosci. 10.1038/nn.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J.; Reznikov L. R.; Price M. P.; Zha X.-M.; Lu Y.; Moninger T. O.; Wemmie J. A.; Welsh M. J.. Protons and ASICs are a neurotransmitter/receptor pair that regulates synaptic plasticity in the lateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 2014, In Press [DOI] [PMC free article] [PubMed]
- Wemmie J. A.; Taugher R. J.; Kreple C. J. (2013) Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci 14(7), 461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P. W. (2009) The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci 10(8), 561–72. [DOI] [PubMed] [Google Scholar]
- Moroz L. L.; Kocot K. M.; Citarella M. R.; Dosung S.; Norekian T. P.; Povolotskaya I. S.; Grigorenko A. P.; Dailey C.; Berezikov E.; Buckley K. M.; Ptitsyn A.; Reshetov D.; Mukherjee K.; Moroz T. P.; Bobkova Y.; Yu F.; Kapitonov V. V.; Jurka J.; Bobkov Y. V.; Swore J. J.; Girardo D. O.; Fodor A.; Gusev F.; Sanford R.; Bruders R.; Kittler E.; Mills C. E.; Rast J. P.; Derelle R.; Solovyev V. V.; Kondrashov F. A.; Swalla B. J.; Sweedler J. V.; Rogaev E. I.; Halanych K. M.; Kohn A. B. (2014) The ctenophore genome and the evolutionary origins of neural systems. Nature 510, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]


