Abstract
Purpose
the aim of this study is to differentiate the behavior of the synovial membrane in the presence of various stimuli in patients who practice sports.
Methods
fifty one patients (30 males and 21 females, mean age 48 years, range 31–59 years) who actively practiced non-competitive sports underwent a biopsy of the synovial membrane during arthroscopic surgery performed for joint effusion secondary to meniscal lesion (24 cases), anterior cruciate ligament injury (ACL) (17 cases), postoperative knee joint stiffness (2 cases), aseptic loosening or dislocation of the polyethylene component of uni-compartmental knee arthroplasty (5 cases), and anterior fibrous impingement of the ankle (3 cases). Synovial tissue samples were obtained during surgery from all the patients and processed for light microscopy, transmission electron microscopy and scanning electron microscopy observation.
Results
circulatory phenomena were observed in acute inflammatory processes, characterized by hyperemia and vasodilation. Exudative and infiltrative phenomena were observed in the presence of foreign bodies and were characterized by leukocytic exudation (presence of polynuclear neutrophils), accompanied by lymphomonocytic infiltration. Proliferative phenomena were observed in post-traumatic forms of synovitis (ACL and meniscal injuries), characterized by hypertrophy and proliferation of villous formations. Degenerative and regressive phenomena were observed in cases of fibrous reaction (ankle impingement and joint stiffness) and were characterized by formation of dense fibrous connective tissue with hyaline patches, evolving towards sclerosis.
Conclusions
the activation of inflammatory processes in patients who expose their joints to excessive stress may lead to the formation of hyperplastic tissue. Ultramicroscopic debris is usually capable of transforming the structural organization of the synovial tissue.
Level of evidence
Level IV, observational case series.
Keywords: athlete, synovitis, synovial membrane, sports, ultrastructure
Introduction
The synovial membrane shows morphological adaptations that are related to the functional, mechanical and metabolic stresses to which it is subjected during articular movement and load bearing. Connective tissue plasticity is such that the original morphology of the synovial villi is restored upon removal of the cause of the stress. Synovial intimal cells, termed synoviocytes, are active cells that interact with different forms of stress in the joint, in particular with excessive physiological loading (1). The particular organization of the synovial membrane, i.e. its extreme sensitivity and reactivity, which is due to its delicate and dense capillary network and absence of a protective coating, explains why significant changes can occur, depending on the pathogen (2).
A trauma affecting the synovial joint constitutes a non-specific irritative stimulus; the components of the synovial membrane may display a hypertrophic or a proliferative response to this stimulus depending on the type of closed trauma sustained (contusion, sprain, dislocation, articular fracture) or the patient’s genotype; however, certain articular pathologies are characterized by the appearance of specific reactions at the level of the synovial joints (1, 3–8). The aim of this study is to differentiate the behavior of the synovial membrane in the presence of various stimuli in patients who practice sports.
Methods
Between October 2008 and June 2010, 51 patients underwent a biopsy of the synovial membrane during arthroscopic surgery performed for joint effusion. All 51 patients (30 males and 21 females, mean age 48 years, range 31–59 years) actively practiced non-competitive sports. Twenty-four patients had meniscal lesions; 17 had suffered anterior cruciate ligament (ACL) ruptures (4 of which were recurrent lesions that had already been treated – 2 with biological reconstructions and 2 with synthetic ligaments); two patients had postoperative joint stiffness; three were suffering from aseptic loosening of uni-compartmental knee arthroplasty (UKA); two patients, each with a UKA, had a dislocation of the polyethylene component due to a sprain trauma; in three cases fibrous impingement syndrome of the ankle was detected.
Fresh synovial tissue was obtained during arthroscopic surgery from all the patients (9). Specimens for light microscopic examination were fixed in 10% formalin, decalcified with formic acid, and embedded in paraffin. Sections were stained with hematoxylin and eosin. Samples for transmission electron microscopic (TEM) observation were fixed for 72 h at room temperature in 2.5% glutaraldehyde in phosphate buffer. After fixation, small pieces of tissue were cut. If these pieces contained hard tissue, i.e. marrow, they were immersed in a decalcifying ethylene-diaminetetraacetic acid (EDTA) solution for 36 hours. If the sample included soft tissue, i.e. collagen, the decalcification stage was omitted. In both cases, the next stage was rinsing of the pieces in cacodylate buffer, followed by fixation with 1% osmium tetroxide in cacodylate buffer at pH 7.4. Subsequently, the samples were rinsed again in cacodylate buffer and dehydrated in ascending concentrations of ethanol (25 to 100%), soaked in acetone and epoxy resin, and cut into sections (700 A thick) using an ultramicrotome. The sections were then stained with uranyl acetate and citrate and examined with TEM equipment, namely a Zeiss EM 109 Turbo microscope (Carl Zeiss Inc., Thornwood, NY, USA).
Samples for scanning electron microscope (SEM) observation were fixed in 2.5% glutaraldehyde in phosphate buffer for 72 hours at room temperature. The pieces were then first washed in phosphate buffer, followed by post-fixation in 1% osmium tetroxide in phosphate buffer and a second washing in phosphate buffer. Dehydration occurred in areas of increasing concentrations of acetone and drying was performed by a critical point drying method. Finally, metallization of the samples with gold palladium allowed them to be observed under a conventional high-vacuum SEM (Zeiss DSM 962; Carl Zeiss Inc.).
Results
The main histopathological findings demonstrating a synovial reaction to trauma were classified as follows (10–13): circulatory phenomena; exudative and infiltrative phenomena; proliferative phenomena; and degenerative and regressive phenomena.
Circulatory phenomena
If trauma has led to a capsular ligamentous lesion the changes that occur are those characteristic of a general inflammatory process: a constant and early finding is vascular congestion, characterized by hyperemia and vasodilation. In normal circumstances the sub-intimal layer of the synovial membrane already has abundant vascularity; in reaction to trauma the vascular network appears richer showing dilated capillaries full of red blood cells which often appear sinusoidal. Frequent reports highlight situations of obvious bleeding, with more or less abundant red blood cells present on the surface but also in the intimal layer, even deep within it (14) (Fig. 1).
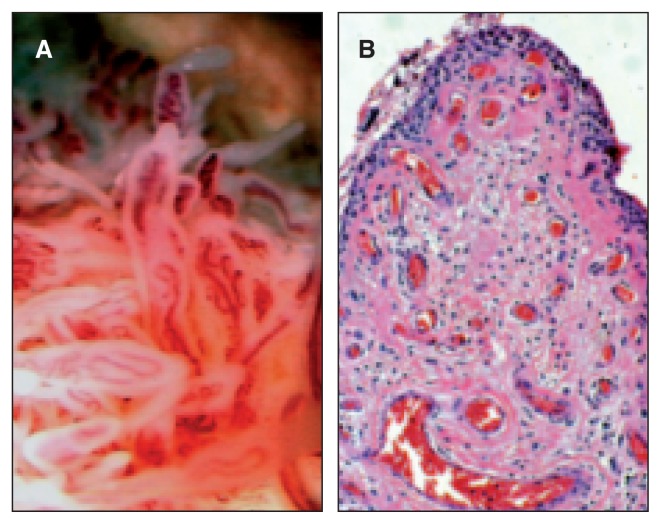
Fig. 1.
A: Post-traumatic subacute arthrosynovitis: the vascular congestion of the villi is easily seen during arthroscopy procedures. B: The intimal hyperplasia of the lining, supported by the accumulation of synoviocytes is indicative of an injury dating back at least 2–3 weeks.
In the two biopsies that were performed during checks for re-tear injuries of the ACL, the membrane was found to be involved in the ligamentization phenomenon and thanks to the penetration of flanges of synovial tissue rich in cells and growth factors, progressive maturation of the neoligament was seen. The thin layer of synovial membrane that surrounds the neoligament was not always visible arthroscopically due to the physiological integration process, however, it can be documented histologically (15) (Fig. 2).
Fig. 2.
The penetration of the synovial tissue (reparative function expression) within the mesh of the tendon tissue (synovialization process) allows the revascularization and colonization of graft. The type B synoviocytes are the main responsible for the repopulation of the graft.
Exudative and infiltrative phenomena
These phenomena are directly connected to hyperemia and to the appearance of elements indicating leukocytic exudation (polynuclear neutrophils), which is accompanied or followed by lymphomonocytic infiltration which is concentrated in the subintimal layer, in proximity to the vascular structures. Infiltrative phenomena are also characterized by the appearance of plasma cells with edema, which soak the intercellular substance, decoupling the structure (16) (Fig. 3). Only in the presence of a foreign body (artificial ligament, prothesis) which is worn by microtrauma and deposits debris that is phagocytized by superficial and deep cells it is possible to see changes in the structure of the synovial tissue. Ultrastructural investigation confirms these changes. With SEM we can observe «activation» of the membrane with the synovicytes proliferating onto the irregular surface, protruding into the cavity and giving rise to irregular furrows, some deep and some less so. Electron microscopy confirms the existence of different cell types, which is a result of different functional behaviors. In traumatic lesions we can observe hyperplasia of the superficial cells, with absolute predominance of intermediate and type B synoviocytes, characterized by abundant rough endoplasmic reticulum and low presence of Golgi apparatus. Metallic debris from 0.4 to l μ in diameter is present in the interstices as indigestible residual material; smaller debris, ranging in size from 0.03 to 0.3 μ, can be found in the cytoplasm within the lysosomes (Fig. 4).
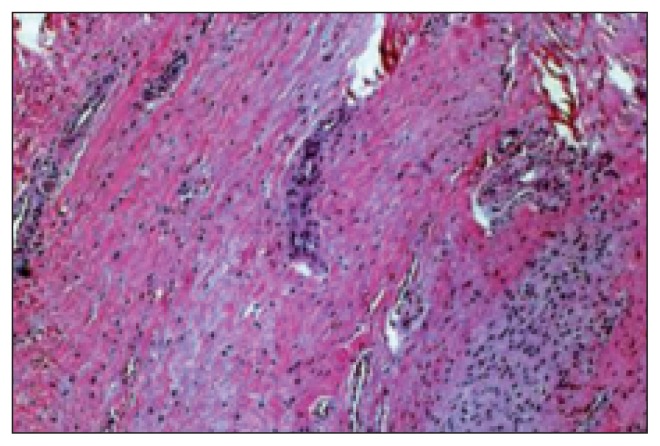
Fig. 3.
Chronic hypertrophic proliferative synovitis.
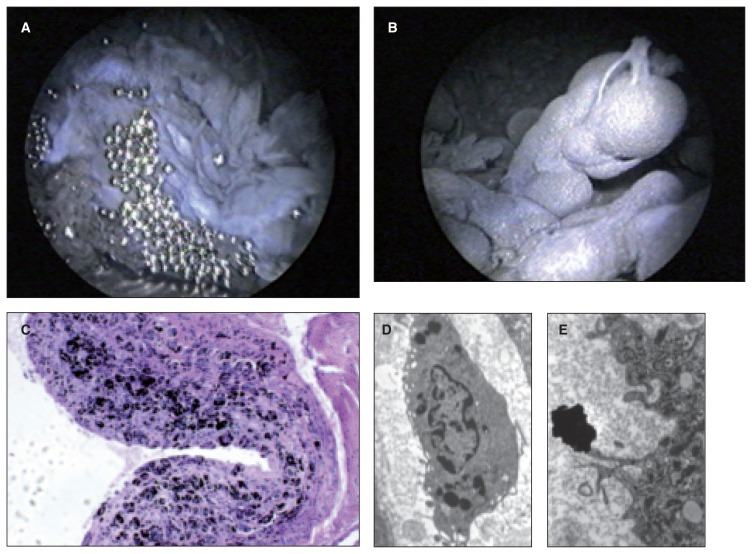
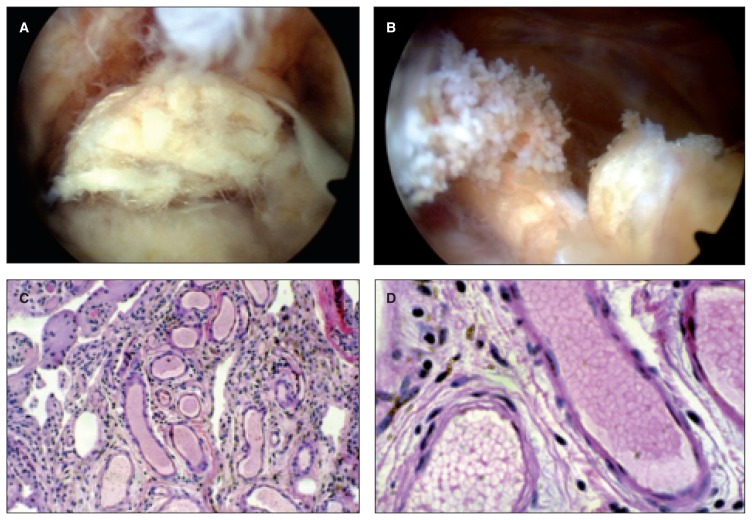
Fig. 4.
A: Arthroscopic examination has allowed us to highlight a dark gray pannus, proliferative, which surrounds the whole joint with the release of a number of metal beads. B: Detail of villus giant piece: numerous pieces of metal inside. C: The histological examination shows the debris of metal, in the form of aggregated blacks within the synovial membrane lined with fibrous connective tissue. D: The transmission electron microscopy shows that small fragments of metal (white arrow) are phagocytosed by macrophages. E: The largest metallic debris are indigestible and remain in the interstitium.
In two patients with synthetic ligaments we found artificial ligament synovitis. In these patients the synoviocytes were not able to break down the synthetic material; the villi activated processes of hypertrophy and hyperplasia in order to dispose of the excess material, however the result was an accumulation of whitish colored villi similar to the original synthetic ligament (Fig. 5).
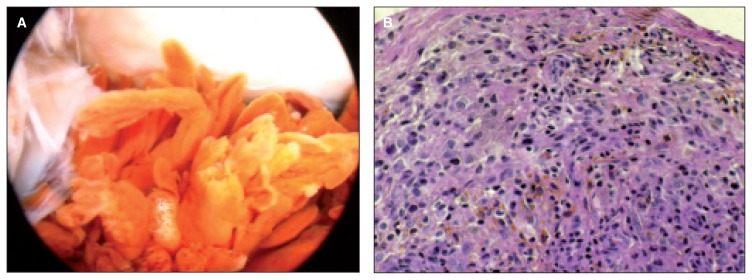
Fig. 5.
A: Arthroscopic view of an artificial ligament broken 1 year by the implantation; it’s possible to appreciate the wear fragments become detached from the synthetic ligament. B: Severe structural disruption of the synovial membrane, especially in the zones of rupture of the ligament. C: Synovial tissue with aspects of papillary hyperplasia, chronic inflammatory pictures, hemosiderin deposit of material and fragments of ligament. D: The task of debris removal is carried mainly by type A synoviocytes, which are able to degrade the material to be deleted.
Proliferative phenomena
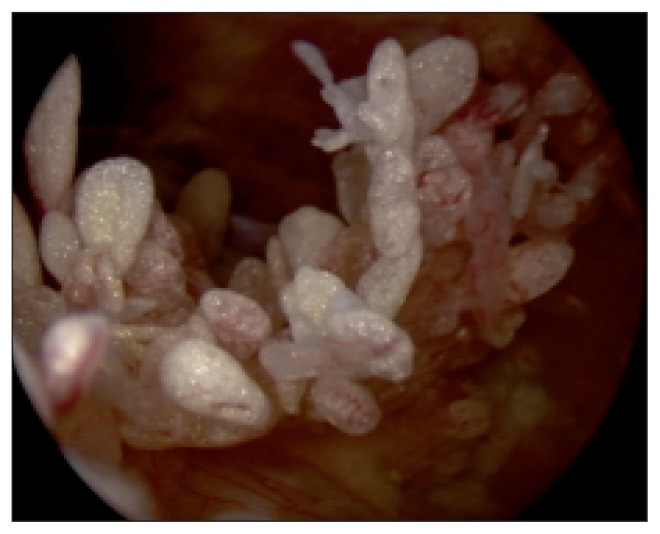
These phenomena may affect elements of intimal cells, vascular structures, the fibroblastic component, and fibroadipose subsynovial layers. A particularly interesting aspect of post-traumatic forms of synovitis is proliferation of the constitutive elements, leading to hypertrophy and proliferation of villous formations, which have a very varied macroscopic morphology, ranging from diffuse and velvety to large and isolated (17–19) (Fig. 6). In secondary forms caused by joint instability and ACL rupture, we observed a hemorrhagic edema-like appearance in subacute forms (3 cases), while in chronic forms (9 cases) the membrane, when cartilage lesions were also present, had a proliferative appearance (16). A meniscal lesion is not normally able to stimulate a synovial reaction like a cartilaginous one does, most likely due to the different ultrastructural composition of the two materials; in fact the cartilage matrix, without lamina splendens, is more antigenic than the collagen meniscus tissue.
Fig. 6.
A: Chronic hyperplastic arthrosynovitis of prevailing inflammatory and reactive. B: The histological examination demonstrated the presence of hemosiderin pigment and villous hyperplasia: these findings do not constitute sufficient evidence to make a diagnosis of SVNP it is in fact necessary to the complete subversion of the structural response of typical granulomatous formations.
Degenerative and regressive phenomena
In the three cases of ankle impingement syndrome we observed development of fibrosis with formation of dense fibrous connective tissue with hyaline patches, evolving towards sclerosis. The vessels were sparse and thick-walled due to the presence of perivascular fibrosis; in such circumstances the cellular component also becomes poor (20). In one of our cases with joint stiffness, the membrane had undergone a metaplastic process whose evolution had significantly reduced the quantity of mature elastic fibers in the joint capsule and in the synovial membrane. In joint stiffness, an increase in immature elastic fibers along with other macromolecules, such as collagen, laminin and fibronectin, can stimulate fibroblast proliferation and activity causing a degeneration of the fibrous tissues (15, 21, 22). The presence of HLA class II antigens in type A synovitis is also presumed to be a factor contributing to maintenance of the joint fibrosis process. Therefore, a histological examination, aimed at defining the molecular composition of the scar tissue, can aid prognosis on joint function. The histological examination shows disruption of the arrangement of collagen fiber bundles and the presence of fibroblasts at different stages of maturation. Normally in cases of severe stiffness it is not the quality of the scar tissue that affects the joints, but rather its location, especially when it is situated in crucial areas such as in fornices or the femoral tibial compartment (23) (Fig. 7). In our second case of joint stiffness the extension deficit was due to the presence of a fibrous connective tissue nodule in the tibial spine, an outcome of the ligament reconstruction (cyclop’s lesion).
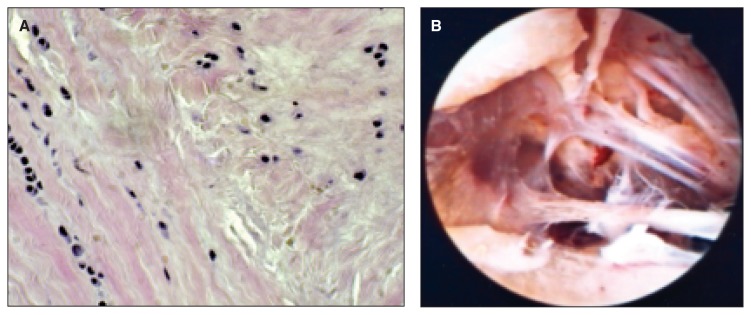
Fig. 7.
A: The alignment of the fibers is subverted; available anarchist fibroblast. B: Appearance arthroscopic intra-articular fibrosis, proliferation of tissue adhesions at the level of the fornix may limit joint movement.
Discussion
The joint of a patient who practices sports and who has suffered a trauma severe enough to produce a lesion will go through a villous morphological evolution that will be more or less marked, depending on the injury sustained and the functional stress experienced. The reaction of synovial membrane stimulated by trauma is non-specific and involves the endo-articular structures (ligament rupture, chondropathies, etc.); only the presence of debris from a foreign body in the joint cavity can stimulate a specific alteration in the synovial lining relative to the material deposits. In patients involved in intensive sports activity who then undergo prosthetic surgery, we may find, years later, synovitis caused by dispersion of metal debris from wear of the prosthetic material. Adhesion, abrasion and corrosion are the mechanisms responsible for the production of debris and which give the synovial membrane a dark color and lead to the proliferation of villi that are dissimilar in size and shape and enlarged by the debris (24). In cases of post-traumatic dislocation of polyethylene from the prosthesis, the membrane only showed signs of inflammation in the absence of the described processes of accumulation. Sports traumas lead to different behaviors of the synovial membrane, which are amplified by the micro-traumas generated by sports activity in patients who continue to expose their joints to demanding activities. Physiological stimuli ensure homeostasis of the joint system and do not alter the morphology of the synovial tissue. When the stimuli are excessive cytokine release may occur; this release constitutes the communication system between mechanical stimulation and synovial homeostasis; mechanical stimuli are, in fact, transduced into intracellular biological events capable of modulating the morphology and functioning of the synovial membrane through gene expression. In addition to the magnitude and direction of a load, the type of load applied is also important in determining the effects on synoviocytes. Cyclic loading brings about increased biosynthetic activity, in cartilage for example, while static loading decreases it. Exercise, continuous passive motion and mobilization seem to have positive effects that do not alter the morphology of the synovial lining, while immobilization causes thinning of the lining, finer consistency of the villous tissue and an alteration of the proteoglycan synthesis. We observed that synovial tissue of different joints (knee, ankle) reacts differently under similar stress conditions; in fact, in post-traumatic synovitis of the knee, the synovial membrane appears non-specific and has hyperemic villous tissue, whereas in the ankle the same type of trauma generates fibrous connective tissue: it is probable in the ankle that torsional trauma stimulates the development of pseudomeniscal tissue in joints without shock absorbers; however, a similar trauma in the knee sees the synovial membrane adapting by developing villous tissue which helps guarantee a complete range of motion of the surface, which is already physiologically predisposed to absorb loading. In conclusion, the plasticity of the synovial membrane is enhanced in joints which are subject to physiological loading; the activation of inflammatory processes in patients who expose their joints to excessive stress may lead to the formation of hyperplastic tissue; ultramicroscopic debris is usually capable of transforming the structural organization of the synovial tissue.
References
- 1.Berumen-Nafarrate E, Leal-Berumen I, Luevano E, et al. Synovial tissue and synovial fluid. J Knee Surg. 2002;15:46–48. [PubMed] [Google Scholar]
- 2.Soeur R. The synovial membrane of the knee in pathological conditions. J Bone Joint Surg Am. 1949;31A:317–340. [PubMed] [Google Scholar]
- 3.Bresnihan B. Are synovial biopsies of diagnostic value? Arthritis Res Ther. 2003;5:271–278. doi: 10.1186/ar1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabby D, Dekel S. Synovial knee pain arising from chronic inflammatory disorders of the knee. J Knee Surg. 2002;15:53–56. [PubMed] [Google Scholar]
- 5.Frassica FJ, Combs JJ, Jr, Sim FH. Synovial proliferative disorders: differential diagnosis. Arthroscopy. 1985;1:183–189. doi: 10.1016/s0749-8063(85)80009-8. [DOI] [PubMed] [Google Scholar]
- 6.Schulte E, Fisseler-Eckoff A, Müller KM. Differential diagnosis of synovitis. Correlation of arthroscopic biopsy to clinical findings. Pathologe. 1994;15:22–27. doi: 10.1007/s002920050020. [DOI] [PubMed] [Google Scholar]
- 7.Pavlovich RI, Lubowitz J. Current concepts in synovial tissue of the knee joint. Orthopedics. 2008;31:160–163. doi: 10.3928/01477447-20080201-24. [DOI] [PubMed] [Google Scholar]
- 8.Pavlovich RI. Synovialis... the forgotten tissue. J Knee Surg. 2002;15:45. [Google Scholar]
- 9.Smith MD, Barg E, Weedon H, et al. Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints. Ann Rheum Dis. 2003;62:303–307. doi: 10.1136/ard.62.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng N, Humby F, Bombardieri M, et al. Clinical, imaging and histological characteristics of patients with rheumatoid arthritis at different stages of disease progression. Rheumatology. 2013;52:71. [Google Scholar]
- 11.van de Sande MG, Gerlag DM, Lodde BM, et al. Evaluating antirheumatic treatments using synovial biopsy: a recommendation for standardisation to be used in clinical trials. Ann Rheum Dis. 2011;70:423–427. doi: 10.1136/ard.2010.139550. [DOI] [PubMed] [Google Scholar]
- 12.Vordenbäumen S, Joosten LAB, Friemann J, Schneider M, Ostendorf B. Utility of synovial biopsy. Arthritis Res Ther. 2009;11:25. doi: 10.1186/ar2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerlag DM, Tak PP. How to perform and analyse synovial biopsies. Best Pract Res Clin Rheumatol. 2009;23:221–232. doi: 10.1016/j.berh.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Fadda M, Zirattu G, Marrone A. The ultrastructural aspects of the synovial intima and subintimal in arthrosis. Arch Putti Chir Organi Mov. 1990;38:195–205. [PubMed] [Google Scholar]
- 15.Manunta A, Gasparini G, Fadda M, Doria C, Lisai P, De Santis E. Il processo di maturazione dell’innesto autologo rotuleo: studio istologico ed ultrastrutturale. Il Ginocchio. 2001;17:20. [Google Scholar]
- 16.Branca A, Calvisi V, Tucci G, De Pamphilis R, Sagarriga Visconti C, Della Rocca C. Modifiche dell’ambiente articolare in seguito a fallimento di ricostruzione del LCA con legamento artificiale. Giornale Italiano di Ortopedia e Traumatologia. 1998;24( Suppl):377–386. [Google Scholar]
- 17.De Santis E, Gasparini G, Zirattu G. L’anatomia patologica dell’artrite reumatoide. Giornale Italiano di Ortopedia e Traumatologia. 1992;20:33–36. [Google Scholar]
- 18.De Santis E, Manunta A, Rinonapoli G, Rosa MA. La sinovite villonodulare pigmentosa del ginocchio. Il Ginocchio. 1994;10:9–20. [Google Scholar]
- 19.Zvijac JE, Lau AC, Hechtman KS, Uribe JW, Tjin-A-Tsoi EW. Arthroscopic treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 1999;15:613–617. doi: 10.1053/ar.1999.v15.015061. [DOI] [PubMed] [Google Scholar]
- 20.Tol JL, van Dijk CN. Etiology of the anterior ankle impingement syndrome: a descriptive anatomical study. Foot Ankle Int. 2004;25:382–386. doi: 10.1177/107110070402500603. [DOI] [PubMed] [Google Scholar]
- 21.Carreri C, Di Leo P, Santucci A, Barbarella R. Le rigidità articolari post- traumatiche e post-chirurgiche: definizione, classificazione, eziopatogenesi. Giornale Italiano di Ortopedia e Traumatologia. 1995;20( Suppl):59–69. [Google Scholar]
- 22.Fabbriciani C, Shiavone Panni A, Sagarriga Visconti C, Milano G. Chirurgia legamentosa e rigidità: eziopatogenesi. Giornale Italiano di Ortopedia e Traumatologia. 1995;21( Suppl):301–311. [Google Scholar]
- 23.Sanguinetti C, De Palma L, Specchia N. Fisiopatologia delle rigidità articolari post-traumatiche e post-chirurgiche. Giornale Italiano di Ortopedia e Traumatologia. 1995;25( Suppl):72–84. [Google Scholar]
- 24.Monteleone M, Maiotti M, Mastidoro L, Bosman C, Boldrini R, Biselli R. La malattia da depositi di pirofosfato di calcio diidrato. Giornale Italiano di Ortopedia e Traumatologia. 1994;20:179–186. [Google Scholar]