Abstract
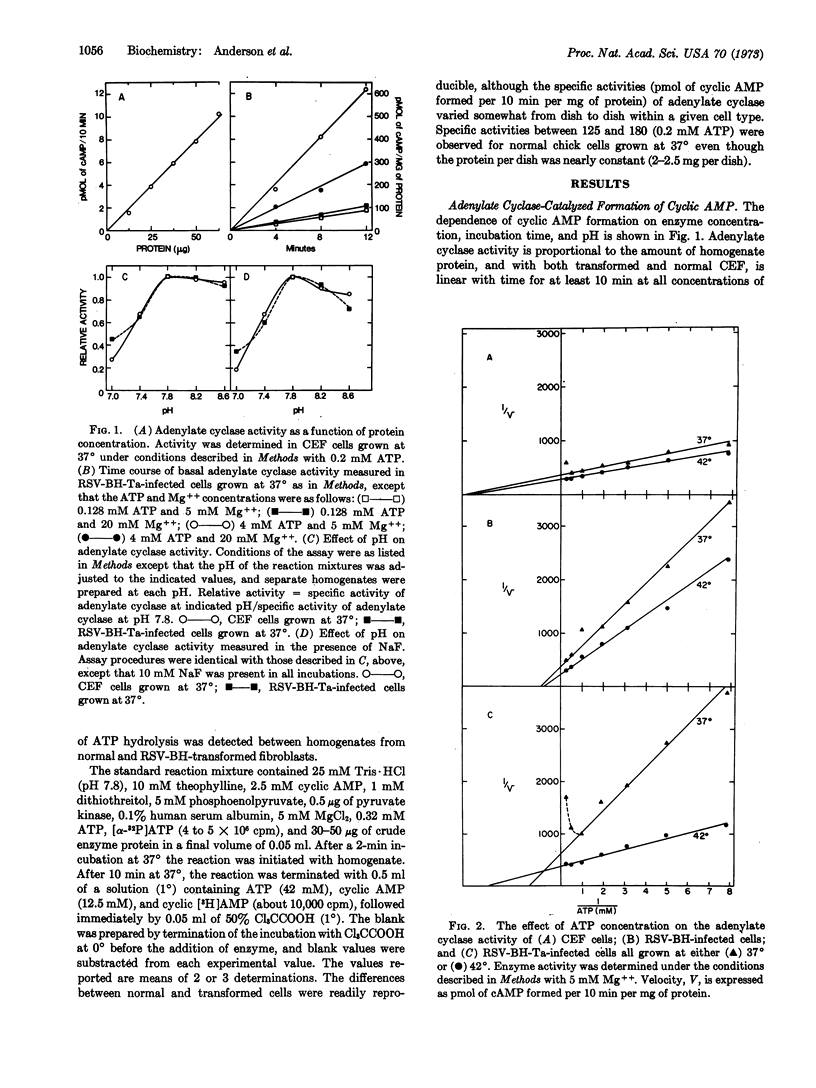
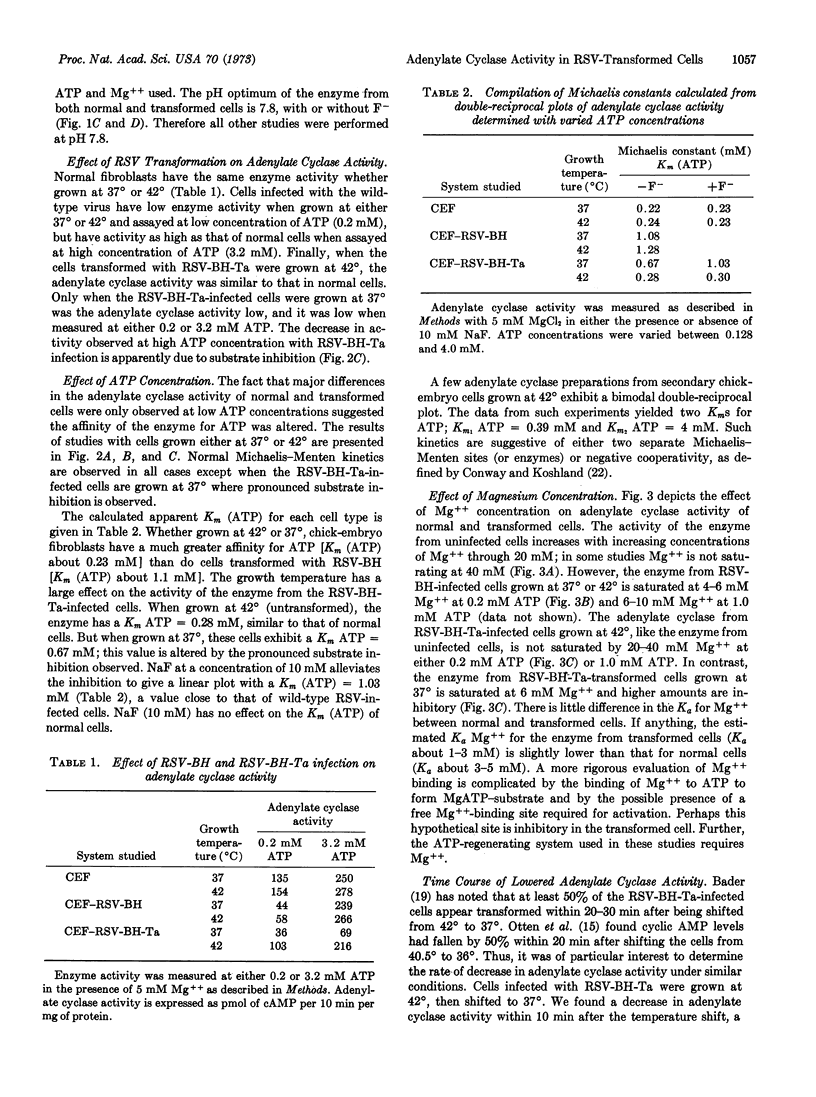
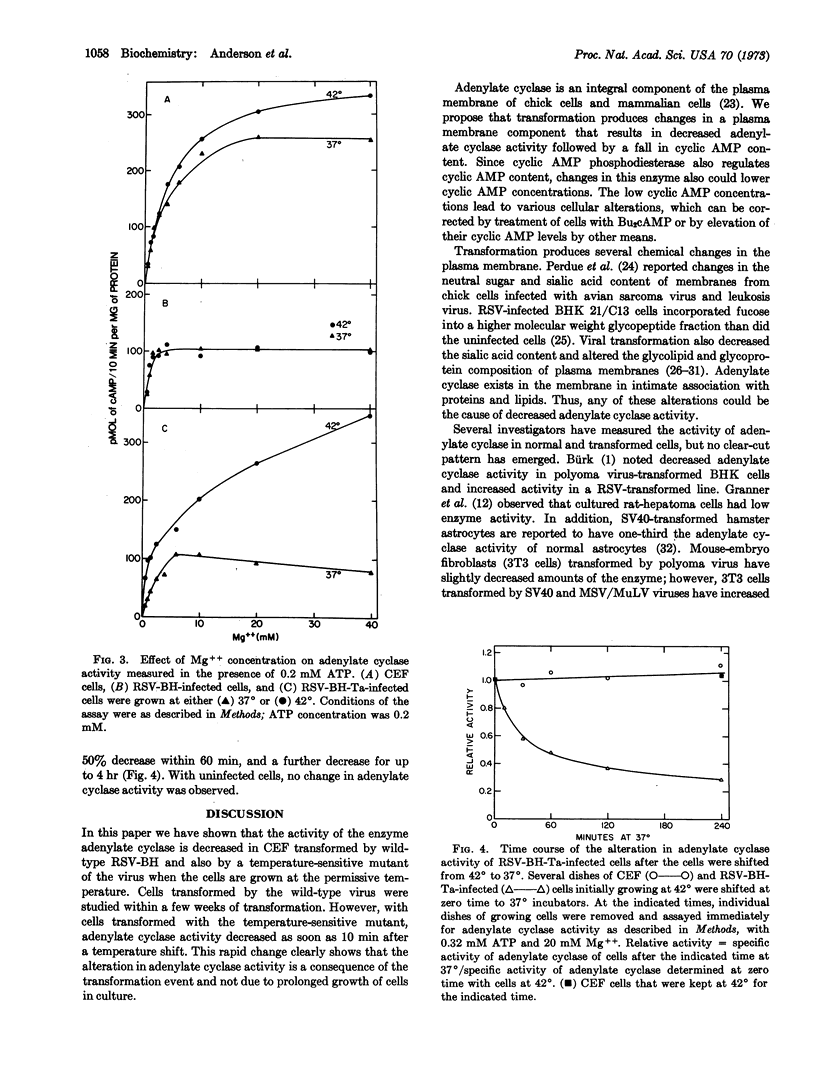
The activity of the enzyme adenylate cyclase, a component of the plasma membrane, has been determined in chick-embryo fibroblasts and in cells transformed by either Bryan high-titer strain of Rous sarcoma virus (RSV-BH) or a temperature-sensitive mutant of this virus (RSV-BH-Ta). Adenylate cyclase activity is reduced in cells transformed by the wild-type virus and also by the temperature-sensitive mutant when the cells are grown at the permissive temperature (37°). Transformation results in an altered affinity (Km) for the substrate (Mg ATP). The apparent Km ATP is 0.23 mM in normal cells and 1.1 mM in cells transformed with wild-type virus. The Km ATP of the cells infected with RSV-BH-Ta is 0.67-1.0 mM at 37° and 0.28 mM at 42°. The enzyme from normal cells appears to have two binding sites for Mg++, one at the catalytic site and a second at a regulatory site. Transformation by RSV-BH or RSV-BH-Ta (37°) apparently alters this second Mg++ site. A decrease in adenylate cyclase activity occurs within 10 min after cells infected with RSV-BH-Ta are shifted from 42° to 37°; the activity falls to one-half that of normal cells 30 min after the temperature shift. Our observations indicate that a viral function lowers cyclic AMP content by lowering the activity of adenylate cyclase, probably through some modification of the plasma membrane.
Keywords: ATP, Mg++, cyclic AMP
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Temperature-dependent transformation of cells infected with a mutant of Bryan Rous sarcoma virus. J Virol. 1972 Aug;10(2):267–276. doi: 10.1128/jvi.10.2.267-276.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Brady R. O., Borek C., Bradley R. M. Composition and synthesis of gangliosides in rat hepatocyte and hepatoma cell lines. J Biol Chem. 1969 Dec 10;244(23):6552–6554. [PubMed] [Google Scholar]
- Brown H. D., Chattopadhyay S. K., Morris H. P., Pennington S. N. Adenyl cyclase activity in Morris hepatomas 7777, 7794A, and 9618A. Cancer Res. 1970 Jan;30(1):123–126. [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Drummond G. I., Severson D. L., Duncan L. Adenyl cyclase. Kinetic properties and nature of fluoride and hormone stimulation. J Biol Chem. 1971 Jul 10;246(13):4166–4173. [PubMed] [Google Scholar]
- Goggins J. F., Johnson G. S., Pastan I. The effect of dibutyryl cyclic adenosine monophosphate on synthesis of sulfated acid mucopolysaccharides by transformed fibroblasts. J Biol Chem. 1972 Sep 25;247(18):5759–5764. [PubMed] [Google Scholar]
- Granner D., Chase L. R., Aurbach G. D., Tomkins G. M. Tyrosine aminotransferase: enzyme induction independent of adenosine 3', 5'-monophosphate. Science. 1968 Nov 29;162(3857):1018–1020. doi: 10.1126/science.162.3857.1018. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Cell density-dependent changes of glycolipid concentrations in fibroblasts, and loss of this response in virus-transformed cells. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1741–1747. doi: 10.1073/pnas.67.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrick M. L., Ryan W. L. Adenosine 3',5'-cyclic monophosphate and contact inhibition. Cancer Res. 1971 Sep;31(9):1313–1315. [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Morgan W. D., Pastan I. Regulation of cell motility by cyclic AMP. Nature. 1972 Jan 7;235(5332):54–56. doi: 10.1038/235054a0. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Role of 3',5'-adenosine monophosphate in regulation of morphology and growth of transformed and normal fibroblasts. J Natl Cancer Inst. 1972 May;48(5):1377–1387. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meezan E., Wu H. C., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969 Jun;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Brady R. O., Bradley R. M., McFarland V. W. Gangliosides in DNA virus-transformed and spontaneously transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1290–1296. doi: 10.1073/pnas.63.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata Y., Bader J. P. Studies on the fixation and development of cellular transformation by Rous sarcoma virus. Virology. 1968 Nov;36(3):401–410. doi: 10.1016/0042-6822(68)90165-7. [DOI] [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Peery C. V., Johnson G. S., Pastan I. Adenyl cyclase in normal and transformed fibroblasts in tissue culture. Activation by prostaglandins. J Biol Chem. 1971 Sep 25;246(18):5785–5790. [PubMed] [Google Scholar]
- Perdue J. F., Kletzien R., Miller K. The isolation and characterization of plasma membrane from cultured cells. I. The chemical composition of membrane isolated from uninfected and oncogenic RNA virus-converted chick embryo fibroblasts. Biochim Biophys Acta. 1971 Dec 3;249(2):419–434. doi: 10.1016/0005-2736(71)90120-9. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Ryan W. L., Heidrick M. L. Inhibition of cell growth in vitro by adenosine 3',5'-monophosphate. Science. 1968 Dec 27;162(3861):1484–1485. doi: 10.1126/science.162.3861.1484. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Drummond G. I., Sulakhe P. V. Adenylate cyclase in skeletal muscle. Kinetic properties and hormonal stimulation. J Biol Chem. 1972 May 10;247(9):2949–2958. [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui B., Hakomori S. Change of glycolipid pattern in Morris hepatomas 5123 and 7800. Cancer Res. 1970 Dec;30(12):2930–2936. [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]



