Our study demonstrated an increased risk of pancreatic adenocarcinoma in patients with an incidental pancreatic cyst found by using CT or MR imaging in a heterogeneous urban population; the relationship between an incidental pancreatic cyst and overall mortality was age-related because the all-cause mortality was considerably increased in those younger than 65 years and unchanged in those 65 years or older.
Abstract
Purpose
To establish the effect of incidental pancreatic cysts found by using computed tomographic (CT) and magnetic resonance (MR) imaging on the incidence of pancreatic ductal adenocarcinoma and overall mortality in patients from an inner-city urban U.S. tertiary care medical center.
Materials and Methods
Institutional review board granted approval for the study and waived the informed consent requirement. The study population comprised cyst and no-cyst cohorts drawn from all adults who underwent abdominal CT and/or MR November 1, 2001, to November 1, 2011. Cyst cohort included patients whose CT or MR imaging showed incidental pancreatic cysts; no-cyst cohort was three-to-one frequency matched by age decade, imaging modality, and year of initial study from the pool without reported incidental pancreatic cysts. Patients with pancreatic cancer diagnosed within 5 years before initial CT or MR were excluded. Demographics, study location (outpatient, inpatient, or emergency department), dates of pancreatic adenocarcinoma and death, and modified Charlson scores within 3 months before initial CT or MR examination were extracted from the hospital database. Cox hazard models were constructed; incident pancreatic adenocarcinoma and mortality were outcome events. Adenocarcinomas diagnosed 6 months or longer after initial CT or MR examination were considered incident.
Results
There were 2034 patients in cyst cohort (1326 women [65.2%]) and 6018 in no-cyst cohort (3,563 [59.2%] women); respective mean ages were 69.9 years ± 15.1(standard deviation) and 69.3 years ± 15.2, respectively (P = .129). The relationship between mortality and incidental pancreatic cysts varied by age: hazard ratios were 1.40 (95% confidence interval [CI]: 1.13, 1.73) for patients younger than 65 years and 0.97 (95% CI: 0.88, 1.07), adjusted for sex, race, imaging modality, study location, and modified Charlson scores. Incidental pancreatic cysts had a hazard ratio of 3.0 (95% CI: 1.32, 6.89) for adenocarcinoma, adjusted for age, sex, and race.
Conclusion
Incidental pancreatic cysts found by using CT or MR imaging are associated with increased mortality for patients younger than 65 years and an overall increased risk of pancreatic adenocarcinoma.
© RSNA, 2014
Introduction
Incidental pancreatic cysts are defined as those discovered on imaging studies performed either for pathologic evaluation in organs other than pancreas or for symptoms not specific to pancreatic disease (1). Detection of incidental pancreatic cysts is increasingly common because of ongoing technical improvements and increases in imaging utilization. The prevalence of pancreatic cysts is reported to be up to 2.6% by using computed tomographic (CT) imaging and up to 20% by using magnetic resonance (MR) imaging (2–5). The majority of the cysts represent mucinous lesions, such as mucinous cystic neoplasms and intraductal papillary mucinous neoplasms, because pseudocysts account for only about 4% of asymptomatic pancreatic cysts and true epithelial cysts of the pancreas are rare in the absence of systemic cystic disease, such as von Hippel-Lindau disease or polycystic kidney disease (6,7).
Although mucinous lesions are considered to be premalignant, current guidelines allow for conservative treatment of patients with pancreatic cysts, particularly those with cysts smaller than 3 cm (8–11). Patients deemed appropriate for conservative treatment undergo repeated follow-up, often without a definable end (10,11). The rate of malignancy in pancreatic cysts selected for radiographic follow-up is reported to be 1%–3% (12,13). In addition to the risk of a cyst-related malignancy, incidental pancreatic cysts have been shown to be associated with pancreatic ductal adenocarcinoma elsewhere in the pancreas among those with familial pancreatic cancer and in the Japanese population (14–17). Despite a large body of literature that assesses the relationship between pancreatic cysts and the risk of malignancy, to our knowledge, no study has evaluated the relationship between incidental pancreatic cysts and mortality.
The goal of our study was to establish the effect of incidental pancreatic cysts found by using CT and MR imaging on the incidence of pancreatic ductal adenocarcinoma and overall mortality in patients from an inner-city urban U.S. tertiary care medical center.
Materials and Methods
Institutional review board granted approval for the study and waived the informed consent requirement. The study was compliant with Health Insurance Portability and Accountability Act regulations. The study was supported by National Institutes of Health Clinical and Translational Science Awards Grant Number 1UL1TR001073 from the National Center for Advancing Translational Sciences. The study was designed as a retrospective cohort, with the presence of an incidental pancreatic cyst on abdominal CT or MR images defined as the exposure.
Cohort Selection
The institutional database was surveyed by using a decision support tool (DST) (Clinical Looking Glass; Streamline Health, Atlanta, Ga) that integrates information from various hospital databases, including medical records, cancer registry, and radiology records (18).
Cyst cohort.—The DST database was searched for abdominal CT or MR imaging performed between November 1, 2001, and November 1, 2011, in patients 18 years and older, for reports that contained the terms “pancr*” and “cyst*” in the same sentence. Reports that contained standardized negations (ie, the term “no cystic pancreatic”) were excluded. For each patient, the date of the earliest study meeting the search criteria was the index date. Patients with a diagnosis of any pancreatic neoplasm within 5 years before the index date were excluded.
All reports extracted from DST by the initial search were manually reviewed by one of two board-certified fellowship-trained abdominal radiologists (V.C. or M.F., with 11 and 9 years of experience, respectively). Patients with the following were excluded: history or signs of acute pancreatitis, autosomal dominant polycystic kidney disease or von Hippel-Lindau syndrome, reports with uncertain origin of the cyst, reports with uncertainty in the cyst description and lack of follow-up study confirming presence of the pancreatic cyst, reports with clinical indication of pancreatic lesion evaluation, or use of a pancreatic protocol.
In patients who had a report that referenced a previous imaging study, the radiology records were reviewed. The index date was changed to the earlier date if the earliest report that described a pancreatic cyst was performed between November 1, 2001, and November 1, 2011, in our institution. If the earliest study that described the pancreatic cyst was performed before November 1, 2001, or if its report was not available in our system, the patient was excluded. The index date was kept as the date of the DST-extracted report if the earlier studies did not describe a pancreatic cyst.
No-cyst cohort.—The pool of patients without a reported pancreatic cyst was established by searching the DST database for the reports of abdominal CT or MR imaging performed between November 1, 2001, and November 1, 2011, in patients who were 18 years or older, where the reports did not contain the terms “pancr*” and “cyst*” in the same sentence. The date of the earliest study was the index date for each patient. Patients with a diagnosis of any pancreatic neoplasm within 5 years before the index date and patients in the cyst cohort were excluded from the pool. The no-cyst cohort was then randomly selected, with frequency match on modality (CT vs MR imaging), age decade, and year of the index date. The ratio of the nonexposed (ie, no cyst) to exposed (ie, cysts) patients was three to one, respectively.
Clinical Data
Demographic data, location of the index imaging study (outpatient, inpatient, or emergency department), date of pancreatic cancer diagnosis, date of death, tumor location, and histologic analysis as recorded in the cancer registry were extracted from DST. For each patient, the date of the most recent encounter, defined as the date of the latest laboratory test, imaging study, or outpatient, inpatient, or emergency department visit was obtained. Modified Charlson comorbidity index was calculated by DST for each patient by using the data from the outpatient, inpatient, and emergency department encounters within 90 days before to the index date. Charlson comorbidity index is a validated tool for prognosis of mortality and for comorbidity adjustment (19–21). The score combines information about the patient regarding age and history of the following: myocardial infarction, congestive heart failure, peripheral vascular disorders, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, moderate or severe liver disease, diabetes without complications, diabetes with chronic complications, hemiplegia or paraplegia, renal disease, any malignancy, metastatic solid tumor, and human immunodeficiency virus or acquired immunodeficiency syndrome.
Data from Imaging Reports
The following imaging characteristics of the pancreatic cysts were recorded for every patient in the cyst cohort from the imaging report: number of cysts, location and the largest dimension of the largest cyst, presence of enhanced component, septations, calcifications, main pancreatic ductal dilatation, and regional lymphadenopathy. The number of the pancreatic cysts was recorded as one or greater than one. Cases with the largest cyst, described as sub-centimeter, were coded separately from those with no reported size. Pancreatic ductal dilatation was present if the report described main pancreatic ductal as either dilated or prominent, or if the report provided main pancreatic ductal measurement larger than 3 mm.
Statistical Analysis
Statistical analysis was performed with statistical software (Stata version 13.1; StataCorp, College Station, Tex). P values less than .05 indicated statistical significance.
Bivariate associations of the continuous variables were compared by using student t test or Wilcoxon rank-sum test, as appropriate. Bivariate associations of the categorical variables were compared by using the χ2 test or Fisher exact test, as appropriate. Cumulative incidences were calculated by using the life table and Kaplan-Meier methods and compared by using the log-rank test.
Cox proportional hazard model with incident pancreatic adenocarcinoma as the outcome was constructed and censored at the date of the latest encounter. Incident pancreatic adenocarcinomas were defined as cases diagnosed at least 6 months after the index date. Cases with pancreatic adenocarcinoma diagnosed less than 6 months after the index date and any with other pancreatic neoplasm histologic results were excluded from this analysis. Only one variable (the presence of a pancreatic cyst) was found to have a P value less than .10 at the bivariate analysis, and it was included in the initial model. Age, sex, and race were also included because they were to be included in the final model a priori. A forward stepwise selection approach was used to evaluate other covariates (modality, study location, and Charlson score), with entry criteria defined as either 10% or greater change in the coefficient of the variable of interest (presence of a pancreatic cyst) or statistical significance of the coefficient for the covariate. First-order interactions with the variable of interest were not evaluated because of insufficient power to detect the interactions in this model.
Cox proportional hazard model with all-cause mortality as the outcome was constructed and censored either at November 1, 2011, or at the date of the most recent encounter, whichever was most recent. This method was used because the DST contains full social security death data until only November 1, 2011, and death data from hospital records thereafter. Covariates with P values less than .10 at the bivariate analysis were included in the initial model. The final model was constructed by using a backward stepwise selection approach, with exit criteria defined as a change in the coefficient of the variable of interest (ie, presence of a pancreatic cyst) of 10% or less and lack of statistical significance of the coefficient for the covariate. Age, sex, and race were to be included in the model a priori. First-order interactions with the variable of interest were evaluated, with interaction considered to be present if an interaction term had P value less than .10.
Proportional hazard assumptions were assessed for all models, and extended Cox model was used where violation was found.
Results
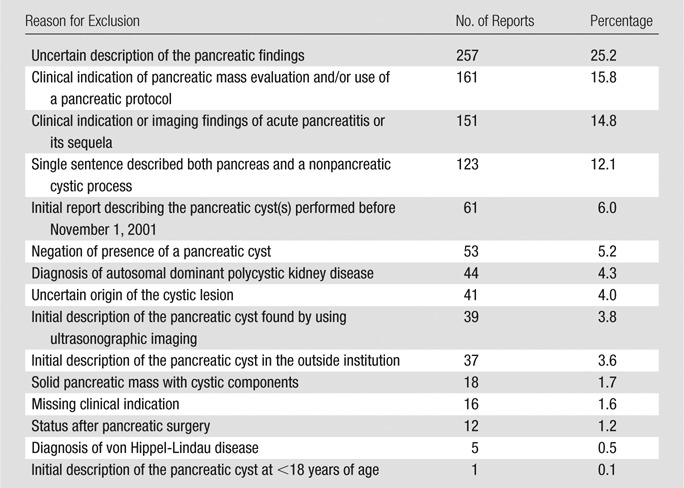
The initial DST cyst cohort search identified 3053 reports. Of these, 1019 reports (33.4%) were excluded after the manual review. The reasons for exclusion are summarized in Table 1. The index date was changed to an earlier one upon manual review of the reports in 252 (12.4%) of the remaining 2034 cases.
Table 1.
Summary of the 1019 Reports Excluded from the Cyst Cohort
The study population included 8052 patients: 2034 in the cyst cohort and 6018 in the no-cyst cohort. Among the 2034 patients in the cyst cohort, incidental pancreatic cysts were diagnosed by using CT imaging in 1524 patients (74.9%) and by using MR imaging in 510 patients (25.1%). In the cyst cohort, intravenous contrast material was administered in 1156 patients (75.8%) who underwent 1524 CT examinations and in 405 patients (79.4%) who underwent 510 MR examinations. MR cholangiopancreatographic imaging sequences were reported to be used in 285 (19.7%) of 1161 MR examinations in the no-cyst cohort and in 113 (22.2%) of 397 MR examinations in the cyst cohort (P = .238). The mean interval between the index date and the date of the most recent encounter was 3.37 years ± 2.7 (standard deviation) in the cyst cohort and 3.23 years ± 2.8 in the no-cyst cohort (P = .04). Table 2 summarizes the demographic characteristics of the study population. There was no statistically significant difference between the cohorts in the mean age, modality, and index year between the cyst and no-cyst cohorts (Table 2). When the cyst cohort was compared with the no-cyst cohort, the cyst cohort contained a higher percentage of women (65.2% vs 59.2%, respectively; P < .001) and patients who were white (31.2% vs 25.9%, respectively; P < .001), and had a higher interquartile range of Charlson score (0–2 vs 0–0, respectively; P < .001).
Table 2.
Demographic Characteristics of Patients in Cyst and No-Cyst Cohorts
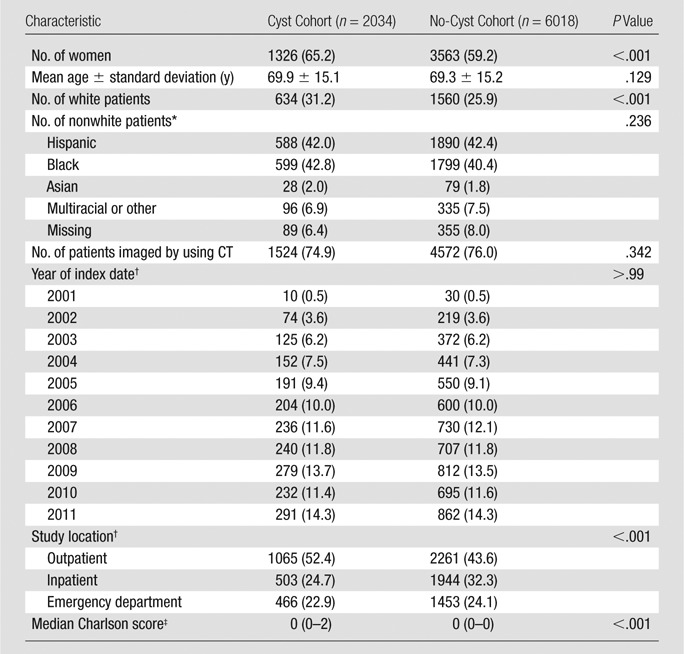
Note.—Data in parentheses are percentages except where indicated.
Percentage is given from a total nonwhite population in each cohort, 1400 patients in cyst cohort, and 4458 in no-cyst cohort.
Data in parentheses are the number of patients.
Data in parentheses are interquartile range.
Table 3 summarizes the imaging characteristics of the incidental pancreatic cystic lesions. We found that 75.4% of pancreatic cysts were solitary with a median cyst diameter of 10 mm. The largest cyst was most commonly in the pancreatic head or uncinate (38.1%). Septations (3.9%), enhanced components (2.5%), and calcification (2.8%) were uncommon. Main pancreatic duct dilatation was reported in 5.8% and regional lymphadenopathy in 1.2%. Cysts were simple (no septations, enhanced components, or calcifications) in 1070 of 1156 cases (92.6%) that were demonstrated by using contrast-enhanced CT examinations. We found 384 (94.8%) of 405 cysts that were demonstrated by using contrast-enhanced MR examinations were simple (ie, no septations or enhanced components).
Table 3.
Comparison of the Patient-specific and Imaging-specific Characteristics between the Patients in the Cyst Cohort Who Developed an Incident Pancreatic Ductal Adenocarcinoma and Those Who Did Not
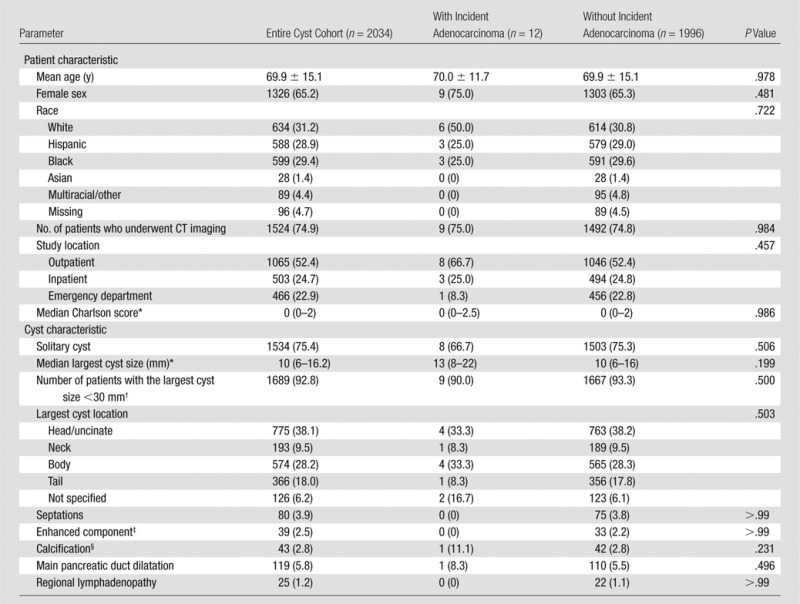
Note.—Data are number of patients unless otherwise indicated; data in parentheses are percentages unless otherwise indicated. Twelve patients in the cyst cohort developed an incident pancreatic ductal adenocarcinoma and 1996 patients did not. These data exclude patients with pancreatic neoplasms with histologic analysis that revealed lesions that were not adenocarcinoma (n = 10) and patients with pancreatic adenocarcinoma diagnosed less than 6 months after the index date (n = 16). The largest cyst size was based on patients with reported exact size of the largest cyst (1748, 10, and 1715 patients in total cyst cohort, incident adenocarcinoma, and without incident adenocarcinoma, respectively).
Data in parentheses are interquartile ratio.
Based on patients with either reported exact size of the largest cyst or the largest cyst reported as sub-centimeter: 1819 patients in total cyst cohort, 10 patients with incident adenocarcinoma, and 1786 patients without incident adenocarcinoma.
Based on studies performed with intravenous contrast administration: 1561 patients in total cyst cohort, eight patients with incident adenocarcinoma, and 1499 patients without incident adenocarcinoma.
Based on the total number of CT examinations performed: 1524 patients in total cyst cohort, nine patients with incident adenocarcinoma, and 1492 patients without incident adenocarcinoma.
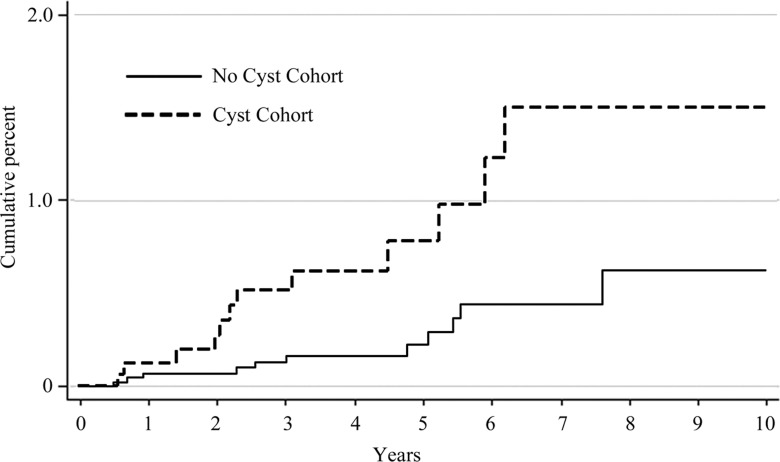
A pancreatic neoplasm was diagnosed after the index date in 72 patients: 38 (1.9%) of 2034 patients in cyst cohort and 34 (0.6%) of 6018 patients in no-cyst cohort (P < .0001). Table 4 summarizes the histologic diagnoses of these tumors. In the entire study population, ductal adenocarcinoma was diagnosed in 51 patients, of which 23 (45.1%) were diagnosed 6 months or longer after the index date (incident adenocarcinoma). Incident adenocarcinoma was diagnosed in 12 (0.6%) of 2034 patients in the cyst cohort and in 11 (0.2%) of 6018 patients in the no-cyst cohort (P = .003). Ten-year cumulative incidences of ductal adenocarcinoma were 1.5% (95% confidence interval [CI]: 0.79, 2.95) and 0.6% (95% CI: 0.29, 1.26) in the cyst and no-cyst cohorts, respectively (P = .004) (Figure). The incidence rates of ductal adenocarcinoma were 1.8 (95% CI: 1.0, 3.1) and 0.6 (95% CI: 0.3, 1.0) per 1000 person-years in the cyst and no-cyst cohorts, respectively (P = .004).
Table 4.
Summary of the Histologic Diagnoses in 72 Patients with Diagnosis of a Pancreatic Malignancy after Index Date
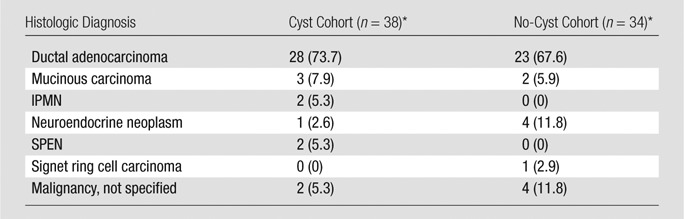
Note.—Data are number of patients; data in parentheses are percentages. P value for cohorts was .341. IMPN = intraductal papillary mucinous carcinoma, SPEN = solid pseudopapillary epithelial neoplasm.
Figure a:
Graph shows Kaplan-Meier analysis of cumulative incidence of (a) pancreatic ductal adenocarcinoma and (b) mucinous malignancies.
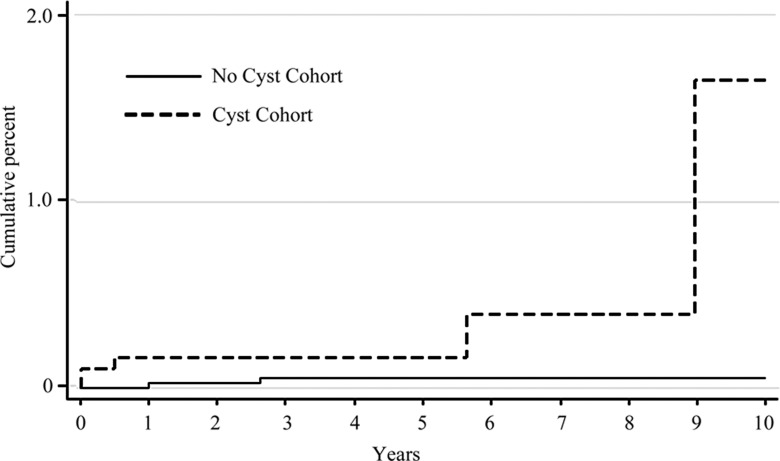
Figure b:
Graph shows Kaplan-Meier analysis of cumulative incidence of (a) pancreatic ductal adenocarcinoma and (b) mucinous malignancies.
Patient-specific and imaging-specific characteristics of the cyst cohort patients who developed an incident adenocarcinoma (n = 12) and those who did not (n = 1996) are summarized in Table 3. There was no statistically significant difference between these two groups based on age, sex, race, or modified Charlson score. Similarly, there was no statistically significant difference between the imaging characteristics of these two groups, including number of cysts, location and size of the largest cyst, presence of septations, enhanced components, calcifications, main pancreatic duct dilatation, or regional lymphadenopathy. Additionally, the two groups did not differ based on a percentage of patients with the largest cyst size smaller than 30 mm (90.0% in patients with incident adenocarcinoma and 93.3% in patients without incident adenocarcinoma; P = .500).
In the Cox regression model, the presence of an incidental pancreatic cyst was associated with a risk of incident adenocarcinoma that is three times higher (95% CI: 1.32, 6.89; P = .009), adjusted for race (white vs nonwhite), age (<65 years vs ≥65 years), and sex. Since main pancreatic duct dilatation has been shown to be a strong predictor of malignancy (14), Cox analysis was repeated by excluding the 119 patients with main pancreatic duct dilatation; the adjusted hazard ratio for the presence of an incidental pancreatic cyst in this group remained similarly elevated at 2.9 (95% CI: 1.25, 6.72; P = .013).
Among 12 patients with incident adenocarcinoma in the cyst cohort, the location of the cyst on the initial study was documented in 10 patients (83.3%). Of these 10 patients, the adenocarcinoma arose in a portion of the pancreas different from the location of the cyst in seven patients (70%).
Thirteen (0.64%) of 2034 patients in the cyst cohort had a pathologic analysis–proven diagnosis of a mucinous lesion. The mean interval between the initial imaging study and the procedure to establish the diagnosis was 4.4 years ± 3.7, and it was longer than 6 months in 10 (76.9%) of 13 cases. Of the 13 mucinous lesions, eight (61.5%) were mucinous cysts or mucinous cystic neoplasms and five (38.5%) were intraductal papillary mucinous neoplasms. Five (38.5%) of 13 mucinous lesions in the cyst cohort were malignant, three (23.1%) were mucinous cystic neoplasms, and two (15.4%) were intraductal papillary mucinous neoplasms. There were two cases of mucinous cystic neoplasm in the no-cyst cohort. Ten-year cumulative incidence of malignant mucinous lesions was 1.3% (95% CI: 0.31, 5.04) and 0.05% (95% CI: 0.01, 0.21) in the cyst and no-cyst cohorts, respectively (P = .006) (Figure). The incidence rates of mucinous malignancy were 0.7 (95% CI: 0.3, 1.8) and 0.1 (95% CI: 0.02, 0.4) per 1000 person-years in the cyst and no-cyst cohorts, respectively (P = .006).
Of 2034 patients in the cyst cohort, 699 patients (34.4%) died, and of the 6018 patients in the no-cyst cohort, 1923 (31.2%) died (P = .045). The all-cause mortality rates in the cyst and no-cyst cohorts were 90.6 (95% CI: 84.1, 97.6) and 83.3 (95% CI: 79.7, 87.2) per 1000 person-years, respectively (P = .09).
In the initial multivariate Cox model with the outcome of all-cause mortality, an interaction between the presence of a pancreatic cyst and age was observed. Stratification of the model based on two age groups removed the interaction from each stratum. The results of the final Cox models are summarized in Table 5. The presence of a pancreatic cyst was associated with 40% increased risk of all-cause mortality in patients younger than 65 years, and no change in all-cause mortality in patients 65 years or older, adjusted for age, sex, race (white vs nonwhite), modified Charlson score, study location, and imaging modality.
Table 5.
Results of Multivariate Cox Analysis with All-Cause Mortality as an Outcome

Model adjusted for age, race (white vs nonwhite patients), sex, Charlson score, modality, and time-dependent function of study location (outpatient, inpatient, or emergency department).
Model adjusted for race (white vs nonwhite patients), sex, modality, and time-dependent functions of age, Charlson score, and study location (outpatient, inpatient, or emergency department).
Discussion
Our retrospective cohort study demonstrated a threefold increased risk of pancreatic ductal adenocarcinoma in patients with incidentally discovered pancreatic cysts. Despite elevated risk of ductal adenocarcinoma and cyst-related malignancies (mucinous cystic neoplasm and intraductal papillary mucinous carcinoma), there was no increase in all-cause mortality in patients over 65 years. Incidental pancreatic cysts were associated with increased all-cause mortality in patients younger than 65 years.
In our cohort of patients with pancreatic cysts, the incidence rate of malignant mucinous neoplasms was 0.7 per 1000 person-years. This is comparable to the reported prevalence rate of mucin-producing adenocarcinoma that arises in patients with pancreatic cysts, which is estimated to be 0.33 per 1000 patients (22). Pancreatic cysts have malignant potential, and several studies (14–16) in a Japanese population demonstrated an association between the pancreatic cysts and pancreatic ductal adenocarcinoma. Tanaka et al (14) demonstrated that in the absence of main pancreatic ductal dilatation, presence of a pancreatic cyst has a hazard ratio of 3.1 (95% CI: 0.19, 49.21; P = .431) for development of pancreatic adenocarcinoma. Our study demonstrated a similar estimate of a hazard ratio despite stark differences in study populations with respect to race of patients (only about 2% of our patients were Asian). Additionally, our study found the hazard ratio to be statistically significant (hazard ratio, 2.9 [95% CI: 1.25, 6.72]; P = .013). This disparity can be explained by the differences in study power: the number of patients with pancreatic cysts in our analysis was 12 times as many as in Tanaka et al.
In patients who have familial pancreatic cancer, small intraductal papillary mucinous neoplasms are associated with the presence of high-grade pancreatic intraepithelial neoplasia (a precursor to adenocarcinoma) elsewhere in the pancreas (17). The results of our study indicate that this may be true in a general population because the tumor is in the part of the pancreas that is different than the location of the original cyst in 70% of our patients with incident pancreatic adenocarcinoma. Similarly, Tada et al (16) found carcinomas in regions remote from preexisting cyst in two of their seven Japanese patients.
Our study demonstrated that the relationship between presence of pancreatic cyst and all-cause mortality is dependent on age: the patients younger than 65 years had a 40% higher risk of mortality if they had a pancreatic cyst, and in the patients 65 years or older, pancreatic cysts were associated with no change in risk of death.
The imaging characteristics of pancreatic cysts in our study population were comparable to those in the published literature. In our cohort of patients with incidental pancreatic cysts, 75% of cysts were solitary, 93% were smaller than 3 cm, 93% were simple, and 94% were not associated with pancreatic duct dilatation. In the literature (2,4,5,23), 60%–85% of pancreatic cysts are reported to be solitary, 89% are reported to be smaller than 3 cm, and 88%–94% are reported to be simple. The pancreatic cysts were most commonly seen in the pancreatic head (38%), which is similar to the 42% in the published literature (23).
Current recommendations of pancreatic cyst follow-up take into account various imaging characteristics of the pancreatic cysts, such as cyst size, presence of solid components, pancreatic ductal dilatation, and various biochemical profiles, but there is no age limit at which the follow-up should be less aggressive (8,10). As a result, incidental discovery of the pancreatic cysts affects health care costs (24). In part, continuous imaging in older patients is thought to be warranted because older age is associated with presence of premalignant or malignant cysts (23,25). Despite increased risk of both mucinous malignancies and ductal adenocarcinoma, the 10-year cumulative incidences of these cancers in patients with pancreatic cysts are still low at 0.7% and 1.5%, respectively. Moreover, our data indicate lack of increase in overall mortality in patients over 65 years with pancreatic cysts, and this indicates that it may be safe to avoid aggressive imaging follow-up and work-up in this population, which relieves some of the burden on health care cost. Given the large size of our study and the fact that our study population of pancreatic cysts is comparable to the published data in terms of imaging characteristics and incidences of cyst-related malignancies and pancreatic ductal adenocarcinoma, we believe that the observed age-related effects of the pancreatic cysts on all-cause mortality may be generalizable.
Our study has several limitations. First, we did not review the images of the studies but rather extracted the information from the reports. This approach introduced some bias due to considerable heterogeneity in reporting of the imaging findings attributable to multiple readers. Additionally, the reports themselves are associated with internal sensitivity and specificity for detection of pancreatic cysts. However, this design assessed the real-world situation where a referring physician is presented with the radiologic report describing a pancreatic cyst, and thus helped to establish the implication of such a report on the patient outcome.
An additional limitation of our study is that the index date in patients whose initial report was not picked up by the DST search was changed to the date of the earliest report that described the pancreatic cyst. Thus, the patients with the reports that described pancreatic cyst in a way that precluded their identification by the DST search criteria and without a follow-up imaging study were excluded. This resulted in having a higher proportion of patients with at least one follow-up study in the cyst cohort, which somewhat biases our all-cause mortality results toward the null hypothesis. Therefore, the true effects of the pancreatic cyst may in fact be slightly higher than what we have observed. However, the index date was changed only in approximately 12% of the cyst cohort.
It is important to note that inclusion criteria of our study did not require pathologic confirmation of either presence of the pancreatic cyst or lack thereof. Because of our study design and imperfect intrinsic sensitivities and specificities of the imaging modalities for the detection of pancreatic cysts, our cyst cohort included a certain number of cases with false-positive findings and our no-cyst cohort included a certain number of cases with false-negative findings. This resulted in nondifferential misclassification bias, which biased the observed results toward the null hypothesis. This study design was chosen to reflect the clinical reality, where the majority of patients do not undergo confirmatory studies once they are diagnosed with a pancreatic cyst based on cross-sectional imaging, and where patients with pancreatic cysts “missed” at imaging remain undiagnosed. Thus, our study evaluated the effect of incidental pancreatic cysts as they were diagnosed by using CT or MR imaging, which is the most clinically relevant scenario. Moreover, if the study were limited to only the patients in whom presence of the pancreatic cysts was confirmed by means of endoscopic ultrasonographic imaging or surgery, a considerable selection bias would have been introduced. Additionally, as lack of the pancreatic cysts is not generally confirmed by pathologic analysis, a differential misclassification would have also been present, and its effect on the observed result would not be easily predictable.
In conclusion, our study demonstrated an increased risk of pancreatic adenocarcinoma in patients with an incidental pancreatic cyst found by using CT or MR imaging in a heterogeneous urban population; the relationship between an incidental pancreatic cyst and overall mortality was related to age, and the all-cause mortality was considerably increased in those younger than 65 years and unchanged in in those 65 years or older. Further study is required to better define the appropriate cost-effective imaging follow-up for incidental pancreatic cysts found by using CT and MR imaging in patients older than 65 years.
Advances in Knowledge
■ Incidental pancreatic cysts are associated with three times increased risk of pancreatic ductal adenocarcinoma in adult population.
■ The effect of an incidental pancreatic cyst on overall mortality is age-related; all-cause mortality is 40% higher in patients older than 65 years and unchanged in patients 65 years or older.
Implications for Patient Care
■ In patients younger than 65 years with a diagnosis of incidental pancreatic cysts by using CT or MR imaging, there is increased risk of both pancreatic adenocarcinoma and overall mortality: therefore, in this group, surveillance is warranted.
■ In patients 65 years or older with incidental pancreatic cysts, there is increased risk of pancreatic adenocarcinoma, but no change in overall mortality compared with patients without cysts; therefore, aggressive surveillance may not be warranted in this age group.
Received April 2, 2014; revision requested May 9; revision received May 26; accepted June 12; final version accepted June 12.
From the 2013 RSNA Annual Meeting.
Funding: This research was supported by the National Institutes of Health (grant 1UL1TR001073).
Disclosures of Conflicts of Interest: V.C. disclosed no relevant relationships. M.F. disclosed no relevant relationships. L.B.H. disclosed no relevant relationships. A.M.R. disclosed no relevant relationships. E.B. disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- DST
- decision support tool
References
- 1.Zárate X, Williams N, Herrera MF. Pancreatic incidentalomas. Best Pract Res Clin Endocrinol Metab 2012;26(1):97–103. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology 2002;223(2):547–553. [DOI] [PubMed] [Google Scholar]
- 3.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg 2004;239(5):651–657; discussion 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008;191(3):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105(9):2079–2084. [DOI] [PubMed] [Google Scholar]
- 6.Tanno S, Obara T, Izawa T, et al. Solitary true cyst of the pancreas in two adults: analysis of cyst fluid and review of the literature. Am J Gastroenterol 1998;93(10):1972–1975. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg 2003;138(4):427–3; discussion 433–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goh BK, Tan YM, Chung YF, et al. Pancreatic cysts: a proposed management algorithm based on current evidence. Am J Surg 2007;193(6):749–755. [DOI] [PubMed] [Google Scholar]
- 9.Das A, Wells CD, Nguyen CC. Incidental cystic neoplasms of pancreas: what is the optimal interval of imaging surveillance? Am J Gastroenterol 2008;103(7):1657–1662. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12(3):183–197. [DOI] [PubMed] [Google Scholar]
- 11.Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 2010;7(10):754–773. [DOI] [PubMed] [Google Scholar]
- 12.Allen PJ, D’Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg 2006;244(4):572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahav M, Maor Y, Avidan B, Novis B, Bar-Meir S. Nonsurgical management of asymptomatic incidental pancreatic cysts. Clin Gastroenterol Hepatol 2007;5(7):813–817. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Nakao M, Ioka T, et al. Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: a prospective study. Radiology 2010;254(3):965–972. [DOI] [PubMed] [Google Scholar]
- 15.Uehara H, Nakaizumi A, Ishikawa O, et al. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 2008;57(11):1561–1565. [DOI] [PubMed] [Google Scholar]
- 16.Tada M, Kawabe T, Arizumi M, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol 2006;4(10):1265–1270. [DOI] [PubMed] [Google Scholar]
- 17.Bartsch DK, Dietzel K, Bargello M, et al. Multiple small “imaging” branch-duct type intraductal papillary mucinous neoplasms (IPMNs) in familial pancreatic cancer: indicator for concomitant high grade pancreatic intraepithelial neoplasia? Fam Cancer 2013;12(1):89–96. [DOI] [PubMed] [Google Scholar]
- 18.Bellin E, Fletcher DD, Geberer N, Islam S, Srivastava N. Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med 2010;85(8):1362–1368. [DOI] [PubMed] [Google Scholar]
- 19.Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser score work. Med Care 2013 May 23. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 20.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 21.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57(12):1288–1294. [DOI] [PubMed] [Google Scholar]
- 22.Gardner TB, Glass LM, Smith KD, et al. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Am J Gastroenterol 2013;108(10):1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SH, Shin CM, Park JK, et al. Outcomes of cystic lesions in the pancreas after extended follow-up. Dig Dis Sci 2007;52(10):2653–2659. [DOI] [PubMed] [Google Scholar]
- 24.Das A, Ngamruengphong S, Nagendra S, Chak A. Asymptomatic pancreatic cystic neoplasm: a cost-effectiveness analysis of different strategies of management. Gastrointest Endosc 2009;70(4):690–699, e6. [DOI] [PubMed] [Google Scholar]
- 25.Atef E, El Nakeeb A, El Hanafy E, El Hemaly M, Hamdy E, El-Geidie A. Pancreatic cystic neoplasms: predictors of malignant behavior and management. Saudi J Gastroenterol 2013;19(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]