Abstract
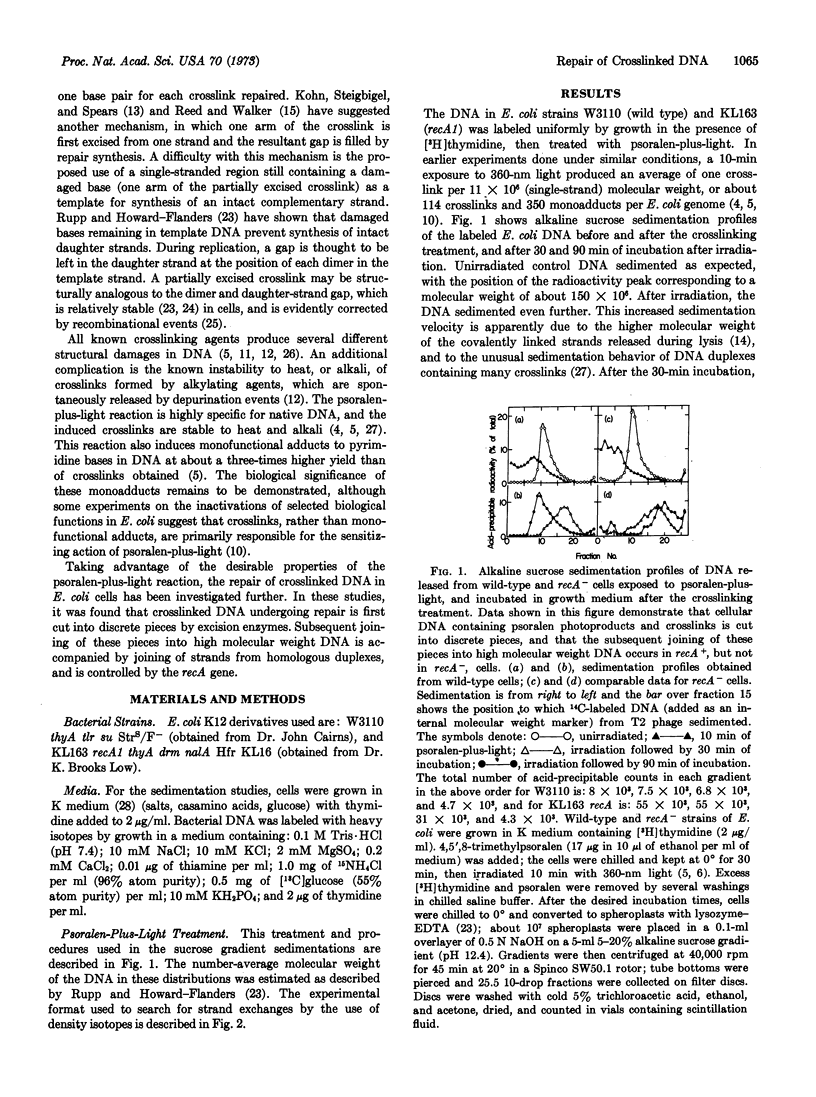
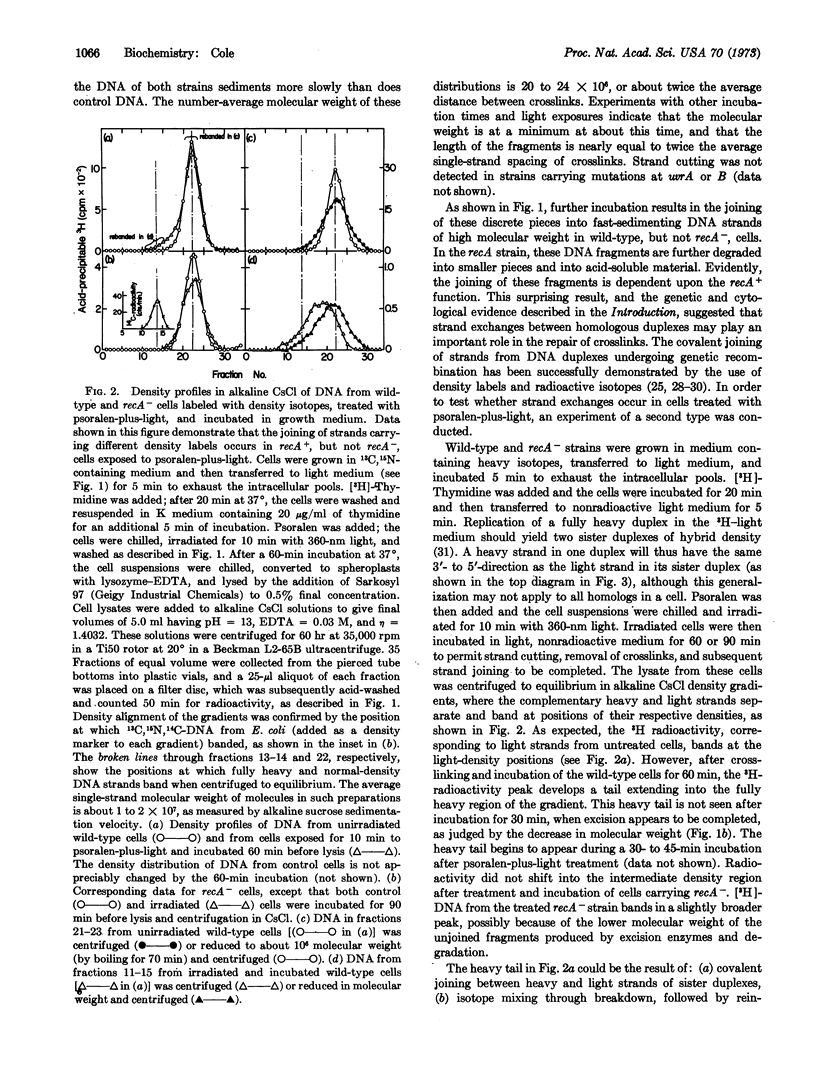
The repair of DNA containing interstrand crosslinks induced by psoralen-plus-light in E. coli cells has been investigated. During a 30-minute incubation after psoralen-plus-light treatment, crosslinks were excised and the cellular DNA was cut into discrete pieces. The molecular weight of these pieces corresponds to about twice the single-strand distance between crosslinks, as measured by sedimentation velocity in alkaline sucrose. During further incubation, these DNA fragments were covalently joined into high molecular weight DNA. This joining did not occur in cells carrying a mutation at recA; in these strains the DNA was further degraded to smaller polynucleotides and acid-soluble material. The possibility that repair of crosslinked DNA involves strand exchanges between homologous duplexes was investigated. Cells were grown in 13C,15N-containing medium for several generations, then switched to medium of normal density that also contained [3H]thymidine for about 0.5 generation. After the crosslinking treatment, the cells were incubated in medium of normal density in order for repair to occur. The DNA was extracted and centrifuged in alkaline CsCl density gradients, where the light and heavy strands were separated. Molecules of intermediate density that contained 3H accumulated during repair in wild-type cells, but not in control cells or treated recA- cells. After molecular weight reduction of the intermediate-density DNA, the 3H could be separated from the heavy strands, demonstrating that covalent joining between heavy and light strands of homologous duplexes accompanies repair. A mechanism involving sequential excision and genetic recombination is proposed for the repair of DNA containing interstrand crosslinks.
Keywords: genetic recombination, psoralen
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brookes P., Lawley P. D. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem J. 1961 Sep;80(3):496–503. doi: 10.1042/bj0800496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Inactivation of Escherichia coli, F' episomes at transfer, and bacteriophage lambda by psoralen plus 360-nm light: significance of deoxyribonucleic acid cross-links. J Bacteriol. 1971 Sep;107(3):846–852. doi: 10.1128/jb.107.3.846-852.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Properties of F' factor deoxyribonucleic acid transferred from ultraviolet-irradiated donors: photoreactivation in the recipient and the influence of recA, recB, recC, and uvr genes. J Bacteriol. 1971 Apr;106(1):143–149. doi: 10.1128/jb.106.1.143-149.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Cole R. S., Zusman D. Sedimentation properties of phage DNA molecules containing light-induced psoralen cross-links. Biochim Biophys Acta. 1970 Dec 14;224(2):660–662. doi: 10.1016/0005-2787(70)90607-6. [DOI] [PubMed] [Google Scholar]
- Crathorn A. R., Roberts J. J. Mechanism of the cytotoxic action of alkylating agents in mammalian cells and evidence for the removal of alkylated groups from deoxyribonucleic acid. Nature. 1966 Jul 9;211(5045):150–153. doi: 10.1038/211150a0. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Rodighiero G. Inter-strand cross-linkages occurring in the photoreaction between psoralen and DNA. FEBS Lett. 1970 Jul 29;9(2):121–123. doi: 10.1016/0014-5793(70)80330-1. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Scott D. The induction of chromosome aberrations by nitrogen mustard and its dependence on DNA synthesis. Proc R Soc Lond B Biol Sci. 1969 Jul 22;173(1033):491–512. doi: 10.1098/rspb.1969.0073. [DOI] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P. "Reversible" DNA. Proc Natl Acad Sci U S A. 1961 Jul 15;47:950–955. doi: 10.1073/pnas.47.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J., La Rock J. Chromosomal effects of mitomycin, a potential recombinogen in mammalian cell genetics. Tex Rep Biol Med. 1969 Summer;27(2):409–418. [PubMed] [Google Scholar]
- HOLLIDAY R. THE INDUCTION OF MITOTIC RECOMBINATION BY MITOMYCIN C IN USTILAGO AND SACCHAROMYCES. Genetics. 1964 Sep;50:323–335. doi: 10.1093/genetics/50.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn K. W., Steigbigel N. H., Spears C. L. Cross-linking and repair of DNA in sensitive and resistant strains of E. coli treated with nitrogen mustard. Proc Natl Acad Sci U S A. 1965 May;53(5):1154–1161. doi: 10.1073/pnas.53.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LETT J. T., STACEY K. A., ALEXANDER P. Crosslinking of dry deoxyribonucleic acids by electrons. Radiat Res. 1961 Apr;14:349–362. [PubMed] [Google Scholar]
- Lawley P. D., Brookes P. Cytotoxicity of alkylating agents towards sensitive and resistant strains of Escherichia coli in relation to extent and mode of alkylation of cellular macromolecules and repair of alkylation lesions in deoxyribonucleic acids. Biochem J. 1968 Sep;109(3):433–447. doi: 10.1042/bj1090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Brookes P. Molecular mechanism of the cytotoxic action of difunctional alkylating agents and of resistance to this action. Nature. 1965 May 1;206(983):480–483. doi: 10.1038/206480a0. [DOI] [PubMed] [Google Scholar]
- Ley R. D., Setlow R. B. Repair replication in Escherichia coli as measured by the photolysis of bromodeoxyuridine. Biophys J. 1972 Apr;12(4):420–431. doi: 10.1016/S0006-3495(72)86094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., GROSSMAN L. Ultraviolet light induced linking of deoxyribonucleic acid strands and its reversal by photoreactivating enzyme. Proc Natl Acad Sci U S A. 1961 Jun 15;47:778–787. doi: 10.1073/pnas.47.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESELSON M. ON THE MECHANISM OF GENETIC RECOMBINATION BETWEEN DNA MOLECULES. J Mol Biol. 1964 Sep;9:734–745. doi: 10.1016/s0022-2836(64)80178-9. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpurgo G. Induction of Mitotic Crossing-over in Aspergillus Nidulans by Bifunctional Alkylating Agents. Genetics. 1963 Sep;48(9):1259–1263. doi: 10.1093/genetics/48.9.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A. B., Riley M. Covalent union of parental DNA's following conjugation in Escherichia coli. J Mol Biol. 1967 Sep 28;28(3):503–511. doi: 10.1016/s0022-2836(67)80100-1. [DOI] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- Reid B. D., Walker I. G. The response of mammalian cells to alkylating agents. II. On the mechanism of the removal of sulfur-mustard-induced cross-links. Biochim Biophys Acta. 1969 Mar 18;179(1):179–188. [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- SHAW M. W., COHEN M. M. CHROMOSOME EXCHANGES IN HUMAN LEUKOCYTES INDUCED BY MITOMYCIN C. Genetics. 1965 Feb;51:181–190. doi: 10.1093/genetics/51.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewe M. J., Suzuki D. T., Erasmus U. The genetic effects of mitomycin C in Drosophila melanogaster. I. Induced mutations and X-Y chromosomal interchanges. Mutat Res. 1971 Jul;12(3):255–267. doi: 10.1016/0027-5107(71)90014-5. [DOI] [PubMed] [Google Scholar]
- Schewe M. J., Suzuki D. T., Erasmus U. The genetic effects of mitomycin C in Drosophila melanogaster. II. Induced meiotic recombination. Mutat Res. 1971 Jul;12(3):269–279. doi: 10.1016/0027-5107(71)90015-7. [DOI] [PubMed] [Google Scholar]
- Venitt S. Interstrand cross-links in the DNA of Escherichia coli B/r and Bs-1 and their removal by the resistant strain. Biochem Biophys Res Commun. 1968 May 10;31(3):355–360. doi: 10.1016/0006-291x(68)90483-x. [DOI] [PubMed] [Google Scholar]



