Abstract
Medulloblastoma, the most common malignant childhood brain tumor, exhibits distinct molecular subtypes and cellular origins. Genetic alterations driving medulloblastoma initiation and progression remain poorly understood. Herein, we identify GNAS, encoding the G-protein Gsα, as a potent tumor suppressor gene that defines a subset of aggressive Sonic Hedgehog (Shh)-driven human medulloblastomas. Ablation of the single Gnas gene in anatomically-distinct progenitors is sufficient to induce Shh-associated medulloblastomas, which recapitulate their human counterparts. Gsα is highly enriched at the primary cilium of granule neuron precursors and suppresses Shh-signaling by regulating both the cAMP-dependent pathway and ciliary trafficking of Hedgehog pathway components. Elevation of a Gsα effector, cAMP, effectively inhibits tumor cell proliferation and progression in Gnas mutants. Thus, our gain- and loss-of-function studies identify a previously unrecognized tumor suppressor function for Gsα that acts as a molecular link across Shh-group medulloblastomas of disparate cellular and anatomical origins, illuminating G-protein modulation as a potential therapeutic avenue.
Keywords: medulloblastoma, G-protein, cAMP, GPCR, cell lineage, sonic hedgehog signaling, cilia, cellular origins
Medulloblastoma (MB) comprises clinically and molecularly distinct that arise either in the cerebellum or brainstem 1-3. Although current treatments improve survival rates, patients suffer severe side effects and relapse of tumors carrying resistance mutations, underscoring an urgent need for alternative targeted therapies. Deregulation of G-protein coupled receptor (GPCR) pathways has been implicated in medulloblastoma 4-6, however, underlying signal transduction events that drive tumor initiation and progression remain obscure. GNAS encodes the heterotrimeric Gs protein alpha-subunit (Gsα) that functions as a molecular switch to transmit various GPCR signals to control cell growth, survival, and motility 7. Recent genome-wide analyses of somatic mutations in cancers identified GNAS as one of the most frequently mutated genes 8. Although most somatic tumor types acquire gain-of-function GNAS mutations 8, analysis of a copy number database (Tumorscape, Broad Institute) surprisingly reveals that MB displays a significant loss of the chromosomal region containing GNAS (Supplementary Fig. 1) compared to other cancers. Furthermore, a recent case report showed that a 14-month-old infant with a novel homozygous nonsense mutation within the GNAS coding region developed MB 9. Herein, we tested whether deregulation of Gsα-coding GNAS may contribute to MB formation.
Results
GNAS defines a subset of aggressive SHH-group tumors
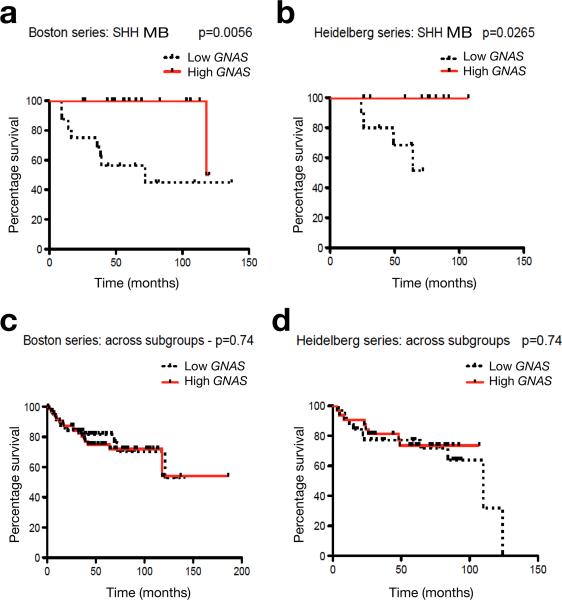
Human MB can be classified into at least four principal subgroups, namely, WNT (Wingless) group, SHH (Sonic hedgehog) group, group 3 and group 4, based on distinct gene expression profiles 1. To define the correlation of GNAS in MB subgroups, we examined GNAS expression from two independent, non-overlapping patient cohorts in the Boston and Heidelberg series 10-12. We found that low GNAS expression was tightly correlated with significantly decreased overall survival within SHH-group tumors (SHH-MB), which comprise approximately 30% of all MBs 1 (Fig. 1a,b). Notably, the prognostic impact of GNAS was not observed in other group tumors and across MB subgroups (Fig. 1c,d; Supplementary Figs. 2,3). These observations suggest that low expression or loss of GNAS specifically defines a subset of aggressive SHH-group MBs.
Figure 1. GNAS defines a subset of aggressive SHH-group tumors.
(a-d) MB patients with available survival information and gene expression profiling studies from both Boston and Heidelberg series of MBs 10,11 were divided into two groups using the median GNAS expression value as the cutoff point. The relationship between GNAS mRNA expression and survival time was analyzed according to the Kaplan-Meier method, using log rank statistics. GNAS levels and patient numbers: a, low (n =16), high (n =17); b, low (n = 10), high (n = 10); c, low (n = 32), high (n = 32); d, low (n = 64), high (n = 65).
Loss of Gnas in neural stem/progenitor cells induces MB formation with full penetrance
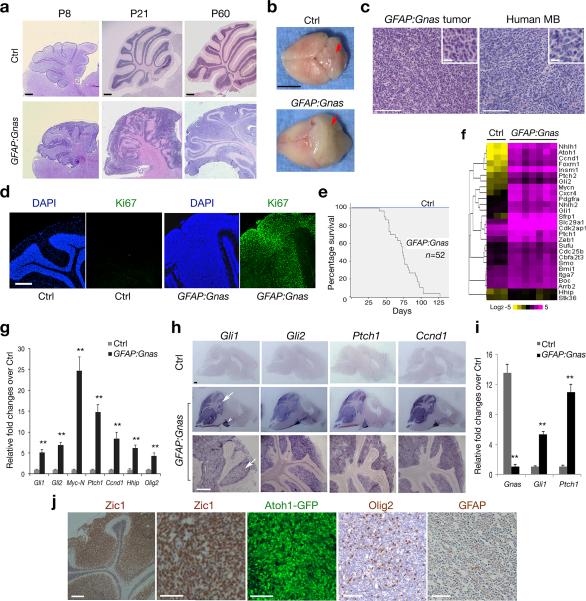
To determine whether Gnas inactivation could lead to brain tumorigenesis, we deleted Gnas in neural stem/progenitor cells by breeding floxed Gnas mice (Gnaslox/lox) with an hGFAP-Cre line 13,14. Strikingly, all resulting hGFAP-Cre+/−:Gnaslox/lox conditional knockout mice (designated as GFAP:Gnas) developed MB-like tumors at adult stages (Fig. 2a,b). We observed expansion of granule neuron progenitors (GNPs) in the cerebellar external granular layer (EGL) beginning at neonatal stages, when the control cerebellum contained only a few rows of GNPs on its surface. Diffuse, continuous GNP expansion continued to increase at postnatal stages. Gnas mutant cerebella were exophytic and delineated by a thick and disorganized EGL (Fig. 2a). By six weeks, GFAP:Gnas mice developed a diffuse MB-like tumor exhibiting the densely-packed, “small round blue” GNP-like histology (Fig. 2c; inset), resembling the histological features of human MB 15. In the mutants at P60, the neoplastic cells were highly proliferative as indicated by extensive expression of Ki67, a proliferative marker, which was barely detectable in controls (Fig. 2d). 100% of animals succumbed to the tumor around 3-4 months of age (Fig. 2e). Although the hGFAP-Cre-recombined cells appear in most brain regions 13, tumor formation was confined only to the cerebellum during the lifespan of Gnas mutants.
Figure 2. Loss of Gnas in neural stem/progenitor cells induces MB formation.
(a) Sagittal brain sections from hGFAPCre:Gnaslox/lox (GFAP:Gnas) and hGFAPCre:Gnaslox/+ (Ctrl) mice at indicated stages were stained with hematoxylin and eosin (H/E).
(b) Brain appearance of control and Gnas mutants at P67. The arrows indicate the cerebellum.
(c) Tumors from Gnas mutants (left) displays similar histology to human MB (right; SHH group). Insets are shown at high magnification.
(d) The cerebella of control and Gnas mutants at P60 were stained with anti-Ki67 and DAPI.
(e) Kaplan-Meier survival curves for control and GFAP:Gnas mice (n = 52).
(f) Heatmap shows expression of Shh pathway components in control cerebella and GFAP:Gnas tumor tissues. The color bar shows expression intensity.
(g) qRT-PCR quantification of Gnas and Shh pathway genes in control and GFAP:Gnas cerebella at P30. Data represent the mean ± SEM (n = six animals). ** P < 0.01; Student's t test.
(h) mRNA expression of Shh target genes as indicated in control and GFAP:Gnas brain sections at P60. Arrow and arrowhead indicate the cerebellum and pontine grey nucleus, respectively.
(i) qRT-PCR analysis of Gnas, Gli1 and Ptch1 in GNPs from control and GFAP:Gnas mice at P7. Data represent the mean ± SEM (n = five animals). ** P < 0.01; Student's t test.
(j) The cerebellar EGL region of GFAP:Gnas mice carrying the Atoh1-GFP reporter at P50 was immunostained with anti-Zic1, Olig2 and GFAP as indicated.
Scale bars in a, 300 μm; b, 5 mm; c, d, h, 200 μm; inset in c, 10 μm; j, 100 μm.
To ascertain gene expression alterations caused by Gnas loss, we examined mRNAs isolated from the cerebella of control and GFAP:Gnas mice at P60 by RNA-deep sequencing. In tumors of Gnas mutants, our data revealed an up-regulation of Shh signaling pathway components (Fig. 2f). Quantitative RT-PCR confirmed that expression of Shh target genes and pathway components was significantly up-regulated (Fig. 2g). Consistently, mRNA in situ hybridization revealed intense expression of Shh downstream genes including Gli1, Gli2, Ptch1 and cyclinD1 (Ccnd1) (Fig. 2h). Furthermore, we observed significant elevation of Shh direct target genes Gli1 and Ptch1 (Fig. 2i) in GNP-like tumor cells compared with normal GNPs, suggesting a cell-intrinsic effect of Gnas mutation on Shh signaling activation. By contrast, expression of Wnt-target genes was not substantially altered (Supplementary Fig. 4), consistent with previous findings that Gnas loss does not affect Wnt signaling in other cellular systems 16. We observed widespread expression of GNP markers Zic1 and Atoh1 (a.k.a. Math1), along with Shh-regulated targets including Olig2 17, but very few astrocytic GFAP+ astrocytes (Fig. 2j). Thus, Gnas loss results in an increase or alternatively a de-repression of physiological levels of Shh pathway activity and over-proliferation of GNP-like tumor cells.
Gsα activity suppresses hedgehog signaling
To test whether the GTPase activity of Gsα protein is required for inhibition of Shh signaling, we treated GNP cells isolated from wildtype neonates with NF449, a selective Gsα antagonist 18, which prevents GTP binding to Gsα and blocks Gsα GTPase activity. Treatment of NF449 resulted in a significant up-regulation of Shh target genes Gli1, Gli2, Ptch1 and Myc-N and caused a decrease of cAMP levels (Fig. 3a), suggesting that inhibition of Gsα GTPase activity activates Shh signaling. To investigate the effect of Gsα gain-of-function activity on Shh signaling, we generated a constitutively activated form of Gsα, GsαQ227L (GsCA), which resulted in a GTPase-defective, active GTP-bound Gsα protein 19. Overexpression of GsCA in GNPs suppressed the upregulation of Shh targets Gli1, Ptch1, Myc-N and Ccnd1 induced by a Shh agonist SAG (Fig. 3b), indicating that Gsα activation inhibits hedgehog signaling.
Figure 3. Gsα and its effector cAMP inhibit Shh signaling and tumor growth in Gnas mutants.
(a) Bar graph (left) depicts expression changes in GNPs treated NF449 over control. Right: cAMP levels measured by ELISA.
(b) GNPs were treated with vehicle and SAG (200 nM) and/or transfected with pcDNA3 control and GsαQ227L (pGsCA) for 48 h. Bar graphs depict Gli1 and Ptch1 expression changes in treated cells over control.
(c) Average cAMP levels of control and mutant GNPs treated with vehicle and forskolin.
(d) GNPs were treated with vehicle or Rolipram (50 μM), forskolin (10 μM) and db-cAMP (100 μM) for 3 h. Gli1 and Ptch1 or Gli3FL and Gli3R were analyzed by qRT-PCR (left) and western blotting (right) as indicated. GAPDH: loading control.
(e) Quantification of Gli1, Ptch1 and Ccnd1 in GNPs treated with H89 or KT5720 over control.
(f) Bar graphs depict the relative fold change of Gli1 and Ptch1 expression in GNPs transfected with pGsCA with or without H89 treatment over control.
(g) Control and Gnas mice (n = eight for each group) were randomized to receive Rolipram or vehicle from P35 to P65. Representative images of H/E staining of cerebellar sections and brain morphology were shown. Arrows: the cerebellum.
(h) Scatter dot-plot depicts average tumor volumes estimated for vehicle- and Rolipram-treated Gnas mutants. Lines: mean values ± SEM.
(i) Images show immunostaining of Zic1 and BrdU in vehicle and Rolipram-treated GFAP:Gnas tumors at P65. Insets: high magnification in boxed areas. Bar graph (right) depicts the percentage of BrdU+/Zic1+ cells (n = eight animals each group).
(j) Kaplan-Meier survival curves for GFAP:Gnas mice (n = 12 per group) were randomized to receive Rolipram or vehicle at P30 for six weeks. P value for the log-rank test =0.0071.
Scale bars in g, 300 μm; i, 20 μm (insets 4 μm). Data shown in a-f are the mean ± SEM representing at least three independent experiments. *P < 0.05, ** P < 0.01; Student's t-test.
Elevation of the Gsα effector cAMP inhibits MB growth
The classic signal transduction pathway of Gsα is through activation of adenylyl cyclase, which, in turn, produces intracellular cAMP 20. cAMP has been shown to activate cAMP-dependent Protein Kinase A (PKA), a negative effector of Shh signaling 21-23. Tumor cells isolated from Gnas mutants had a significant reduction in intracellular cAMP levels, while treatment with the adenylyl cyclase agonist forskolin (FSK) elevated cAMP levels (Fig. 3c). To test the hypothesis that cAMP elevation could inhibit Shh signaling activation, we treated Gnas mutant GNPs with Rolipram, which elevates cAMP levels by selectively inhibiting phosphodiesterase-4 activity to block cAMP degradation 24,25, FSK or a non-hydrolysable cAMP analog db-cAMP. Each of these cAMP-raising agents significantly reduced expression of Gli1 and Ptch1 (Fig. 3d). In addition, in wildtype GNPs, treatment with FSK and Rolipram enhanced the proteolytic processing of full-length Gli3 into a repressive form Gli3R (Fig. 3d), an inhibitor of Shh target expression 26,27. In contrast, inhibition of cAMP-dependent PKA with two different small molecule inhibitors, H89 and KT570, significantly increased expression of Gli1, Ptch1 and Ccnd1 (Fig. 3e). Furthermore, mitigation of PKA activity by H89 (Supplementary Fig. 5) can restore Shh target expression suppressed by constitutively activated Gsα (Fig. 3f). Thus, our data are in keeping with previous observations that Gsα-mediated signaling can elevate cAMP levels to inhibit hedgehog target gene expression through cAMP-dependent PKA activity in other cellular systems 26,28,29.
To determine the effects of cAMP elevation on tumor growth in vivo, we evaluated the efficacy of Rolipram, which is well tolerated and readily crosses the blood brain barrier in vivo 24,30. Control and GFAP:Gnas mutants at the young adult stage P35 were randomized to receive either daily i.p. vehicle or Rolipram for 30 days with a dosage exhibiting effective anti-tumor activity in vivo 30, and assessed for tumor development. Rolipram administration during this period did not affect overall cerebellar structure and myelin formation (Fig. 3g; Supplementary Fig. 6). Vehicle-treated Gnas mutants displayed extensive tumor cell expansion and bulged cerebella (Fig. 3g). In contrast, in Rolipram-treated mutants, the tumor size and proliferation of Zic1+ GNP-like tumor cells were substantially reduced (Fig. 3g-i). In addition, Rolipram treatment exhibited a significantly extended lifespan of Gnas mutants (Fig. 3j). Thus, elevation of the Gsα effector cAMP by Rolipram could lead to inhibition of tumor growth in Gnas mutants.
Gsα controls ciliary trafficking of hedgehog signaling components in GNPs
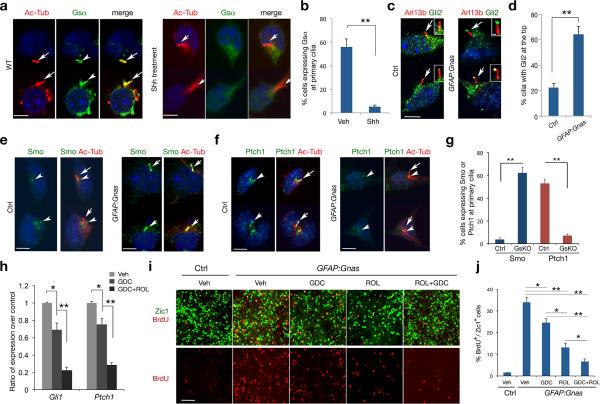
Ciliary trafficking of signaling components has an important role in regulating Shh pathway activity and MB formation 22,31,32. Strikingly, we observed that Gsα was highly enriched at the primary cilium of GNPs (Fig. 4a) but hardly detectable in mouse embryonic fibroblasts (Supplementary Fig. S7), suggesting a unique ciliary function of Gsα for GNP development. In the presence of Shh, ciliary localization of Gsα is diminished (Fig. 4a,b). Treatment of Shh and its agonist SAG 33 did not alter the total amount of Gsα protein, but rather reduced the amount of the GTP-bound, active form of Gsα protein (Gsα-GTP; Supplementary Fig. 8).
Figure 4. Gsα regulates ciliary trafficking of hedgehog signaling components in GNPs.
(a-b) GNPs without or with Shh treatment (3 μg ml–1) for 16 hr were immunostained with anti-Gsα and acetylated α-tubulin (Ac-Tub) (arrows). b: quantitation of Gsα fluorescence at the primary cilium (≥ 300 cell counts each group per experiment).
(c-g) GNPs from control or GFAP:Gnas mice were immunostained with anti-Gli2, Smo, Ptch1 and Ac-Tub. Insets in c show cilia at a high magnification. Bar graphs in d, g depict the percentage of Gli2 accumulation at cilium tips, and Smo or Ptch1 fluorescence at the primary cilium, respectively (≥ 300 cell counts per genotype from each experiment). Arrows and arrowheads indicate primary cilia and their base, respectively.
(h) GNPs from Gnas mutants were treated with GDC-0449 (1 μm) or both GDC-0449 and Rolipram (50 μm). Bar graphs depict relative Gli1 and Ptch1 expression by qRT-PCR in drug-treated vs. vehicle-treated cells.
(i) Zic1 and BrdU immunostaining in GNPs from Gnas mutants treated GDC-0449, Rolipram or both and labeled with BrdU for 48 hr.
(j) Bar graph depicts the average percentage of BrdU+ cells among Zic1+ GNPs. Experiments were performed three times with at least n = 3 for each treatment. One-way ANOVA with post hoc Newman–Keuls multiple comparison test. **P < 0.01.
Scale bars in a-f, 3 μm; c insets, 0.5 μm; i, 30 μm. Representative images and quantifications from at least three independent experiments are shown. Data represent the mean ± SEM. *P < 0.05, **P < 0.01, Student's t-test or one-way ANOVA.
In GNPs from GFAP:Gnas mutants, Gsα expression was essentially depleted, while total levels of Smoothened (Smo) were comparable to controls (Supplementary Fig. 9). At the tips of cilia, approximately 64.3 ± 6.2% cells exhibited strong immunoreactivity for Gli2, a Shh downstream effector (Fig. 4c, insets), whereas Gli2 was hardly detectable or weakly presented in control GNPs (Fig. 4c,d). Consistent with a principal function of PKA in restraining Gli2 activation 21,23, our observation suggests that Gnas loss reduces cAMP-dependent PKA activity and increases Gli2 accumulation at ciliary tips, leading to Shh signaling activation. We further detected ciliary translocation of Smo seven-transmembrane protein in the majority of Gnas mutants, but not in control GNPs, where Smo was diffusely localized in the cytoplasm near the base of cilia but absent from ciliary shafts (Fig. 4e,g). Conversely, a Smo-inhibiting protein, Ptch1, was mainly detected in the ciliary shaft of control GNPs (Fig. 4f) but absent from cilia in Gnas mutants (Fig. 4f,g). In contrast, ciliary trafficking of a Shh signaling regulator Gpr16129 was not altered in Gnas mutants (Supplementary Fig. 10), suggesting a specificity of Gsα in regulating ciliary localization of GPCRs. Consistent with the existence of multiple Gsα-mediated GPCRs, Gpr161−/− embryos exhibited milder developmental defects than Gnas−/− animals 28. Given that PKA null mutation could lead to Gli2 accumulation at the tips of cilia, but not the ciliary trafficking of Smo in neural progenitors 21, our observations of both strong Gli2 signal at the ciliary tip and Smo ciliary translocation in Gnas mutants suggest that Gsα might have an additional role in regulating Smo trafficking in primary cilia independent of cAMP-dependent PKA activity.
cAMP elevation and Smo inhibition augment suppression of tumor cell proliferation
Our data indicate that Gsα suppresses Shh signaling not only by stimulating intracellular cAMP levels to activate PKA, but also perhaps independently by inhibiting Smo activation. Treatment of Gnas mutant GNPs with GDC-0449, which blocks SMO activation induced by SAG (Supplementary Fig. 11)34, led to a reduction in Gli1 and Ptch1 expression (Fig. 4h). Combined treatment of GDC-0449 and Rolipram however resulted in further inhibition of Shh target expression (Fig. 4h). Since Gnas mutant GNPs are highly proliferative (Fig. 4i), we then determined whether cell proliferation is responsive to cAMP elevation and Smo inhibition. The proliferation rate was reduced in Gnas-mutant GNPs treated with Rolipram or GDC-0449 to a certain extent, where Rolipram exhibited a relatively stronger effect than GDC-0449 (Fig. 4i,j). However, combinatorial treatment of both drugs was found to cause a greater inhibition of cell proliferation in Gnas mutants (Fig. 4i,j).
Loss of Gnas in Atoh1+ or Olig1+ progenitors leads to anatomically distinct MBs
SHH-driven MBs may arise from multiple cellular origins in human patients 3. hGFAP-Cre mediated Gnas deletion might affect multiple progenitor populations in the posterior fossa. To examine whether Gnas loss in committed GNPs could result in MB formation, we crossed Gnasfloxed mice with an Atoh1-Cre line, which directs Cre expression in GNPs of the cerebellum and dorsal brainstem cochlear nuclei 3,35. The resulting Atoh1-Cre+/−:Gnaslox/lox (Atoh1:Gnas) mutant mice developed MB-like tumors with an expansion of tumor cells in the EGL layer (Fig. 5a). Tumor cells expressed neuronal markers Tuj1 and Zic1 extensively with few Olig2 and GFAP-expressing glial cells (Fig. 5b), and exhibited a significant up-regulation of Shh signaling target genes Ptch1, Gli1 and Hhip (Fig. 5c). This suggests that ablation of Gnas selectively in committed cerebellar GNPs is sufficient to cause Shh-associated MB formation.
Figure 5. Loss of Gnas in Atoh1+ or Olig1+ progenitors leads to an anatomically distinct Shh-associated MB.
(a) A sagittal hindbrain section from a Atoh1:Gnas mouse at P50 was stained with H/E. The boxed area is shown at a high magnification in the right panel.
(b) Tumor tissues were immunostained with anti-Tuj1, Olig2, Zic1 and GFAP as indicated.
(c) Bar graphs depict expression of Ptch1, Gli1 and Hhip in Atoh1:Gnas cerebella over control at P40. Data represent the mean ± SEM from five animals each group. ** P < 0.01; Student's t test.
(d) Olig1 expression (arrow) was detected in the progenitors of the dorsal brainstem at sagittal levels at E15.5 by in situ hybridization.
(e) The dorsal brainstem region from Olig1-Cre:Rosa-tdTomato mice at P7 was immunostained with Zic1. Arrows indicates a population of tdTomato+ cells Zic1.
(f-g) H/E staining of the sagittal sections of Olig1:Gnas brains at 3 or 5 month ages. Arrows indicate the tumor tissue. Boxed region in f is shown at high magnification in g. BS: brainstem.
(h-j) Sections of Olig1:Gnas tumor tissues were immunostained with anti-Ki67, NeuN, Zic1, Pax6 and GFAP as indicated.
Scale bars in a, 50 μm. b, 20 μm, d, f, 200 μm; e, 20 μm; g-j, 50 μm.
To test whether other Shh-responsive progenitor cells were susceptible to oncogenic transformation due to Gnas loss, we ablated Gnas in the progenitors expressing a Shh-regulated gene Olig136,37. During embryogenesis, Olig1 is mainly expressed in the specified progenitors of the brainstem around rhombomeres r2-r4, which can give rise to oligodendrocyte precursors (OPC) and granule neuron lineage cells 37,38 (Fig. 5d). The Olig1+ progenitors were mainly detected in the rostral brainstem but separated from caudal brainstem progenitors in rhombomeres r6-r8, a source of Wnt-associated MBs 39 (Fig. 5d). They were essentially undetectable in the upper rhombic lip, EGL and cochlear nucleus (Fig. 5d; Supplementary Fig. 12), the other sources for Shh-associated MBs 3,35. Lineage tracing analysis indicated that the progeny of Olig1+ progenitors were present in a population of Zic1+ GNPs in the dorsal brainstem (Fig. 5e). Notably, Olig1-Cre+/−:Gnaslox/lox mutant mice (designated as Olig1:Gnas) generated by breeding Gnas-floxed and Olig1-Cre mice developed anatomically-distinct tumors that were largely restricted between the caudal posterolateral lobe and the dorsal brainstem around the fourth ventricle (Fig. 5f), and contiguous within the rostral brainstem (Fig. 5g). Olig1:Gnas mice developed relatively intact cerebellar structure and morphology, displaying tumor formation around 3 months of age. Approximately 37.4 ± 4.1% of cells in the tumor tissues expressed Ki67 (Fig. 5h), suggesting that the neoplastic cells are highly proliferative. In tumors of Olig1:Gnas mice, we detected extensive expression of the neuronal markers NeuN, Zic1 and Pax6 (Fig. 5i), a hallmark feature of a primitive neuroectodermal tumor-like MB 40. In contrast, only a few scattered cells in the tumor were positive for an astrocytic marker GFAP (Fig. 5j).
Anatomically-distinct tumors from different Gnas mutants resemble SHH-associated MB
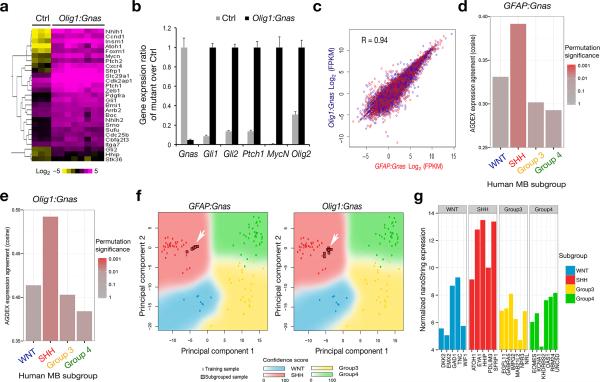
Although the localization of MBs is distinct between Olig1:Gnas and GFAP:Gnas mutants (Fig. 1), transcriptome profiling by RNA-seq (Fig. 6a) and qRT-PCR (Fig. 6b) analysis revealed a significant up-regulation of Shh pathway components in tumors of either origin. Regression analysis reveals a direct correlation of gene transcription profiles between GFAP:Gnas and Olig1:Gnas tumors (Fig. 6c). Cross-species comparison of gene expression profiles of Gnas tumors with data from human MB subgroups using two different class prediction algorithms AGDEX41 (Fig. 6d,e) and PAM42 (Fig. 6f) revealed that the tumors derived from both GFAP:Gnas and Olig1:Gnas mice showed a gene expression signature most closely resembling the SHH-group human MB (Fig. 6df). Therefore, our data highlight Olig1+ progenitors in the posterior fossa as an important source of a subset of Shh-associated MBs with heterogeneous cellular origins3.
Figure 6. Tumors in Gnas mutants exhibit a gene expression signature resembling SHH-MB.
(a) Heatmap analysis of gene profiling of control cerebella (n = 3) and tumor tissues (n = 8) from Olig1:Gnas mutants by RNA-sequencing shows upregulation of Shh pathway components in tumors. The color bar shows expression intensity.
(b) Relative expression of Shh pathway components between control and Olig1:Gnas tumors from four month old animals (n = eight per group) was assayed by qRT-PCR. ** p < 0.01; Student's t test.
(c) Regression analysis of gene expression profiles indicates a direct correlation of gene transcription profiles between GFAP:Gnas and Olig1:Gnas tumors (n = eight per group).
(d-e) Cross-species comparison of global differential expression from Affymetrix microarray analysis of mouse tumors (n = eight per group) with bona fide human MB subgroups by AGDEX3 R algorithm. Bar graphs represent the cosine similarity measure and reflect the similarity of global expression profile between each mouse tumor subtype and each human MB subgroup.
(f) Principal component analysis (PCA) of expression profiles between human and above mouse tumor samples. Arrows indicate that gene expression profiles of mouse tumors match to Shh-subgroup.
(g) Subgrouping analysis by nanoString technology indicates the MB from the patient with a GNAS homozygous nonsense mutation resembles a SHH-group tumor with high confidence (PAM prediction score = 0.999996).
To further examine the tumorigenic capacity of neoplastic cells in these Gnas mutants, we transplanted cells isolated from tumor tissues from GFAP:Gnas or Olig1:Gnas mutants by stereotactically injecting them into the forebrain in immuno-compromised nude mice. Tumor cells were able to propagate into the brain in the transplanted animals and became obvious one month after transplantation (Supplementary Fig. 13). Tumors formed in mouse allografts persisted with GNP-like tumor cells exhibiting MB histology (Supplementary Fig. 13). Congruent with gene profiling and histological data, this suggests that the tumors in GFAP:Gnas or Olig1:Gnas mutants comprise transformed neoplastic cells, resembling SHH-driven MBs. Moreover, to identify the subgroup affiliation of MB in the patient with a homozygous GNAS-inactivating mutation 9, we carried out targeted gene-expression profiling analysis and observed a significant upregulation of signature genes in the SHH-group but not in WNT, group 3 or 4 MB (Fig. 6g). Thus, our results predict that the tumor carrying the GNAS nonsense mutation is a SHH-subgroup MB with high confidence (Fig. 6g). Furthermore, a recent genome sequencing study identified eight cases out of 133 SHH-MBs carrying GNAS mutations including one case with a frame-shift mutation 5 (Supplementary Table 1). Together with tumor formation in Gnas mutants, these studies suggest that GNAS inactivating mutations could lead to SHH-MB formation in patients.
Discussion
We demonstrate here that the Gnas-encoded GPCR signal transducer Gsα is a potent tumor suppressor in Shh-driven MBs. Gnas expression determines progenitor cell competency in initiation of MBs among distinct cells of origin. Low levels of GNAS define a subset of aggressive SHH-MB, highlighting GNAS as a prognostic biomarker for treatment stratification of SHH-associated tumors. The case report that the patient with a homozygous GNAS nonsense mutation 9 developed SHH-MB provides additional clinical relevance of GNAS deregulation in tumor formation. Our gain- and loss-of-function studies suggest that Gsα inhibits MB formation at least in part by suppressing Shh signaling through activating the cAMP-dependent pathway to regulate Gli3 processing and Gli2 activation, as well as modulating ciliary trafficking of hedgehog signaling components in GNPs. Thus, a dual-mode regulation of the intracellular cAMP signaling cascade and Smo activation at the primary cilium by Gsα reinforces the inhibition of Shh signaling and blocks MB initiation. Our data further suggest that Gsα might serve as a point of convergence between Smo and various Gsα-coupled GPCR signaling pathways 4,6,29,43 to modulate Shh signal strength and control MB formation. We did not detect significant alterations of gene loci encoding hedgehog-pathway components such as PTCH1, SMO or SUFU in GNAS-low SHH-MBs 44 and in Gnas tumors (Supplementary Fig. 14, Table 1), suggesting that GNAS deregulation or inactivation may represent a unique subset of SHH-MBs. Nonetheless, our copy number variation study revealed a number of other genetic alterations occurred in both GFAP:Gnas and Olig1:Gnas mutants. The altered genetic loci harboring homozygous deletions include Tulp4, a candidate tumor suppressor gene in MB 39 and Hjurp, a critical factor for chromosome segregation and stability 45,46 (Supplementary Fig. 14). These genetic alterations might potentially contribute to transforming mechanisms in Gnas mutants.
We identify Olig1+ progenitors as a cellular source for Shh-associated tumors localized to the dorsal brainstem, demonstrating the cellular and anatomic heterogeneity within SHH-MB 2,3,5,47. Recent studies indicate that Olig1 may regulate the fate switch between OPC and cerebellar interneurons 48, raising the possibility that interneuron precursors might contribute to MB formation. However, this seems unlikely because Shh-driven MBs are derived from lineage-restricted GNPs even with SmoM2 activation or Ptch1 mutation in multipotent cerebellar progenitors 13,35. Intriguingly, the ventral brainstem of GFAP:Gnas mice exhibits an expanded pontine grey nucleus derived from lower rhombic lip progenitors, which could also act as a source of Wnt-subgroup tumor 39. Thus, Gsα may suppress MB formation in different types of progenitor cells. Together, our studies uncover Gnas as a potential molecular link among anatomically distinct MBs, pointing to a previously unrecognized tumor suppressor function of Gnas in the initiation of diverse MBs in light of other tumor types often caused by activating GNAS mutations 8,49.
Although Smo inhibitors show efficacy regarding the inhibition of MB growth in animal models such as Ptch1 mutants 50 and adult Shh-driven MB patients 51, drug responses were only transient due to the emergence of drug resistance. Our data suggest that the cAMP stimulants such as Rolipram, which possesses anti-tumor activity and is clinically approved already as an antidepressant in humans in Japan and Europe 52, might be a potent alternative agent against aggressive SHH-MBs caused by GNAS inactivation. Although other cAMP raising agents have been previously proposed for the treatment of human tumors exhibiting overactive Shh pathways 52, our current spontaneous MB model with Gnas inactivation provides important and novel validation of the efficacy of Rolipram in vivo. This suggests that Rolipram and perhaps other cAMP-raising agents including bioflavonoids, which overcome multi-drug resistance in cancer therapy 53,54, might be repurposed for treating MB. The profound and specific impact of Gsα on MB tumorigenesis illuminates a new alternative treatment avenue, such that co-targeting of Gsα or its signaling effectors together with Smo inhibition might circumvent the drug resistance seen with Smo antagonists alone 51,55 and could be beneficial in treatment of aggressive SHH-MB with GNAS deregulation.
Online Methods
Animals
We bred Gnaslox/lox mice 56 with hGFAP-Cre (Jackson laboratory), Atoh1-Cre (gift from Dr. Bernd Fritzsch, University of Iowa) and Olig1-Cre 36 mice to generate hGFAPCre+/−:Gnaslox/+, Atoh1Cre+/−;Gnaslox/+ mice and Olig1Cre+/−;Gnaslox/+ mice, respectively, which were then bred with Gnaslox/lox mice to produce control mice (hGFAPCre+/−:Gnaslox/+, Atoh1Cre+/−;Gnaslox/+ and Olig1Cre+/−:Gnaslox/+), and Gnas mutant offspring hGFAPCre+/−:Gnaslox/lox (GFAP:Gnas), Atoh1-Cre+/−:Gnaslox/lox (Atoh1:Gnas) and Olig1Cre+/−;Gnaslox/lox(Olig1:Gnas) mice, respectively. The above control mice developed and behaved the same as wildtype. Rosa-tdTomato reporter mice (Jackson laboratory) and Atoh1-GFP reporter line (gift from Dr. Jane Johnson) were also bred with Gnas mutants to monitor gene deletion and Atoh1-expressing GNP cells, respectively. We used both male and female mice for the study. The mouse strains used in this study were generated and maintained on a mixed C57Bl/6;129Sv;CD-1 background. BALB/c nude mice (Charles River Laboratories) were used for allograft transplantation study. All animal use and studies were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center, Cincinnati Children's Hospital Medical Center, USA and animal use committee at Sichuan University in Chengdu, China. All human patient samples were obtained with consent as outlined by individual institutional review boards.
Tissue processing, antibodies, immunostaining and microscopy
Mouse brains at defined ages were dissected and fixed overnight in 4% (w/v) paraformaldehyde (PFA) and processed for cryosectioning or paraffin embedding and sectioning. The procedure for immunostaining was described previously 37. Briefly, for tissue immunostaining, cryosections or pre-deparaffinized tissue sections were incubated overnight in primary antibodies diluted in block solution (PBS with 5% v/v normal goat serum (Sigma-Aldrich) and 0.3% v/v Triton X-100). After washing with PBS for five times, sections were then incubated overnight in appropriate either biotinylated secondary antibodies, followed by using the ABC avidin/biotin method to visualize staining signals under light microscopy with the peroxidase/diaminobenzidine (DAB) method, or incubated with corresponding fluorophore-conjugated secondary antibodies (Jackson ImmunoResearch) under fluorescent microscopy. For cell immunostaining, cultured cells were fixed with 4% PFA for 10 min and washed five times with PBS, then placed in blocking solution for 30 min. We incubated primary antibodies in blocking solution with proper dilutions and stain cells for 1 h at room temperature. For BrdU staining, cells or tissue sections were denatured with 0.1N HCl for 1 h in 37°C water bath. After denaturation, sections were neutralized with 0.1 M Borax, pH 8.5 (Sigma) for 10 min. Sections were washed with 0.3% Triton X-100/1 × PBS (wash buffer) for 3 times and blocked with 5% normal donkey serum (Sigma-Aldrich) contained wash buffer for 1 h at room temperature. Mouse-anti BrdU (BD Bioscience, 1:500) antibody was used to label BrdU overnight at 4°C. DAPI was included in the final washes before the samples were mounted in Fluoromount G (SouthernBiotech) for microscopy. Primary antibodies used in this study were as follows: Zic1 (Rockland, 200-401-159), Olig2 (gift of C. Stiles, Harvard Medical School), GFAP (Sigma, G3893), Gsα (Santa Cruz, Sc-823), Smoothened (Smo) (LS-C47301, LSBio), acetylated α-tubulin (mAb 6-11B-1, Sigma), Patched1 (gift of Rajat Rohatgi, Stanford University), Gpr161 (gift of Suzie Scales, Genentech, Inc), Ki67 (Thermo Sci, Clone: SP6), Pax6 (Developmental Studies Hybridoma Bank-University of Iowa), Tuj1 (Covance, MMS-435P), BrdU (BD Bioscience 347580), NeuN (Millipore, MAB377), Arl13b (Santa Cruz, sc-102318), Gli2 and Gli3 (gift of Suzie Scales, Genentech, Inc.).
For microscopy and image acquisition, images of stained samples used in figures were collected on an inverted laser scanning confocal microscope (Carl Zeiss LSM 510) microscope equipped with high-efficiency fluorochrome specific filter sets for DAPI, Cy2, Cy3 and Cy5. For quantification of the number of Ki67, BrdU or Zic1-expressing cells, areas to be counted were traced with a 40× objective lens and sample frames (40 μm × 40 μm) were selected at least 10 random but non-overlap regions per section by the image analysis software. At least five sections at different hindbrain levels per animal were selected for quantification. Imaging of cilia was taken with a Zeiss immersion oil objective lens (63 ×) with 2× optical zoom magnifications. Experiments were performed at least three times for each genotype. At least 300 cells with visible cilia from each independent experiment per genotype were analyzed. Images were quantified in a double-blinded manner.
RNA in situ hybridization of brain sections was performed using digoxigenin-labeled riboprobes as described previously 17. The probes used were: murine Ptch1, Gli1, Gli2 and Cyclin D1. Detailed protocols are available upon request.
Cerebellar GNP culture and proliferation assays
Cerebella from P6 or P7 mice were digested with Trypsin/DNase (1 mg ml–1; Worthington), triturated to obtain a single-cell suspension, and then centrifuged through a 35%–65% Percoll gradient (Sigma) according to Yang et al., 2008 13. Cells from the 35%–65% interface were suspended in the GNP culture medium [Neurobasal (Gibco) with 2 mM L-glutamine, 0.45% D-glucose, B27 supplement, 16 μg ml–1 N-Acetyl-LCysteine and penicillin/streptomycin]. We pre-plated GNPs onto poly-D-lysine (100 μg ml–1) coated plates for 1 hr at 37°C twice, and then transferred them to Poly-D/L-ornithine coated plates for culture. We treated GNP cells from control and Gsα mutants with following agents: SAG (Enzo, Lx-270-426) 200 nM; forskolin (Sigma, F3917) 10 μM; a selective Gαs antagonist NF449 (http://www.tocris.com/dispprod.php?ItemId=1801#.UwpK4v3j7Ko) (Tocris, Cat. # 1391) 200 μM; GDC-0449 (Selleckchem, S1082) 1 μM and Rolipram 50 μM (R&D, Cat.# 0905) or transfected the cells with pcDNA3 or pGsCA for 48 hr. For in vitro proliferation assays, we labeled GNP cells with BrdU (50 μg ml–1) for 48 hr followed by immunostaining.
Real-time RT-PCR Analysis
RNAs were isolated with the RNeasy Plus Mini kit (Qiagen) from GNP cells or snap-frozen tumors. Reverse transcription was performed with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). We analyzed each gene with at least two different primer sets. qRT-PCR was carried out using the ABI Prism 7700 Sequence Detector System (Perkin-Elmer Applied Biosystems) using Gapdh (TaqMan kit, Applied Biosystems) as an internal control. Each analysis was performed in triplicate, and the results were normalized to Gapdh for each sample. The primer sequences for qRT-PCR are available upon request.
Western blotting
GNP cells were rinsed in PBS and lysed in modified RIPA buffer (50 mM Na-Tris, pH 7.4, 150 mM NaCl, 1% (v/v) NP-40, 0.25% sodium deoxycholate, 1 mM dithiothreitol, 10 mM NaF, 1 mM active sodium vanadate, 1 mM PMSF and 1 × a cocktail of cOmplete protease inhibitors (Roche Applied Science) and centrifuged at 13,000 rpm for 15 min at 4 C. After the determination of protein concentration (Bio-Rad), the lysates were separated by 4–12% SDS-PAGE. We performed western blotting using standard protocols. The antibodies used were as follows: rabbit antibodies to Gli3 for detecting Gli3FL and Gli3R forms (gift of Suzie Scales), Gsα (Santa Cruz, Sc-823), anti-phospho-PKA substrates (P-PKAs, Cell Signaling, 9624s) and GAPDH (Santa Cruz, FL-335). Bands were visualized with secondary antibodies conjugated to horseradish peroxidase (Bio-Rad) and ECL western blotting detection reagents (Pierce) per the manufacturer's instructions. We used ImageJ densitometry software for western blot quantification.
Assay for activated Gsα (Gsα-GTP)
We performed activated Gsα-GTP pull-down assays following the manufacturer's protocol (NewEast Bioscience, 80801). Briefly, GNPs were incubated with Shh (3 μg ml–1, R & D), XAV-939 (1 μM) and SAG (200 nM) for 1 h. Cell lysates were incubated with the antibody against the active form Gsα-GTP followed by protein A/G agarose beads for 1 h. Activated Gsα and Gsα proteins were detected by western blot with Gsα antibody.
Enzyme-linked immunoassay (ELISA) for cAMP
We plated GNP cells on 96-well tissue culture plates and cultured them for 1 day. The cAMP level in the GNPs was assayed following the manufacturer's protocol (Cell Signaling, 4339). In brief, GNPs were treated with SAG (200 nM), NF449 (100 μM) or forskolin (10 μM) for 24 hr. The amount of cAMP in lysates from 8,000 GNP cells was measured by ELISA.
RNA-seq data analysis
We isolated RNAs from the cerebella of adult wildtype mice and tumor tissue from individual GFAP:Gnas or Olig1:Gnas mutants and subject to RNA deep sequencing. RNA-seq libraries were prepared using Illumina RNA-seq Preparation Kit (Illumina) and sequenced in HiSeq 2000 sequencer. RNA-seq reads were mapped using TopHat with default settings. TopHat output data was then analyzed by Cufflinks to (1) calculate FPKM values for known transcripts in mouse genome reference and (2) test for changes of gene expression of control and tumor tissues.
Copy number variation analysis
We isolated genomic DNAs from the cerebellum of adult wildtype mice and tumor tissue from individual GFAP:Gnas or Olig1:Gnas mutants (n = 3 for each genotype) using a DNA preparation kit (Zymo Inc.) and were hybridized on aCGH arrays (Nimblegen, Mouse CGH 3x720K Whole-Genome Tiling Array) according to the manufacturer's protocol. Data from the aCGH arrays were analyzed using the Nexus aCGH software with recommended normalization settings. Each sample was compared to a distributed baseline to identify amplified and deleted regions using a segmentation algorithm within Nexus Suite using a cutoff with gain (≥ 0.23) and loss (≤ –0.5) and significance threshold = 1.0E–5. Segments showing copy number variation were only reported if they occurred in list those regions of gain or loss with an individual False Discovery Rate (FDR) no greater than 5%.
Classification of medulloblastoma patient subgroups
Classification of a group of 103 paraffin-embedded MB samples was established using unsupervised hierarchical clustering (HCL) as the training series described previously 57,58. Unsupervised HCL of MB expression data identified the following four unique sample clusters: WNT group, SHH group, group C, and group D. Briefly, gene expression data from MB patient samples were generated by using Affymetrix_HTHGU133Achips (Affymetrix, Santa Clara, CA). Molecular subgroups were classified using TM4 Microarray Software Suite (MeV v4.4; Dana-Farber Cancer Institute, Boston, MA). Subgroup-specific signature genes were identified by a multivariate permutation test. We performed principal component analysis (PCA) of gene expression data using Partek Genomics Suite (Partek, St Louis, MO).
Subgroup determination of the tumor with a homozygous GNAS nonsense mutation from a 14-month-old post-mortem infant: Total RNA was extracted from FFPE tumor tissue using the Qiagen RNeasy FFPE Kit (Qiagen, Hilden, Germany) and 200 ng of total RNA were analysed on a nanoString nCounter using a custom 25 gene probeset as previously described 58. Counts were normalized to the three housekeeping genes (GAPDH, ACTB and LDHA) and subgroup prediction was done using PAM (Prediction analysis of microarrays) as previously described using the R-statistical environment (v2.15.1)58.
Molecular classification of mouse tumors
Mouse tumors and normal cerebella (n = 8 each group) were profiled on the Affymetrix GeneChip Mouse Gene 1.1 ST v1 platform. Transcript level Robust Multi-array Average (RMA) normalization was performed using the oligo package (v 1.14) 59 in the R environment (v 2.15). Mouse transcripts were mapped to Human transcripts using gene orthology predicted by EnsemblCompara GeneTrees60 available on Ensembl BioMart (GRCm38 dataset). Subsequently, the expression profiles were analyzed to assign molecular MB subgroup to the mouse tumors, using the AGDEX R package (v 1.0.1) 41 and human MB expression data from Northcott et al 57. In this cross-species comparison of global differential expression, mouse normal cerebellum and human normal cerebellum were used as references. The degrees of agreement in differential expression of the mouse tumors between each of the four human MB subgroups were assessed separately and tested for statistical significance using permutation tests.
The molecular classification of the mouse tumors were also performed using a class prediction algorithm, Prediction Analysis for Microarrays (PAM)42, as implemented in the pamr package (v 1.51). The mouse and human samples first were normalized to their respective cerebellar references. Subgroup-specific markers were identified based on Kruskal-Wallis tests with multiple hypothesis correction by the Benjamin-Hochberg method, using a false discovery rate threshold of 0.01 and a signal-to-noise ratio threshold of 1.5. The resulting 545 subgroup-specific signature markers were used as features for class prediction of the mouse tumors using a PAM classifier trained on the human MB samples. Predicted subgroups with confidence probabilities higher than established thresholds 58 were considered bona fide subgroup assignments. Plots were generated by Principal Component Analyses (PCA) on the expression profiles of human medulloblastomas (training data). The resulting eigen vectors were used to project the expression profiles of the classified samples onto the vector space spanned by the first two eigen vectors of the training data. The background confidence score gradient was generated using 200 replicates of the training data with added Gaussian noise and subsequently smoothed by Nadaraya-Watson normalization (fields v6.7.6 R package).
Rolipram treatment in mice and volumetric measurement of tumors
Control and GFAP:Gnas mutant mice at P30-P35 were randomized to receive either Rolipram (5 mg kg–1) or vehicle control [5% (v/v) DMSO] administered twice daily via intraperitoneal injection as previously described 30. Brain tissues were harvested and processed into 8-μm sections in the sagittal plane and stained with hematoxylin and eosin (H/E) or subject to immunostaining. Abnormal tissue area with densely packed cells and round-to-oval hyperchromatic nuclei was assumed to be tumor tissue in the tissue section. For volumetric measurement, sections of cerebella were scanned by using a ScanScope XT from Aperio (Vista, CA) to acquire serial section images, then stacked and aligned with the StackReg function of ImageJ to generate three-dimension models and analyzed with Imaris Software (Bitplane) to calculate the volume of tumor tissues.
Intracranial transplantation
Tumor cells isolated from Gsα mutants were plated in GNP culture medium, and harvested with 0.25% trypsin and 0.02% EDTA for 2 minutes, washed twice with Hank's balanced salt solution (HBSS), and resuspended in Ca2+ and Mg2+-free HBSS. Cell viability was determined by trypan blue exclusion. Only single-cell suspensions with more than 90% viability were used for in vivo allograft studies. Cells (5 × 105) were stereotactically injected into the lateral ventricle of nude mice (6-week-old BALB/c nu/nu; coordinates: anterior–posterior, +1.8; medial–lateral, +2.2; dorsal–ventral, -2.0 mm from Bregma).
Statistical analysis
All analyses were done using Microsoft Excel or Prism GraphPad 6.00 for Mac OS (San Diego California, www.graphpad.com). Quantifications were performed from at least three independent experimental groups. Data are presented as mean ± S.E.M. in the graphs. p-values are from Student's two-tailed t test to compare two sets of data. To compare more than two sets, one-way analysis of variance analysis (ANOVA) with a Newman–Keuls multiple comparison test for post-hoc analysis. Survival analyses used animal death times and censoring times when animals were sacrificed or as otherwise stated. Survival curves were plotted with the Kaplan– Meier method and compared by using a two-sided log-rank test. In human tumor data analysis, Fisher's exact test was used for data in Tumorscape database unless otherwise specified. p < 0.05 is considered to be statistically significant.
Supplementary Material
Acknowledgements
The authors would like to thank A. Gilman, L. Lum, E. Hurlock, K. Campbell, J. Chan, H. Li, A. Hassan, D. He, E. Lu and W. Ding for comments and technical support. We thank B. Fritzsch (U. Iowa) for Atoh1-Cre line, R. Rohatgi (Stanford U.) for Ptch1 antibody and S. Scales (Genentech Inc.) for Gli2, Gli3 and GPR161 antibodies. This study was funded in part by grants from the US National Institutes of Health (R01 NS078092 and R01 NS075243) to QRL and from Canadian Institutes of Health Research (CIHR) to MDT, and postdoctoral fellowship by the Mildred-Scheel foundation/German Cancer Aid (MR).
Footnotes
Author contributions
X. H., and Q. R. L. designed the experiments, analyzed the data and wrote the manuscript with input from all authors. X. H., L. Z., Y. C., M. R., D. S., F. L., H. W., Y. Y., Y. X., Y. D., V. R., X. W. and T. H. carried out the in vitro, in vivo, gene profiling or in silico studies. M. K. and S. M. P. provided whole-genome sequencing data. Y. H., F. W. and W. Z. provided resources and supervision. L.S.W. provided the Gnas floxed mice. D. K. B. and S. H. K. diagnosed and confirmed the GNAS mutated MB. S. L. P., R. J. G., J. B. R. and R. W-R provided conceptual advice and edited the manuscript. M. D. T. and Q. R. L. supervised the project.
Accession codes: The RNA-seq, mRNA affymetrix GeneChip microarray and aCGH microarray data are deposited in the NCBI Gene Expression Omnibus (GEO) under accession number GSE53248.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Taylor MD, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta neuropathologica. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. doi:10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Northcott PA, et al. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta neuropathologica. 2011;122:231–240. doi: 10.1007/s00401-011-0846-7. doi:10.1007/s00401-011-0846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grammel D, et al. Sonic hedgehog-associated medulloblastoma arising from the cochlear nuclei of the brainstem. Acta neuropathologica. 2012;123:601–614. doi: 10.1007/s00401-012-0961-0. doi:10.1007/s00401-012-0961-0. [DOI] [PubMed] [Google Scholar]
- 4.Sasai K, et al. Medulloblastomas derived from Cxcr6 mutant mice respond to treatment with a smoothened inhibitor. Cancer research. 2007;67:3871–3877. doi: 10.1158/0008-5472.CAN-07-0493. [DOI] [PubMed] [Google Scholar]
- 5.Kool M, et al. Genome Sequencing of SHH Medulloblastoma Predicts Genotype-Related Response to Smoothened Inhibition. Cancer cell. 2014;25:393–405. doi: 10.1016/j.ccr.2014.02.004. doi:10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewiadomski P, Zhujiang A, Youssef M, Waschek JA. Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA. Cellular signalling. 2013;25:2222–2230. doi: 10.1016/j.cellsig.2013.07.012. doi:10.1016/j.cellsig.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. doi:10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 8.Kan Z, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. doi:10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 9.Huh JY, et al. Novel nonsense GNAS mutation in a 14-month-old boy with plate-like osteoma cutis and medulloblastoma. The Journal of dermatology. 2014 doi: 10.1111/1346-8138.12284. doi:10.1111/1346-8138.12284. [DOI] [PubMed] [Google Scholar]
- 10.Cho YJ, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29:1424–1430. doi: 10.1200/JCO.2010.28.5148. doi:10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remke M, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29:2717–2723. doi: 10.1200/JCO.2011.34.9373. doi:10.1200/JCO.2011.34.9373. [DOI] [PubMed] [Google Scholar]
- 12.Remke M, et al. FSTL5 is a marker of poor prognosis in non-WNT/non-SHH medulloblastoma. J Clin Oncol. 2011;29:3852–3861. doi: 10.1200/JCO.2011.36.2798. doi:10.1200/JCO.2011.36.2798. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZJ, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer cell. 2008;14:135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuo L, et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 15.Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol. 2012;8:340–351. doi: 10.1038/nrneurol.2012.78. doi:10.1038/nrneurol.2012.78. [DOI] [PubMed] [Google Scholar]
- 16.Regard JB, et al. Wnt/beta-catenin signaling is differentially regulated by Galpha proteins and contributes to fibrous dysplasia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20101–20106. doi: 10.1073/pnas.1114656108. doi:10.1073/pnas.1114656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu QR, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 18.Hohenegger M, et al. Gsalpha-selective G protein antagonists. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:346–351. doi: 10.1073/pnas.95.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graziano MP, Gilman AG. Synthesis in Escherichia coli of GTPase-deficient mutants of Gs alpha. The Journal of biological chemistry. 1989;264:15475–15482. [PubMed] [Google Scholar]
- 20.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nature reviews. Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 21.Tuson M, He M, Anderson KV. Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development. 2011;138:4921–4930. doi: 10.1242/dev.070805. doi:10.1242/dev.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozawa YI, Lin C, Chuang PT. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Current opinion in genetics & development. 2013 doi: 10.1016/j.gde.2013.04.008. doi:10.1016/j.gde.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Y, Wang C, Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Developmental biology. 2009;326:177–189. doi: 10.1016/j.ydbio.2008.11.009. doi:10.1016/j.ydbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conti M, Jin SL. The molecular biology of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol Biol. 1999;63:1–38. doi: 10.1016/s0079-6603(08)60718-7. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. doi:10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regard JB, et al. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nature medicine. 2013;19:1505–1512. doi: 10.1038/nm.3314. doi:10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukhopadhyay S, et al. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. doi:10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, et al. Blocking CXCR4-mediated cyclic AMP suppression inhibits brain tumor growth in vivo. Cancer research. 2007;67:651–658. doi: 10.1158/0008-5472.CAN-06-2762. doi:10.1158/0008-5472.CAN-06-2762. [DOI] [PubMed] [Google Scholar]
- 31.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. doi:10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 32.Goetz SC, Ocbina PJ, Anderson KV. The primary cilium as a Hedgehog signal transduction machine. Methods in cell biology. 2009;94:199–222. doi: 10.1016/S0091-679X(08)94010-3. doi:10.1016/S0091-679X(08)94010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. doi:10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dijkgraaf GJ, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer research. 2011;71:435–44. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 35.Schuller U, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xin M, et al. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1354–1365. doi: 10.1523/JNEUROSCI.3034-04.2005. doi:10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu QR, et al. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 38.Machold R, Klein C, Fishell G. Genes expressed in Atoh1 neuronal lineages arising from the r1/isthmus rhombic lip. Gene expression patterns : GEP. 2011;11:349–359. doi: 10.1016/j.gep.2011.03.007. doi:10.1016/j.gep.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson P, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. doi:10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 41.Pounds S, et al. A procedure to statistically evaluate agreement of differential expression for cross-species genomics. Bioinformatics. 2011;27:2098–2103. doi: 10.1093/bioinformatics/btr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. doi:10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sengupta R, et al. CXCR4 activation defines a new subgroup of Sonic hedgehog-driven medulloblastoma. Cancer research. 2012;72:122–132. doi: 10.1158/0008-5472.CAN-11-1701. doi:10.1158/0008-5472.CAN-11-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Northcott PA, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488:49–56. doi: 10.1038/nature11327. doi:10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra PK, et al. Misregulation of Scm3p/HJURP causes chromosome instability in Saccharomyces cerevisiae and human cells. PLoS Genet. 2011;7:e1002303. doi: 10.1371/journal.pgen.1002303. doi:10.1371/journal.pgen.1002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunleavy EM, et al. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137:485–497. doi: 10.1016/j.cell.2009.02.040. doi:10.1016/j.cell.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 47.Parsons DW, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331:435–439. doi: 10.1126/science.1198056. doi:10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silbereis JC, et al. Olig1 function is required to repress dlx1/2 and interneuron production in Mammalian brain. Neuron. 2014;81:574–587. doi: 10.1016/j.neuron.2013.11.024. doi:10.1016/j.neuron.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Hayre M, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nature reviews. Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. doi:10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romer JT, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Yauch RL, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. doi:10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sengupta R, Sun T, Warrington NM, Rubin JB. Treating brain tumors with PDE4 inhibitors. Trends Pharmacol Sci. 2011;32:337–344. doi: 10.1016/j.tips.2011.02.015. doi:10.1016/j.tips.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: A versatile source of anticancer drugs. Pharmacognosy reviews. 2011;5:1–12. doi: 10.4103/0973-7847.79093. doi:10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nikaido T, et al. Inhibition of adenosine 3′,5′-cyclic monophosphate phosphodiesterase by flavonoids. III. Chemical & pharmaceutical bulletin. 1989;37:1392–1395. doi: 10.1248/cpb.37.1392. [DOI] [PubMed] [Google Scholar]
- 55.Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nature reviews. Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. doi:10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen M, et al. Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. The Journal of clinical investigation. 2005;115:3217–3227. doi: 10.1172/JCI24196. doi:10.1172/JCI24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Northcott PA, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. doi:10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Northcott PA, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta neuropathologica. 2012;123:615–626. doi: 10.1007/s00401-011-0899-7. doi:10.1007/s00401-011-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–2367. doi: 10.1093/bioinformatics/btq431. doi:10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vilella AJ, et al. EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 2009;19:327–335. doi: 10.1101/gr.073585.107. doi:10.1101/gr.073585.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.